Abstract
Introduction
Herpes Zoster in humans is the result of varicella zoster virus (VZV) infection. Injecting rats with varicella zoster virus produces pain similar to herpes zoster “shingles” pain in humans. . In a previous study, orofacial pain was induced by injecting the whisker pad of male rats with VZV and the pain response increased after attenuating neurexin 3 (Nrxn3) expression in the central amygdala. Neurons descend from the central amygdala to the lateral parabrachial nucleus and orofacial pain signals ascend to the lateral parabrachial nucleus. GABAergic neurons within the central amygdala regulate pain by inhibiting activity within the lateral parabrachial nucleus. Attenuating Nrxn3 expression in the central amygdala increased GABA release in the lateral parabrachial nucleus suggesting Nrxn3 controls pain by regulating GABA release. Nrxn3 can also control synaptic connections between neurons, and we hypothesized that Nrxn3 knockdown in the central amygdala would reduce the number of GABAergic synaptic connections in the lateral parabrachial nucleus and increase VZV associated pain.
Methods
To test this idea, the number of synaptic connections between GABAergic cells of the central amygdala and excitatory or dynorphin positive neurons within the lateral parabrachial nucleus were quantitated after infusion of a virus expressing synaptophysin. Synaptophysin is a synaptic vesicle protein that labels neuronal synaptic connections. These connections were measured in rats with and without whisker pad injection of VZV and knockdown of Nrxn3 within the central amygdala. Orofacial pain was measured using a place escape avoidance paradigm.
Results
GABAergic synaptic connections were reduced in the lateral parabrachial nucleus after Nrxn3 knockdown. Rats with a reduction in the number of connections had an increase in VZV associated orofacial pain. Immunostaining with the pain marker prodynorphin indicated that the reduction in GABAergic connections was primarily associated with prodynorphin positive neurons.
Discussion
The results suggest Nrxn3 reduces VZV associated orofacial pain, in part, by enhancing synaptic connections between GABA cells of the central amygdala and pain neurons within the lateral parabrachial nucleus.
Introduction
Varicella zoster virus (VZV) infects axons within the skin, then migrates to the neuronal cell body. After entering the neurons, the virus replicates resulting in chickenpox and later the virus becomes latent. Once the virus becomes latent the virus DNA remains, but the virus does not replicate.Citation1 In older individuals or when an individual is under stress the VZV reactivates causing herpes zoster (HZ) sometimes referred to as shingles.Citation1 HZ symptoms include pain that is severe enough to require medical treatment.Citation2 Up to 56% of HZ cases have facial involvementCitation3–5 and this includes herpes zoster ophthalmicus. Injecting VZV into rodents induces pain.Citation6–11 Our lab reported that affective/motivational orofacial pain significantly increases after injecting VZV into the whisker pad.Citation12
Importantly, orofacial pain is controlled in the lateral parabrachial nucleusCitation13 and it has been reported that the central amygdala and the lateral parabrachial form a pathway that suppresses this pain.Citation14 The amygdala and lateral parabrachial pathway controls affective/motivational aspects of the pain response.Citation15 GABAergic neurons within the central amygdalaCitation15,Citation16 have axons that project to the lateral parabrachial regionCitation13,Citation15,Citation17 and activation of these neurons could suppress the pain response. Recently, our lab reported neurexin 3 (Nrxn3) was expressed in the central amygdala and in the parabrachial of rats injected with VZV.Citation18 Neurexins are needed for synapse formation, synaptic transmission, and neurotransmitter release.Citation19,Citation20 Because Nrxn3α modulates presynaptic GABA releaseCitation19 reducing Nrxn3 within neurons located in the central amygdala reduced GABA release and prevented the inhibition of the ascending pain signals from the trigeminal nucleus and trigeminal gangliaCitation13,Citation15 resulting in increased VZV associated orofacial pain.Citation21
Evidence suggests Nrxn3 can strengthen the connection to inhibitory GABAergic neuronsCitation19 by control of synaptic connections between neurons.Citation22 Moreover, stimulation of dynorphin positive neurons in the lateral parabrachial nucleus induces aversive behaviors and is considered critical in the pain pathway.Citation23 Thus, we hypothesized that Nrxn3 knockdown in the central amygdala would reduce the number of GABAergic synaptic connections to dynorphin positive cells in the lateral parabrachial nucleus and increase VZV associated pain. This reduced GABAergic connection would result in increased affective orofacial pain as the pain neurons would no longer be inhibited.
