Abstract
Opioids remain an essential part of the treatment of chronic pain. However, their use and increasing rates of misuse are associated with high morbidity and mortality. The development of tolerance to opioids and analgesics further complicates dosing and the need to reduce side effects. First-generation digital systems were developed to improve analgesics but are not always capable of making clinically relevant associations and do not necessarily lead to better clinical efficacy. A lack of improved clinical outcomes makes these systems less applicable for adoption by clinicians and patients. There is a need to enhance the therapeutic regimens of opioids. In the present paper, we present the use of a digital analgesic that consists of an analgesic administered under the control of a second-generation artificial intelligence system. Second-generation systems focus on improved patient outcomes measured based on clinical response and reduced side effects in a single subject. The algorithm regulates the administration of analgesics in a personalized manner. The digital analgesic provides advantages for both users and providers. The system enables dose optimization, improving effectiveness, and minimizing side effects while increasing adherence to beneficial therapeutic regimens. The algorithm improves the clinicians’ experience and assists them in managing chronic pain. The system reduces the financial burden on healthcare providers by lowering opioid-related morbidity and provides a market disruptor for pharma companies.
Introduction
Treatment of chronic pain and loss of analgesic effects is a significant unmet need. Over 100 million individuals in the United States experience chronic pain, and approximately a quarter of them experience daily pain.Citation1–Citation4 Using opioid therapy for pain has increased in the past two decades.Citation5 Concurrently, there is a significant increase in the misuse and abuse of prescribed opioids and inadvertent opioid overdoses.Citation6 Opioid analgesics are essential therapeutic options for chronic non-cancer pain (CNCP) and cancer-related pain.Citation7–Citation9 Regular use of opioids in CNCP leads to increased all-cause mortality, thus highlighting the need for educated prescribing.Citation10 Half of the patients with advanced malignancy experience chronic pain.Citation11 Most subjects undergoing pharmacologic interventions do not experience the desired effect, and many develop drug tolerance or resistance.Citation2
Current regimens of opioid prescription lead to high morbidity and mortality.Citation12,Citation13 Abuse of opioids has a devastating influence on public health worldwide.Citation14 This epidemic involves increased misuse and overdose of opioids, leading to increased mortality. Prescribers play a vital role in the ongoing opioid epidemics by overprescribing these medications.Citation15–Citation17 Unintentional drug overdose involving prescription opioids is increasing with marked heterogeneity in dosages.Citation18 During the coronavirus disease 2019 pandemic, it has become even more apparent that this at-risk group faces many challenges to recovery, including an increase in overdoses.Citation19–Citation21
There are many causes of opioid dependence or lack of proper adherence to therapeutic regimens. A lack of standardization of use and the development of tolerance that leads to increasing dosages associated with increased severe side effects and risks of dependency are some of the challenges, further complicating beneficial and safe usage of opioids.Citation22,Citation23
Opioids remain a vital part of the treatment regimens for chronic pain, making better methods for using them an unmet need.Citation12 There remains a need for better approaches that balance treating chronic pain while minimizing risks for opioid abuse, misuse, and diversion.Citation24 This paper introduces the digital analgesic, comprising an opioid or any other type of analgesic, including nerve stimulators, controlled by a second-generation digital health system to optimize the dose, reduce tolerance, and minimize side effects. The digital analgesic can overcome several of the challenges raised by the opioid epidemic, in an attempt to increase the safety and effectiveness of the use of these drugs.
The Need for Improving the Therapeutic Regimens of Opioids and Other Types of Analgesics
Opioids are the utmost analgesic medications used for severe pain.Citation25 Opioids activate the endogenous opioid system, comprising four prominent peptide families (beta-endorphin, enkephalins, dynorphins, nociceptin/orphanin FQ), and four G protein-coupled receptors (mu, delta, kappa, and nociceptin). Opioids activate several signaling pathways to control neuronal function and neurotransmission.Citation25
The obstacles to using opioids consist of patient barriers, including reluctance to report pain to take analgesics, and professional barriers, including inadequate pain assessment and lack of knowledge of opioid-based regimens. Prescribing opioids is associated with long-term continued opioid use.Citation26 Their use in patients with chronic pain requires precision in management. The current recommendations are to use opioids only when the benefits outweigh the risks.Citation23 Caregivers need to establish their patients’ treatment goals and consider stopping the therapy if the risks outweigh the benefits. Clinicians need to prescribe the lowest effective dosage and look into the prescription drug monitoring program data, when available, for high-risk combinations or dosages. Patients suffering from opioid use disorders should receive an evidence-based treatment regimen.Citation27 The risks of long-term adverse effects associated with opioids are significant obstacles to their use in chronic pain. Respiratory depression is a side effect that causes worry and distress in patients and their care givers.Citation28–Citation30 Aspects related to high risk of misuse are younger age, a history of a substance use disorder, major depression, and psychotropic medications.Citation31,Citation58,Citation59
Increased dosing is not always associated with an improved result. A recent study determined the physical and emotional dimensions of health for CNCP patients receiving opioid therapy using the 36-Item Short-Form Health Survey (SF-36). The data showed that patients receiving low-dose or high-dose opioid treatment do not have significantly different quality-of-life outcomes. Patients on high doses had lower item scores, indicating poorer health.Citation32 Opioids may sometimes increase rather than decrease pain. Central sensitization (hyperalgesia) underlies pain chronification generated by high dose and high potency opioids.Citation23,Citation33
Implementing various interventions for dealing with increased opioid consumption; however, their effects on abuse and overdose-related death have been conflicting.Citation34 The route of administration of systemic opioids affects their potency.Citation35 Monitoring of prescribing practices, urine testing, prescription monitoring programs, opioid treatment agreements, and utilization of universal precautions are essential. Using a combination of strategies to stratify risk, identify abnormal drug-related behaviors, enabling tailoring treatments accordingly.Citation24 Prescription monitoring programs targeting health care providers (HCPs) aim to reduce opioid prescriptions. However, their effect on the correctness of use according to published guidelines is inconsistent. Continuing medical education (CME) and pain management programs improve chronic pain management under specific settings.Citation34 Prescription monitoring programs (PMPs) vary between countries. A retrospective study of PMPs’ controlled substance database showed that the regular monthly total prescriptions written within the state decreased by 16%.Citation36 A pragmatic approach to the careful use of opioids has for different settings to overcome some of these barriers.Citation37 These programs do not provide solutions for the challenges of optimizing dosing for better effectiveness while minimizing side effects.
The challenges of standardizing treatment regimens involve the hard-to-control patient-, disease-, and environment-related variables that affect the response’s magnitude and the development of complications. Inter- and intra-subject variability in pain alleviation and side effects further complicate the attempts to formulate therapy guidelines and monitor these patients.
Development of Tolerance to Opioids and Analgesics Complicates Appropriate Dosing Regimens
Development of tolerance to chronic opioid use is the main reason for inadequate pain control, leading to dose escalation, hence making the related side effects severe and widespread.Citation38 Long-term opioids use associated with the development of analgesic tolerance. Tolerance reduces the impact following prolonged drug administration that decreases drug potency, characterized by a shift to the right in the dose-response curve.Citation39 Multiple mechanisms are associated with tolerance development, including patient and disease-related parameters.Citation40,Citation41 Opioid tolerance manifests in reduced analgesic effect and respiratory depression, sedation, nausea, and other signs of depression of the central nervous system.Citation42 Interactions between the opioid receptors and the medications, frequency of administration, and doses are factors associated with the development of tolerance.
A rightward move in the agonist dose-response curve after repeated administration exemplifies tolerance.Citation43 Intracellular neural machinery involves desensitization and downregulation of the opioid receptors that underlie opioid tolerance.Citation43 Opioid tolerance is an adaptive process resulting from modifications at the level of the µ-opioid receptors (MORs) and in the circuit and synaptic levels in the central and peripheral nervous systems.Citation44 Morphine is associated with alterations of expression of miRNAs in neuronal tissues or cells. Different types of opioids are associated with modifications in the degrees and types of miRNAs expressions.Citation45–Citation47 Neuroadaptation and downregulation of MORs underlie tolerance development. Downregulation of MORs results from increased degradation decreased expression, and neuroadaptation.Citation48,Citation49 The binding of the opioid to MORs induces an inhibitory G protein signal associated with an analgesic effect. Side effects include respiratory depression, analgesic tolerance, and constipation, related to MOR modulation.Citation50–Citation52 Several types of machinery underlie behavioral tolerance to opioids, including desensitization of receptor signaling; downregulation of receptors; induction of drug metabolism, termed metabolic tolerance; and compensatory processes.Citation53
Studies showed marked inter and intra-individual variability in the development of opioid tolerance following short- or long-term dosing.Citation39 Genetic and epigenetic variations in receptors contribute to the numerous different responses to opioids between individuals.Citation54–Citation56 Individual parameters associated with the loss of response include the source of pain, age and sex; pharmacokinetics and pharmacodynamics; genetic polymorphisms; comorbidities and environmental factors; medication interference; and treatment adherence.Citation57 Opiate dependency can result from modifications in the opioid receptor genes and other genes. The preproenkephalin gene encodes peptides’ modulation of pain perception, contributing to addiction development.Citation58 Prior opioid exposure, the use of medications that interact with or augment the effect of opioids, and end-organ function are relevant to the outcome of therapy.Citation59,Citation60
The proportion of opioid-tolerant-only extended-release and long-acting (ER/LA) opioid analgesics studied for new users of extended-release oxycodone (ERO) and other ER/LA opioid analgesics. 64% of ERO, 64% of extended-release hydromorphone, and 40% of transdermal fentanyl developed tolerance.Citation61 In a recent study of 372,038 initiators, only 38% of subjects did not meet the opioid tolerance criteria before starting high dosage treatment.Citation62 Extended-release opioids administered at short intervals increased the daily doses, increasing the risk of opioid-related mortality.Citation63
Dose Alterations and Drug Holidays Can Improve the Effectiveness of Chronic Use of Opioids
Treatment regimens based on fixed dosing and times of administration of chronic drugs are not in line with the variability inherent in biological systems. These regimens may contribute to a high primary and secondary lack of response to chronic drug administration.Citation64–Citation68 The development of tolerance to opioids is a significant challenge for their safe use. It is associated with a loss of response and increased dosages while increasing the risk of side effects.Citation69–Citation71
Strategies for mitigating opioid tolerance and opioid-induced hyperalgesia include lowering dosages, shortening the duration of treatment by interrupting infusions of sedative or analgesic agents daily, modulating infusions based on analgesic assessment and sedation scores, using multimodal analgesic agents (nerve blocks and non-opioid analgesics), and by rotating analgesic agents sequentially.Citation72
Improving opioid effectiveness and minimizing side effects can be achieved through drug holidays and dose alterations. Drug holidays and intermittent dose escalations and reductions can improve the response rate to chronic therapies.Citation73–Citation79 Models of drug holidays developed to regenerate responsiveness to opioids lost during the development of tolerance.Citation80 Drug holidays may help manage opioid tolerance.Citation35,Citation81,Citation82 There have been reports of positive outcomes of drug holidays in patients with chronic pain and opioid addiction, including anesthesia-based detoxification in extreme cases.Citation83 There is an increase in post-surgical complications that are dose-dependent on opioids. A retrospective study of 8559 patients undergoing revision of total hip arthroplasty showed that an “opioid holiday” in patients with chronic treatment lowers complications.Citation84