To test this idea, our lab quantitated synaptic connections within the parabrachial of Gad1-iCre male rats after infusing the amygdala with a Cre-dependent synaptophysin gene linked to a fluorescent marker. Synaptophysin is an integral membrane protein localized to synaptic vesicles and can mark synaptic terminals.Citation24 In this study, the number of connections between GABAergic neurons projecting from the central amygdala to the lateral parabrachial nucleus was quantitated on excitable and prodynorphin neurons after infusing the central amygdala with Nrxn3 shRNA and after injecting the whisker pad of male rats with VZV.
Materials and Methods
Animal Husbandry
This study was approved by the Texas A&M University School of Dentistry Institutional Animal Care and Use Committee (IACUC 2022–0102) using the guidelines from the most current Guide for the Care and Use of Laboratory Animals. Male rats were obtained from the Rat Resource and Research Center with a cre driven by the Gad1 promoter [LE-Tg(Gad1-iCre)3Ottc, RRRC#: 00751]. This strain was developed by Brandon Harvey and Jim Pickel. Male 300 gram rats were kept on a 14:10 light/dark cycle. The rats were given food and water ad libitum.
Experimental Paradigm
First surgery was completed where virus was infused into the central amygdala and lateral parabrachial nucleus. Second, four weeks after surgery, the whisker pad was injected with vehicle or VZV. Third, the animal’s pain response was measured two weeks after whisker pad injection and then the animals were sacrificed for tissue collection and further molecular analysis.
Treatment and Experimental Groups
Surgery was performed using male rats after administering 2% isoflurane in an airflow of 2 liter per minute. Using sterile technique, infusions were completed using a Hamilton syringe (Neuros #7002) which was inserted into the brain. The central amygdala was infused bilaterally at coordinates anterior-posterior = 2.2 mm from Bregma, midline 4.2 mm and depth 8.4 mm, flat skull. In this surgery, the central amygdala of Gad1-iCre Long Evans rats was infused with 1.0 µL of 1 × 1013 TU/mL AAV1 virus containing the construct AAV1-GFP-U6-mNRXN3-shRNA (Vector Biolabs, Malvern, PA) mixed one to one with AAV1 containing pAAV hSyn FLEx mGFP-2A-Synaptophysin-mRuby (Addgene, Watertown, MA). Synaptophysin is used in studies of synaptic plasticity and integrity.Citation24 The Nrxn3 shRNA sequence was (5’-CACCGCCAGTGAATGAGCACTATCCCTCGAGGGATAGTGCTCATTCACTGGCTTTTT-3’). In a separate group of Long Evans rats, the central amygdala was infused with 1 µL of 1 × 1013 TU/mL AAV1 containing a scrambled shRNA sequence (AAV1-GFP-U6-shRNA, Vector Biolabs) mixed 1 to 1 with the synaptophysin construct. Next, the right lateral parabrachial nucleus of all the rats was infused with AAV5 containing pAAV-CaMKIIa-EGFP (Addgene, Watertown, MA) at stereotaxic coordinates anterior-posterior = 0.0 mm from Lambda, 2.4 mm from midline at a depth of 6.6 mm, flat skull. The virus solutions were infused at a rate of 50 nanoliters per minute using a Stoelting stereotaxic syringe pump. After infusion, the needle was left in place for 5 minutes and then removed. Four weeks after infusion, half of the infused rats (Nrxn3 shRNA or scrambled shRNA group) were injected with 100 µL of MeWo cells without VZV (no VZV) and half were injected with 100 µL of MeWo cells containing VZV (50,000 pfu/µL). All VZV injections were into the left whisker pad using the VZV Parent of Oka (pOka). This strain was originally obtained from M. Takahashi (Osaka University, Japan) and propagated as detailed previouslyCitation25 on the VZV permissive MeWo human cell line (ATCC, Manassas, VA). Injections were completed using a 1 mL syringe with a 29 gauge needle. Use of the virus and cell lines was approved by Texas A&M University Institutional Biosafety Committee.