Patients suffering from cancer-associated pain suffer from episodic breakthrough pain while receiving a stable dose of opioids. Dosing of an immediate-release opioid administered in a proportion of the total daily dose in many cases. In a double-blind, randomized trial, three-dose proportions (1/6, 1/8, and 1/12 of the total daily dose) were administered in two parts, each with three dose proportions in random order. When patients required opioid breakthrough doses, they took the next numbered bottle rather than their usual breakthrough dose. The study results did not show any difference between the three-dose proportions regarding the decrease in pain intensity, pain control on a subsequent day, time to pain relief, or any difference in side effects. The study showed that 1/12 the 24 hourly doses should be the lowest dose that delivers benefit.Citation85 In a retrospective trial of patients with chronic osteoarthritis pain, a short-acting opioid provided a safer and cheaper alternative to long-acting opioid therapy.Citation86
In a retrospective study, drug holidays improved the tolerance in patients with nonmalignant pain who received continuous intrathecal opioid therapy using implantable infusion pump devices.Citation35 In intractable cancer pain during methadone substitution therapy, an antagonist supported opioid detoxification, and a drug holiday reestablished sensitivity to opioids and improved pain.Citation83 An abstinence-oriented approach enhanced the outcomes by treating patients with chronic pain and aberrant drug-taking behavior. This method involves patients initially discontinuing opioid use for a drug holiday.Citation87
These data support the option of changing dosage to improve the response rate to chronic opioid use.
Using First-Generation Digital Systems for Improving the Use of Analgesics
First-generation artificial intelligence (AI) systems promote the 4Ps- personalized, preventive, predictive, and participatory-medicine model, describing patient autonomy.Citation88 They focus on clinical big data-based decision-making and produce evidence-based information. Their real-world utilization is inadequate as they do not necessarily result in a better response or lower rates of unwanted side effects.Citation89–Citation94
Developing big data resources is associated with biases that affect the products of treatment algorithms.Citation90 First-generation algorithms are not always capable of making clinically relevant associations and do not signify better clinical efficacy.Citation95 A lack of improved clinical outcomes makes these systems less applicable for adoption by clinicians and patients.Citation96,Citation97 Apps that provide opioid dose double-checking can prevent mistakes in dosing and misuse, lack accuracy, and proper clinical validation.Citation98 An opioid dose conversion app, the Safer Prescription of Opioids Tool (SPOT), developed as a clinical decision support (CDS) system, was proposed as a method for improving prescription accuracy.Citation99 A review of multiple studies on mobile phones reminding patients to take their drugs correctly showed a relatively limited effect on adherence.Citation100
Advantages of Using Second-Generation Digital Health Systems
Second-generation AI comprises personalized closed-loop systems that improve patient responses to chronic drugs.Citation96 These systems focus on improved patient outcomes measured by the clinical response and reduced side effects in a single subject.Citation96 They add the “5th P,” progress, to improve clinically meaningful results in a subject-tailored manner.Citation96 These systems implement an n=1 concept to personalized therapeutic regimens to overcome big data biases associated with big data.Citation96
The improved clinical outcomes confirm increased adherence of patients to appropriate regimens and maintainable response to chronic drugs while dealing with the compensatory mechanisms which underlie tolerance and progression of disease.Citation23,Citation96,Citation101
A second-generation system introduces personalized variability signatures into an algorithm to improve the beneficial effects of chronic drugs.Citation66–Citation68,Citation96,Citation102–Citation113 This system can quantify subject-tailored variability patterns and implement them through individualized algorithms.Citation66,Citation67,Citation96,Citation105 This approach can deal with drug tolerance and ensure the sustainable beneficial effects of chronic drug use. At the first level of this second-generation system, it implements variability in dosage and administration times of drugs to patients who lose their responses to chronic drugs while keeping these changes within the approved therapeutic windows. Ongoing clinical trials evaluate these regimens in patients with inflammatory bowel disease who have lost their response to anti-TNFs and in patients with drug-resistant epilepsy. Higher levels of the system comprise closed-loop algorithms designed to receive inputs about clinical outcomes and side effects and respond appropriately by suiting the variability in clinical outcomes. Disease and host-linked patterns of variability are followed, quantified, and implemented into the algorithm. The system disregards genotypic and phenotypic parameters by summing up the total effects of these parameters on the outcomes.Citation96
a. The digital analgesic: An analgesic where second-generation AI systems control its administration for optimizing the dose, improving effectiveness, and minimizing side effects
Digital analgesics comprise an opioid formulation or any other analgesic, including nerve stimulators and opioid pumps. A second-generation AI system controls their administration applied through a user-friendly app downloaded to the subject’s cellphone. The algorithm regulates the administration of analgesics in a personalized manner, with two endpoints: effectiveness of pain relief and reduction of unwanted side effects.Citation23,Citation96
The digital analgesic enables patients and their physicians to follow personalized therapeutic regimens to improve responses, overcome tolerance, optimize dosages, and minimize side effects. Improving pain relief while reducing side effects increases patients’ adoption of the system and improves physicians’ experience.
Version 1.0 of the digital analgesic remains within the digital reminders to improve patient adherence. It comprises an open-loop system that does not collect data. The system provides variability in dosing and drug administration times within the approved range as pre-determined by the caregivers for each patient. The physician enters the minimal and maximal daily dosage range and the minimal and maximum dosages per day. The digital system provides patients with a regimen that continuously changes within the approved boundaries. These systems may be exempt from all regulatory processes.Citation114 Later versions use closed-loop regimens for personalized therapies by implementing personal variability signatures into therapeutic protocols and continuously adapting the regimens to the clinical outcomes.Citation23,Citation96
b. The digital analgesic as a market differentiator: Advantages for patients, clinicians, healthcare authorities, payers, and companies
The digital analgesic improves global health by improving pain, reducing side effects, and increasing users’ and institutions’ savings by avoiding the need for more expensive interventions. The highly competitive analgesic market necessitates companies to develop market differentiators. The digital analgesic provides a clinical benefit while using the same generic product, offering a clear advantage for companies, translated to increased sales and better pricing. Combining a generic analgesic with a second-generation AI system generates intellectual property (IP). Multiple users’ use of the digital pill creates big data resources based on clinical outcomes, which can overcome biases inherent in first-generation systems. The new IP and generated big data can serve as additional market differentiators.
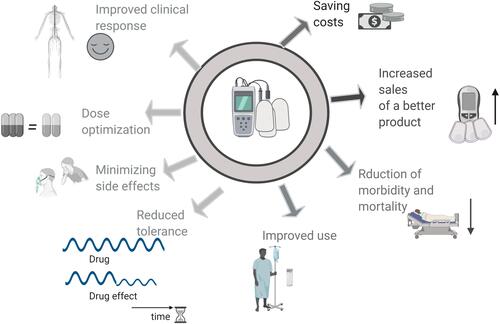
presents the advantages of digital analgesics. Digital analgesics provide advantages to all players in the health care system. Patients and caregivers benefit from the improved clinical response, reduced tolerance, dose optimization, and minimal side effects. The healthcare system benefits from saving costs. Public health benefits from improved use, reduced misuse, and high morbidity and mortality rates. Drug manufacturers can expect increased sales of better products.
Figure 1 The advantages of the digital analgesic over regular therapeutic regimens. Digital analgesics provide advantages to all participants of the healthcare system. Patients and physicians benefit from the improved clinical response, reduced tolerance, dose optimization, and minimizing side effects. Healthcare providers benefit from the reduction in costs.
Summary
The digital analgesic consists of an opioid formulation or any other analgesic controlled by a second-generation AI system that implements variability into dosing regimens. Digital analgesics may improve patient responses to drugs by reducing tolerance, optimizing dosages, and lowering the rates of side effects. Maximizing the therapeutic benefits of pain medications is anticipated to improve overall patients’ wellbeing and reduce healthcare costs. Future studies and real-world data collection will assist in implementing these systems in routine care.
Abbreviations
CNCP, chronic non-cancer pain; PMPs, prescription monitoring programs; HCPs, health care providers; CME, continuing medical education; MORs, μ opioid receptors; ER/LA, extended-release and long-acting; ERO, extended-release oxycodone; AI, artificial intelligence; SPOT, Safer Prescription of Opioids Tool; CDS, clinical decision support; IP, intellectual property.
Disclosure
YI is the founder of Oberon Sciences. The authors report no other conflicts of interest in this work.
References
- Borsook D, Youssef AM, Simons L, et al. When pain gets stuck: the evolution of pain chronification and treatment resistance. Pain. 2018;159:2421–2436. doi:10.1097/j.pain.0000000000001401
- Moore A, Derry S, Eccleston C, et al. Expect analgesic failure; pursue analgesic success. BMJ. 2013;346:f2690. doi:10.1136/bmj.f2690
- National Academies Press. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): National Academies Press; 2011.
- Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16:769–780. doi:10.1016/j.jpain.2015.05.002
- Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948–956. doi:10.1097/ALN.0b013e3181cdef98
- Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. doi:10.7326/0003-4819-152-2-201001190-00006
- Nijland L, Schmidt P, Frosch M, et al. Subcutaneous or intravenous opioid administration by patient-controlled analgesia in cancer pain: a systematic literature review. Support Care Cancer. 2019;27:33–42.
- Xing F, Yong RJ, Kaye AD, et al. Intrathecal Drug Delivery and Spinal Cord Stimulation for the Treatment of Cancer Pain. Curr Pain Headache Rep. 2018;22:11. doi:10.1007/s11916-018-0662-z
- Novaes MA, Knobel E, Bork AM, et al. Stressors in ICU: perception of the patient, relatives and health care team. Intensive Care Med. 1999;25:1421–1426. doi:10.1007/s001340051091
- Hauser W, Schubert T, Vogelmann T, et al. All-cause mortality in patients with long-term opioid therapy compared with non-opioid analgesics for chronic non-cancer pain: a database study. BMC Med. 2020;18(1):162. doi:10.1186/s12916-020-01644-4
- van den Beuken-van Everdingen MHJ, van Kuijk SMJ, Janssen DJA, et al. Treatment of Pain in Cancer: towards Personalised Medicine. Cancers. 2018;2:10.
- Fanelli G, Tolle TR, Dea J, et al. Opioids for chronic non-cancer pain: a critical view from the other side of the pond. Minerva Anestesiol. 2016;82:97–102.
- Volkow ND. Collision of the COVID-19 and Addiction Epidemics. Ann Intern Med. 2020;173:61–62. doi:10.7326/M20-1212
- Slater RR, Uong J, Gupta R, et al. The Opioid Epidemic: risk Evaluation and Management Strategies for Prescribing Opioids. Instr Course Lect. 2020;69:405–414.