Behavioral Testing
In these experiments, the motivational and affective aspect of the pain was measured in male rats.Citation26,Citation27 Pain testing was completed two weeks after whisker pad injection. The specific test is referred to as the Place Escape/Avoidance Paradigm (PEAP). All tests were completed during the beginning of the light phase between 7 am and 11 am. Rats are first placed in an acrylic box with the dimensions of 30 cm × 30 cm × 30 cm. This box is open on the top so that testing of the animals with filaments can be completed. Half of the box is clear acrylic and half is covered with black material using a design developed by the Fuchs’s laboratory.Citation26 Once placed in the box the rats are immediately poked with a 60-gram filament every 15 seconds on the injected side if the rat was on the dark side. Once the animal moves to the light side or clear acrylic side of the box it will receive a poke to the non-injected side. The filament was targeted to the same spot as the VZV injection. Animals that have pain on the injected side will move from the preferred dark side to the clear acrylic side upon poke to the sore injected side. Control animals without pain will not move as readily to the clear acrylic side when poked on the injection site.
Immuno-Fluorescent Staining
After measuring the pain response, a set of the treated animals were sacrificed for immunostaining and fluorescent imaging. The rats were sacrificed by injecting 100 mg/kg ketamine and 10 mg/kg xylazine. The rats are first perfused with 9% sucrose and then 4% paraformaldehyde in PBS pH 7.4. Fixed tissues are then stored in a 25% sucrose solution. For sectioning, tissues were covered in frozen embedding medium (PolarStat Plus, StatLab, McKinney, TX) cooled to −20 Celsius. The tissues were sectioned (32 µm) with a cryostat and placed on Histobond slides (VWR International, Radnor, PA). The tissue was post-fixed for 5 minutes in 4% paraformaldehyde. After fixation, the tissue was rinsed in PBS containing 0.3% Triton-X and then blocked for 2 hours at room temperature with a PBS solution containing 5% normal goat serum and 0.3% Triton-X 100. The slides were incubated in a primary antibody overnight at 4°C after dilution in PBS containing 5% BSA and 0.3% Triton X-100. Lateral parabrachial sections were placed in a primary antibody solution containing mouse monoclonal antibody prodynorphin (Millipore Sigma catalog #MABT882, Burlington, MA) at a 1:200 dilution. Sections of the central amygdala were placed in a mixture of a rabbit polyclonal NeuN antibody (Millipore Sigma catalog #ABN78) diluted to 1:200 and a mouse monoclonal antibody GAD67 (ie, GAD1) (Millipore Sigma catalog #MAB5406) diluted to 1:150. After incubation in primary antibody, the slides were then rinsed three times in PBS solution containing 0.3% Triton-X 100 for a total of 45 minutes and placed for 2 hours in secondary antibody and PBS and 0.3% Triton X-100. Secondary antibodies (1:500 dilution) included goat anti mouse 568 and goat anti-rabbit 647 or goat anti-mouse 647 (Invitrogen, Carlsbad, CA). After incubation in the secondary antibody, the slides were rinsed three times using the same PBS and 0.3% Triton X-100 for a total of 45 min. After rinsing, the slides were processed using a TrueVIEW Autofluorescence quenching kit (Vector Labs, Burlingame, CA). Fluoromount-G mounting medium containing Hoechst 33342 stain was added to the slides (Electron Microscopy Sciences, Hatfield, PA) and a coverslip was placed. Slides were imaged on a Leica Stellaris 8 confocal microscope (Leica Microsystems, Wetzlar, Germany). No primary control showed no signal (data not shown).
Cell or Puncta Counts
A reviewer blinded to the identity of the treatment groups completed the cell counts. Slide images were captured on the Stellaris 8 confocal microscope and these images were analyzed using Leica software. After subtracting the average background from images, any fluorescent signal associated with a cell nucleus was counted as a positive cell. In the lateral parabrachial nucleus of rats, the number of EGFP positive cells colocalizing with synaptophysin puncta was counted and then the number of EGFP positive cells with synaptophysin puncta colocalizing with prodynorphin was counted in a 0.04 mm2 field. To count the number of synaptophysin puncta, a three-dimensional scan of the lateral parabrachial tissue was completed to produce 0.3 µm optical sections through the z-plane. The number of puncta on each cell was counted from two 0.04 mm2 fields on each section and averaged. Cell counts for three sections were averaged for each animal. In the central amygdala, the number of cells having a NeuN fluorescent signal that colocalized with GAD1 was quantitated after immunostaining. Cell counts from two fields on each section were averaged, and three sections were averaged for each animal.