- Kuehn BM. Efforts aim to curb opioid deaths, injuries. JAMA. 2009;301:1213–1215. doi:10.1001/jama.2009.367
- Morasco BJ, Gritzner S, Lewis L, et al. Systematic review of prevalence, correlates, and treatment outcomes for chronic non-cancer pain in patients with comorbid substance use disorder. Pain®. 2011;152(3):488–497. doi:10.1016/j.pain.2010.10.009
- McEwen S, Prakken S. Reducing the oversupply of prescription opioids. N C Med J. 2018;79:175–180. doi:10.18043/ncm.79.3.175
- Adewumi AD, Hollingworth SA, Maravilla JC, et al. Prescribed Dose of Opioids and Overdose: a Systematic Review and Meta-Analysis of Unintentional Prescription Opioid Overdose. CNS Drugs. 2018;32(2):101–116. doi:10.1007/s40263-018-0499-3
- Alexander GC, Stoller KB, Haffajee RL, et al. An Epidemic in the Midst of a Pandemic: opioid Use Disorder and COVID-19. Ann Intern Med. 2020;173(1):57–58. doi:10.7326/M20-1141
- Slavova S, Rock P, Bush HM, et al. Signal of increased opioid overdose during COVID-19 from emergency medical services data. Drug Alcohol Depend. 2020;214:108176. doi:10.1016/j.drugalcdep.2020.108176
- Dubey MJ, Ghosh R, Chatterjee S, et al. COVID-19 and addiction. Diabetes Metab Syndr. 2020;14:817–823. doi:10.1016/j.dsx.2020.06.008
- Helmerhorst GT, Teunis T, Janssen SJ, et al. An epidemic of the use, misuse and overdose of opioids and deaths due to overdose, in the United States and Canada: is Europe next? Bone Joint J. 2017;99-B:856–864. doi:10.1302/0301-620X.99B7.BJJ-2016-1350.R1
- Azmanov HRE, Ilan Y. Establishment of an individualized chronotherapy, autonomic nervous system, and variability-based dynamic platform for overcoming the loss of response to analgesics. Pain Physician. 2020;1:324.
- Orleans L, Kaye AD. Prescription Opioid Abuse in Chronic Pain: an Updated Review of Opioid Abuse Predictors and Strategies to Curb Opioid Abuse: part. Pain Physician. 2017;20:S93–S109.
- Corder G, Castro DC, Bruchas MR, et al. Endogenous and Exogenous Opioids in Pain. Annu Rev Neurosci. 2018;41:453–473. doi:10.1146/annurev-neuro-080317-061522
- Sanger N, Bhatt M, Singhal N, et al. Adverse Outcomes Associated with Prescription Opioids for Acute Low Back Pain: a Systematic Review and Meta-Analysis. Pain Physician. 2019;22:119–138.
- Control CfD, Prevention. CDC guideline for prescribing opioids for chronic pain—United States, 2016. Morbidity Mortality Weekly Rep. 2016;65:1–49. doi:10.15585/mmwr.mm6501a1
- Edwards H, Bennett M. Access to Opioids for Patients with Advanced Disease. Curr Pharm Des. 2019;25:3203–3208. doi:10.2174/1381612825666190716095337
- Rose ME. Are Prescription Opioids Driving the Opioid Crisis? Assumptions vs Facts. Pain Med. 2018;19:793–807. doi:10.1093/pm/pnx048
- Ballantyne JC. Opioids for the Treatment of Chronic Pain: mistakes Made, Lessons Learned, and Future Directions. Anesth Analg. 2017;125:1769–1778. doi:10.1213/ANE.0000000000002500
- Cragg A, Hau JP, Woo SA, et al. Risk Factors for Misuse of Prescribed Opioids: a Systematic Review and Meta-Analysis. Ann Emerg Med. 2019;74:634–646. doi:10.1016/j.annemergmed.2019.04.019
- Denawa Y, Kurtz W, Conermann T. The Social and Functional Implications of High- Versus Low-Dose Opioids on Chronic Non-Cancer Pain. Pain Physician. 2019;22:401–411. doi:10.36076/ppj/2019.22.401
- Rivat C, Ballantyne J. The dark side of opioids in pain management: basic science explains clinical observation. Pain Rep. 2016;1:e570. doi:10.1097/PR9.0000000000000570
- Moride Y, Lemieux-Uresandi D, Castillon G, et al. A Systematic Review of Interventions and Programs Targeting Appropriate Prescribing of Opioids. Pain Physician. 2019;22:229–240. doi:10.36076/ppj/2019.22.229
- Winkelmuller M, Winkelmuller W. Long-term effects of continuous intrathecal opioid treatment in chronic pain of nonmalignant etiology. J Neurosurg. 1996;85:458–467. doi:10.3171/jns.1996.85.3.0458
- Manders L, Abd-Elsayed A. Mandatory Review of Prescription Drug Monitoring Program Before Issuance of a Controlled Substance Results in Overall Reduction of Prescriptions Including Opioids and Benzodiazepines. Pain Physician. 2020;23:299–304.
- Gaertner J, Boehlke C, Simone CB, et al. Early palliative care and the opioid crisis: ten pragmatic steps towards a more rational use of opioids. Ann Palliat Med. 2019;8:490–497. doi:10.21037/apm.2019.08.01
- Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Eng J Med. 2016;374:1253–1263. doi:10.1056/NEJMra1507771
- Mercadante S, Arcuri E, Santoni A. Opioid-Induced Tolerance and Hyperalgesia. CNS Drugs. 2019;33:943–955. doi:10.1007/s40263-019-00660-0
- Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154:384–396. doi:10.1038/bjp.2008.100
- Bailey CP, Connor M. Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol. 2005;5:60–68. doi:10.1016/j.coph.2004.08.012
- Rivat C, Ballantyne J. The dark side of opioids in pain management: basic science explains clinical observation. Pain Reports. 2016;1:54.
- Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol. 2011;164:1322–1334. doi:10.1111/j.1476-5381.2011.01335.x
- Zhang TJ, Qiu Y, Hua Z. The emerging perspective of morphine tolerance: microRNAs. Pain Res Manage. 2019;2019:34. doi:10.1155/2019/9432965
- Tapocik JD, Ceniccola K, Mayo CL, et al. MicroRNAs are involved in the development of morphine-induced analgesic tolerance and regulate functionally relevant changes in Serpini1. Front Mol Neurosci. 2016;9:20. doi:10.3389/fnmol.2016.00020
- Toyama K, Kiyosawa N, Watanabe K, et al. Identification of circulating miRNAs differentially regulated by opioid treatment. Int J Mol Sci. 2017;18:1991. doi:10.3390/ijms18091991
- Wang J, Xu W, Zhong T, et al. miR-365 targets β-arrestin 2 to reverse morphine tolerance in rats. Sci Rep. 2016;6:38285. doi:10.1038/srep38285
- Christie M. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154:384–396.
- Kieffer BL, Evans CJ. Opioid tolerance–in search of the holy grail. Cell. 2002;108:587–590. doi:10.1016/S0092-8674(02)00666-9
- Matthes HW, Maldonado R, Simonin F, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the µ-opioid-receptor gene. Nature. 1996;383:819–823. doi:10.1038/383819a0
- Raehal KM, Walker JK, Bohn LM. Morphine side effects in β-arrestin 2 knockout mice. J Pharmacol Exp Therapeutics. 2005;314:1195–1201. doi:10.1124/jpet.105.087254
- Bohn LM, Gainetdinov RR, Lin F-T, et al. μ-Opioid receptor desensitization by β-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi:10.1038/35047086
- Cahill CM, Walwyn W, Taylor AM, et al. Allostatic mechanisms of opioid tolerance beyond desensitization and downregulation. Trends Pharmacol Sci. 2016;37:963–976. doi:10.1016/j.tips.2016.08.002
- Zhang G, Yang P. Bioinformatics Genes and Pathway Analysis for Chronic Neuropathic Pain after Spinal Cord Injury. Biomed Res Int. 2017;2017:6423021. doi:10.1155/2017/6423021
- Louwies T, Ligon CO, Johnson AC, et al. Targeting epigenetic mechanisms for chronic visceral pain: a valid approach for the development of novel therapeutics. Neurogastroenterol Motil. 2018;3:e13500.
- Hajj A, Khabbaz L, Laplanche JL, et al. Pharmacogenetics of opiates in clinical practice: the visible tip of the iceberg. Pharmacogenomics. 2013;14:575–585. doi:10.2217/pgs.13.13
- George B, Minello C, Allano G, et al. Opioids in cancer-related pain: current situation and outlook. Support Care Cancer. 2019;27:3105–3118. doi:10.1007/s00520-019-04828-8
- Nitsche JF, Schuller AG, King MA, et al. Genetic dissociation of opiate tolerance and physical dependence in δ-opioid receptor-1 and preproenkephalin knock-out mice. Journal of Neuroscience. 2002;22:10906–10913. doi:10.1523/JNEUROSCI.22-24-10906.2002
- Scarborough BM, Smith CB. Optimal pain management for patients with cancer in the modern era. CA Cancer J Clin. 2018;68:182–196. doi:10.3322/caac.21453
- Alexander GC, Kruszewski SP, Webster DW. Rethinking opioid prescribing to protect patient safety and public health. JAMA. 2012;308:1865–1866. doi:10.1001/jama.2012.14282
- Larochelle MR, Cocoros NM, Popovic J, et al. Opioid tolerance and urine drug testing among initiates of extended-release or long-acting opioids in Food and Drug Administration’s Sentinel System. J Opioid Manag. 2017;13:315–327. doi:10.5055/jom.2017.0407
- Young JC, Lund JL, Dasgupta N, et al. Opioid tolerance and clinically recognized opioid poisoning among patients prescribed extended-release long-acting opioids. Pharmacoepidemiol Drug Saf. 2019;28:39–47. doi:10.1002/pds.4572
- Murphy L, Brands B, Grant D, et al. Exploring the use of extended release opioids at shortened dosing intervals in people with chronic pain and high risk medication or substance use. Int J Clin Pharm. 2020;1:e4.