Enzyme-Linked Immunosorbent Assay (ELISA)
Two weeks after VZV injection animals a set of the treated animals were sacrificed by exposure to CO2 and the brain was isolated after decapitation. The brain was placed in a Zivic device (Zivic Instruments, Pittsburgh, PA), the device with a brain was placed on dry ice and sectioned into 2 mm slices using razor blades. A frozen 2 mm slice is placed on a glass slide and frozen on dry ice. Then, a 2 mm circular punch of the central amygdala is moved to a tube containing 500 µL of T-Per tissue protein extraction reagent containing Halt Protease Inhibitor (Thermo Scientific, Rockford, IL). The punch samples are ground and the solid material separated by centrifugation and decanting of the supernatant. Within the supernatant, the amount of Nrxn3α was determined using ELISA following the manufacturer’s directions (MyBiosource, San Diego, CA). Analysis was completed in duplicate using 100 µL of supernatant in each well. Next, to compare samples the amount of total protein was measured in each sample using a BCA protein assay (Thermo Scientific, Waltham, WA). Values were represented as the pg of Nrxn3 per µg of total protein.
Statistics
The behavioral data was analyzed by two-way ANOVA with repeated measures. In the PEAP assay, there were multiple time bins: 5 minutes, 10 minutes, 15 minutes, 20 minutes, 25 minutes, 30 minutes. The independent variables for the behavioral test were virus (VZV, no VZV) and treatment (Nrxn3 shRNA, control shRNA) and the dependent variable was the PEAP measurement. Cell count and puncta count data were analyzed using a two-way ANOVA. The independent variables were virus (VZV, no VZV) and treatment (Nrxn3 shRNA, control shRNA) and the dependent variable was the cell or puncta count. Tukey’s post-hoc tests were completed on Prism, 7.05, GraphPad Software (La Jolla, CA). PEAP, cell count and puncta count data had normal distributions and equal variances. All data are given as a mean and standard error of the mean (SEM). Each point on a histogram represents a different animal within that treatment group.
Results
Infusing the Central Amygdala with Nrxn3 shRNA Reduced Nrxn3α Expression
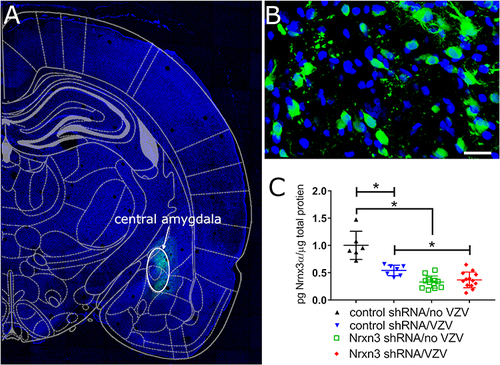
Infusion of shRNA constructed with a EGFP gene results in green cells () localized to the central amygdala region () and the cells had a neuronal morphology (). Nrxn3 shRNA treatment significantly reduced Nrxn3α protein levels in the central amygdala in contrast to control, scrambled shRNA, F(1, 34)=67.3, P < 0.001. Post-hoc testing indicated Nrxn3α protein expression significantly decreased within the central amygdala after Nrxn3 shRNA treatment in VZV treated rats (, compare blue triangles to red diamonds) and in no VZV treated rats (, compare black triangles to green squares). VZV injection of the whisker pad significantly decreased Nrxn3α expression in the central amygdala in contrast to vehicle, F(1, 34)=16.6, P = 0.003. Post-hoc testing indicated that in rats that received control shRNA a significantly lower amount of Nrxn3α protein was measured when comparing no VZV and VZV treated animals (, compare black triangles to blue triangles). In addition, a significant interaction between shRNA treatment and VZV administration was observed, F(1, 34)=23.3, P < 0.001.
Figure 1 Infusion of virus expressing Nrxn3 shRNA reduced the number of Nrxn3α positive cells. The central amygdala of Long Evans rats was infused with AAV1 virus containing the construct AAV1-GFP-mNRXN3-shRNA or a scrambled shRNA. Four weeks after infusion the whisker pad was injected with MeWo cells without varicella zoster virus (no VZV) or MeWo cells containing varicella zoster virus. Six weeks after infusion the brain was isolated and the sections imaged. (A) is a low magnification image of a brain slice indicating green GFP fluorescence from expression of the virus construct in the central amygdala region (white oval). A high magnification image of the GFP positive cells (green) within the central amygdala is shown in (B). Bar = 20 micrometers. (C) shows a histogram for Nrxn3 expression within the central amygdala after infusion of AAV1 and injection of the whisker pad. Each point is from an individual animal. An asterisk indicates a significant difference of α=0.05.
Reducing Nrxn3 Expression Increased the Orofacial Pain Response
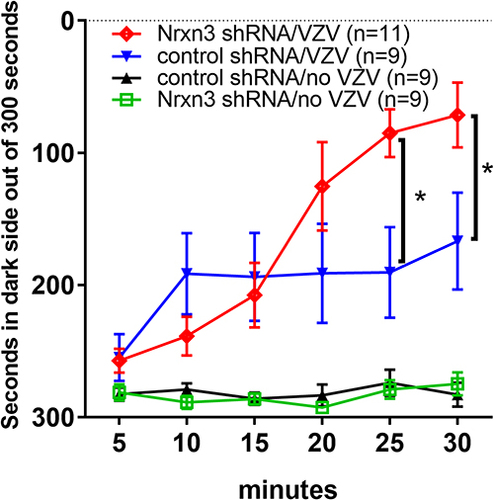
In this study VZV was injected in the whisker pad of male rats to induce orofacial pain. VZV injection and shRNA treatment had a significant main effect on the pain response F(3, 34)=11.54, P < 0.0001. Post-hoc testing indicates that Nrxn3α knockdown increased the nociceptive response after VZV injection, comparing Nrxn3α shRNA/VZV group (, red line) to control shRNA/VZV group ( blue line). VZV injection increased the pain response in animals treated with Nrxn3α shRNA, comparing Nrxn3α shRNA/VZV group (, red line) to Nrxn3α shRNA/no VZV group ( green line) at 15, 20, 25 and 30 minutes of testing. VZV injection also increased the pain response in animals treated with control shRNA, comparing control shRNA/VZV group (, blue line) to control shRNA/no VZV group (, black line) at 10, 15, 20, 25 and 30 minutes of testing. A significant interaction between treatment and time was observed F(15, 170)=6.9, P < 0.0001.
Figure 2 Knock down of Nrxn3 expression increased orofacial pain resulting from injection of the whisker pad with varicella zoster virus. The central amygdala of rats was infused with virus for knockdown of Nrxn3 expression using shRNA. Both scrambled or Nrxn3 shRNA viral constructs were infused. Four weeks after infusion the whisker pad was injected with either no VZV or VZV. Two weeks after whisker pad injection the rats were tested for a pain response using the Place Escape/Avoidance Paradigm (PEAP). The testing data is collected in 5 minute bins over a 30 minute testing period. The time spent on the dark side of the testing chamber within each 5 minute bin is recorded. Four different treatment groups were tested with 9 animals in each group. An asterisk indicates a significant difference of α=0.05 between treatment groups.
GABAergic Neurons from the Central Amygdala Express the Synaptic Marker Synaptophysin
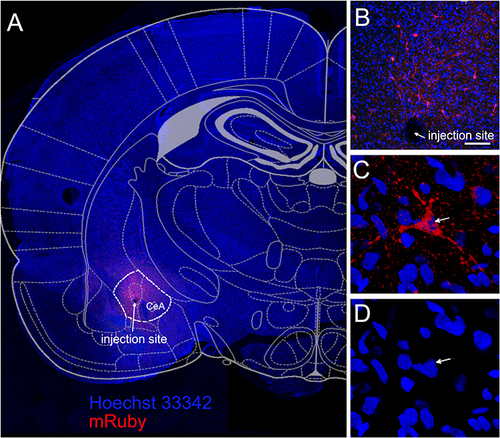
Synaptophysin is an integral membrane protein localized to synaptic vesicles and can mark synaptic terminals. The central amygdala was infused with virus that expressed synaptophysin in GAD1 positive neurons and this protein was linked to the fluorescent protein mRuby, ( and , red). High magnification images of transfected cells suggest mRuby was localized within cells having neuronal morphology ( and ).