- Weiner WJ, Koller WC, Perlik S, et al. Drug holiday and management of Parkinson disease. Neurology. 1980;30:1257–1261. doi:10.1212/WNL.30.12.1257
- Toni T, Tidor B. Combined model of intrinsic and extrinsic variability for computational network design with application to synthetic biology. PLoS Comput Biol. 2013;9:e1002960. doi:10.1371/journal.pcbi.1002960
- Kenig A, Ilan Y. A Personalized Signature and Chronotherapy-Based Platform for Improving the Efficacy of Sepsis Treatment. Front Physiol. 2019;10:1542. doi:10.3389/fphys.2019.01542
- Khoury T, Ilan Y. Introducing Patterns of Variability for Overcoming Compensatory Adaptation of the Immune System to Immunomodulatory Agents: a Novel Method for Improving Clinical Response to Anti-TNF Therapies. Front Immunol. 2019;10:2726. doi:10.3389/fimmu.2019.02726
- Ilan Y. Generating randomness: making the most out of disordering a false order into a real one. J Transl Med. 2019;17:49. doi:10.1186/s12967-019-1798-2
- Hayhurst CJ, Durieux ME. Differential Opioid Tolerance and Opioid-induced Hyperalgesia: a Clinical Reality. Anesthesiology. 2016;124:483–488. doi:10.1097/ALN.0000000000000963
- Naliboff BD, Wu SM, Schieffer B, et al. A randomized trial of 2 prescription strategies for opioid treatment of chronic nonmalignant pain. J Pain. 2011;12(2):288–296. doi:10.1016/j.jpain.2010.09.003
- Chen L, Vo T, Seefeld L, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14:384–392. doi:10.1016/j.jpain.2012.12.012
- Martyn JJ, Mao J, Bittner EA. Opioid tolerance in critical illness. N Eng J Med. 2019;380:365–378.
- Rensing N, Han L, Wong M. Intermittent dosing of rapamycin maintains antiepileptogenic effects in a mouse model of tuberous sclerosis complex. Epilepsia. 2015;56:1088–1097. doi:10.1111/epi.13031
- Ben-Horin S, Kopylov U, Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev. 2014;13:24–30. doi:10.1016/j.autrev.2013.06.002
- Pontes C, Gratacos J, Torres F, et al. Evaluation of dose reduction versus standard dosing for maintenance of remission in patients with spondyloarthritis and clinical remission with anti-TNF (REDES-TNF): study protocol for a randomized controlled trial. Trials. 2015;16:370. doi:10.1186/s13063-015-0828-5
- Inciarte-Mundo J, Hernandez MV, Rosario V, et al. Reduction of biological agent dose in rheumatic diseases: descriptive analysis of 153 patients in clinical practice conditions. Reumatol Clin. 2014;10:10–16. doi:10.1016/j.reuma.2013.04.012
- Strik AB, Mathôt D, Ponsioen R. Dashboard driven vs. conventional dosing of infliximab in inflammatory bowel disease patients: the PRECISION trial. J Crohn’s Colitis. 2019;13:S063. doi:10.1093/ecco-jcc/jjy222.090
- Stacey BR, Emir B, Petersel D, et al. Pregabalin in treatment-refractory fibromyalgia. Open Rheumatol J. 2010;4:35–38. doi:10.2174/1874312901004010035
- Dais J, Khosia A, Doulatram G. The Successful Treatment of Opioid Withdrawal-Induced Refractory Muscle Spasms with 5-HTP in a Patient Intolerant to Clonidine. Pain Physician. 2015;18:E417–20. doi:10.36076/ppj.2015/18/E417
- Dumas EO, Pollack GM. Opioid tolerance development: a pharmacokinetic/pharmacodynamic perspective. AAPS J. 2008;10:537–551. doi:10.1208/s12248-008-9056-1
- Yu ZQ, Zhang CL, Xu YJ, et al. Chronopharmacology of analgesic effect and tolerance induced by six narcotic analgesics in mice. Drug Res. 2015;65:141–146. doi:10.1055/s-0034-1374617
- Gazelka HM, Leal JC, Lapid MI, et al. Opioids in Older Adults: indications, Prescribing, Complications, and Alternative Therapies for Primary Care. Mayo Clin Proc. 2020;95:793–800. doi:10.1016/j.mayocp.2020.02.002
- Breitfeld C, Eikermann M, Kienbaum P, et al. Opioid “holiday” following antagonist supported detoxification during general anesthesia improves opioid agonist response in a cancer patient with opioid addiction. Anesthesiology. 2003;98:571–573. doi:10.1097/00000542-200302000-00039
- Wilson JM, Farley KX, Erens GA, et al. Preoperative opioid use is a risk factor for complication following revision total hip arthroplasty. Hip Int;2020. 1120700020947400. doi:10.1177/1120700020947400
- Currow DC, Clark K, Louw S, et al. A randomized, double-blind, crossover, dose ranging study to determine the optimal dose of oral opioid to treat breakthrough pain for patients with advanced cancer already established on regular opioids. Eur J Pain. 2020;24:983–991. doi:10.1002/ejp.1548
- Ghodke A, Barquero S, Chelminski PR, et al. Short-Acting Opioids Are Associated with Comparable Analgesia to Long-Acting Opioids in Patients with Chronic Osteoarthritis with a Reduced Opioid Equivalence Dosing. Pain Med. 2018;19:2191–2195. doi:10.1093/pm/pnx245
- Blondell RD, Ashrafioun L. Treating opioid dependency and coexistent chronic nonmalignant pain. Am Fam Physician. 2008;78:1132–1133.
- Orth M, Averina M, Chatzipanagiotou S, et al. Opinion: redefining the role of the physician in laboratory medicine in the context of emerging technologies, personalised medicine and patient autonomy (‘4P medicine’). J Clin Pathol. 2019;72:191–197. doi:10.1136/jclinpath-2017-204734
- Buch VH, Ahmed I, Maruthappu M. Artificial intelligence in medicine: current trends and future possibilities. Br J Gen Pract. 2018;68:143–144. doi:10.3399/bjgp18X695213
- Kelly CJ, Karthikesalingam A, Suleyman M, et al. Key challenges for delivering clinical impact with artificial intelligence. BMC Med. 2019;17:195. doi:10.1186/s12916-019-1426-2
- Briganti G, Le Moine O. Artificial Intelligence in Medicine: today and Tomorrow. Front Med. 2020;7:27. doi:10.3389/fmed.2020.00027
- Overley SC, Cho SK, Mehta AI, et al. Navigation and Robotics in Spinal Surgery: where Are We Now? Neurosurgery. 2017;80:S86–S99. doi:10.1093/neuros/nyw077
- Tepper OM, Rudy HL, Lefkowitz A, et al. Mixed Reality with HoloLens: where Virtual Reality Meets Augmented Reality in the Operating Room. Plast Reconstr Surg. 2017;140:1066–1070. doi:10.1097/PRS.0000000000003802
- Malloy KM, Milling LS. The effectiveness of virtual reality distraction for pain reduction: a systematic review. Clin Psychol Rev. 2010;30:1011–1018. doi:10.1016/j.cpr.2010.07.001
- Keane PA, Topol EJ. With an eye to AI and autonomous diagnosis. NPJ Digit Med. 2018;1:40. doi:10.1038/s41746-018-0048-y
- Ilan Y. Second-Generation Digital Health Platforms: placing the Patient at the Center and Focusing on Clinically Meaningful Endpoints Title: second-Generation Artificial Intelligence Algorithms. Front Digital Health. 2020. doi:10.3389/fdgth.2020.569178
- Murali NS, Deao CE. Patient Engagement. Prim Care. 2019;46:539–547. doi:10.1016/j.pop.2019.07.007
- Ashton-James CE, Glare P, Darnall BD. Out of office hours: scalable, on-demand, digital support for patients tapering prescription opioids. Pain. 2020;161:2252–2254. doi:10.1097/j.pain.0000000000001947
- Flint R, Buchanan D, Jamieson S, et al. The Safer Prescription of Opioids Tool (SPOT): a Novel Clinical Decision Support Digital Health Platform for Opioid Conversion in Palliative and End of Life Care-A Single-Centre Pilot Study. Int J Environ Res Public Health. 2019;1:16.
- Demena BA, Artavia-Mora L, Ouedraogo D, et al. A Systematic Review of Mobile Phone Interventions (SMS/IVR/Calls) to Improve Adherence and Retention to Antiretroviral Treatment in Low-and Middle-Income Countries. AIDS Patient Care STDS. 2020;34(2):59–71. doi:10.1089/apc.2019.0181
- Ilan Y. Overcoming Compensatory Mechanisms toward Chronic Drug Administration to Ensure Long-Term, Sustainable Beneficial Effects. Mol Ther Methods Clin Dev. 2020;18:335–344. doi:10.1016/j.omtm.2020.06.006
- Potruch A, Khoury ST, Ilan Y. The role of chronobiology in drug-resistance epilepsy: the potential use of a variability and chronotherapy-based individualized platform for improving the response to anti-seizure drugs. Seizure. 2020;80:201–211. doi:10.1016/j.seizure.2020.06.032
- Kolben Y, Weksler-Zangen S, Ilan Y. Adropin as a potential mediator of the metabolic system-autonomic nervous system-chronobiology axis: implementing a personalized signature-based platform for chronotherapy. Obes Rev. 2020;22. doi:10.1111/obr.13108
- Kessler A, Weksler-Zangen S, Ilan Y. Role of the Immune System and the Circadian Rhythm in the Pathogenesis of Chronic Pancreatitis: establishing a Personalized Signature for Improving the Effect of Immunotherapies for Chronic Pancreatitis. Pancreas. 2020;49:1024–1032. doi:10.1097/MPA.0000000000001626
- Ilan Y. Advanced Tailored Randomness: a Novel Approach for Improving the Efficacy of Biological Systems. J Comput Biol. 2020;27:20–29. doi:10.1089/cmb.2019.0231
- Ilan Y. Order Through Disorder: the Characteristic Variability of Systems. Front Cell Dev Biol. 2020;8:186. doi:10.3389/fcell.2020.00186
- Gelman R, Bayatra A, Kessler A, et al. Targeting SARS-CoV-2 receptors as a means for reducing infectivity and improving antiviral and immune response: an algorithm-based method for overcoming resistance to antiviral agents. Emerg Microbes Infect. 2020;9(1):1397–1406. doi:10.1080/22221751.2020.1776161
- Forkosh E, Kenig A, Ilan Y. Introducing variability in targeting the microtubules: review of current mechanisms and future directions in colchicine therapy. Pharmacol Res Perspect. 2020;8:e00616. doi:10.1002/prp2.616
- Ilan Y. Overcoming randomness does not rule out the importance of inherent randomness for functionality. J Biosci. 2019;1:44.
- Ilan Y. Why targeting the microbiome is not so successful: can randomness overcome the adaptation that occurs following gut manipulation? Clin Exp Gastroenterol. 2019;12:209–217. doi:10.2147/CEG.S203823
- Ilan Y. beta-Glycosphingolipids as Mediators of Both Inflammation and Immune Tolerance: a Manifestation of Randomness in Biological Systems. Front Immunol. 2019;10:1143. doi:10.3389/fimmu.2019.01143
- Ilan Y. Randomness in microtubule dynamics: an error that requires correction or an inherent plasticity required for normal cellular function? Cell Biol Int. 2019;43:739–748. doi:10.1002/cbin.11157
- El-Haj M, Kanovitch D, Ilan Y. Personalized inherent randomness of the immune system is manifested by an individualized response to immune triggers and immunomodulatory therapies: a novel platform for designing personalized immunotherapies. Immunol Res. 2019;67:337–347. doi:10.1007/s12026-019-09101-y
- FDA Guidances with Digital Health Content. Available from: https://www.fda.gov/media/122535/download2020;https://www.fda.gov/medical-devices/digital-health. Accessed April 6, 2022.