Figure 3 Synaptophysin was expressed in GABAergic cells of the central amygdala. The central amygdala of Gad1-iCre Long Evans rats was infused with AAV1 containing pAAV hSyn FLEx mGFP-2A-Synaptophysin-mRuby. During this same surgery the central amygdala was infused with the shRNA viral construct. Image is from a representative rat infused with control shRNA and injected with no VZV. (A) Six weeks after infusion mRuby fluorescent signal (red) was detected within the central amygdala (CeA). White dotted line shows the borders of the central amygdala. Arrow points to the injection site (A and B). Enlarged image of synaptophysin positive cell (red, arrow) within the central amygdala is shown in (C). Hoechst 33342 stain of the nuclei from the same cell (arrow) is shown in blue (D). Bar= 100 µm.
Axons Project from the Central Amygdala to the Lateral Parabrachial Nucleus and Terminate on CaMKIIa Positive Cells
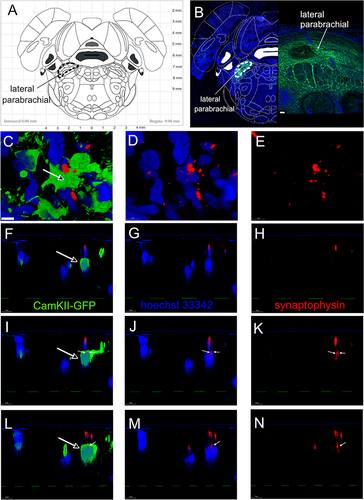
The lateral parabrachial nucleus was transfused with a virus that expressed the green fluorescent marker EGFP under the direction of a CaMKIIa promoter (). CaMKIIa is a marker for excitable neurons.Citation28 Six weeks after transfusion excitatory CaMKIIa positive neurons expressed EGFP in the lateral parabrachial nucleus (, green). CaMKIIa positive neurons expressing EGFP colocalized (, open arrow, green) with synaptophysin positive puncta (ie, synaptic terminals, and , red) within the lateral parabrachial nucleus. Cell nuclei are in blue (). Three different optical sections were taken through the z plane. Panels F, G and H are one slice along the z plain, panels I, J and K are for another slice along the z plain and panels L, M and N are for a third slice along the z plain. Synaptophysin positive puncta (, red) were observed and small white arrows in panels I, J, K, L, M, N show synaptophysin positive puncta colocalized with EGFP.
Figure 4 Synaptophysin positive terminals colocalize with excitable cells in the lateral parabrachial nucleus. Atlas image of coronal brain section from a rat with the lateral parabrachial region outlined with a black dotted line in (A). The central amygdala of Gad1-iCre Long Evans rats was infused with AAV1 containing pAAV hSyn FLEx mGFP-2A-Synaptophysin-mRuby. Excitable cells within the lateral parabrachial nucleus were labeled by infusing the lateral parabrachial nucleus with AAV5 virus containing pAAV-CaMKIIa-EGFP. Six weeks after infusion EGFP positive cells (green, (B) were present within the lateral parabrachial region (white dotted line). In (B), a low magnification image is shown on the left and a higher magnification image is shown on the right. EGFP positive cells (green) are shown in (C, F, I and L) and synaptophysin (red) is shown in (C (through (N)). Cell nuclei are labeled blue with Hoechst 33342 stain in (B–D, F, G, I, J, L and M). (F) through (N) are images through the z plane of the lateral parabrachial tissue. (F, I and L) are three different z plane cross sections, respectively, through the cell in (C) (open arrow). (F–H) show one z plane slice through the cell, (I–K) show the second slice and (L–N) are the third slice through the cell. Synaptophysin labeled puncta on the CaMKII positive cell was indicated by small white arrows in (I–N). Bar = 50 µm in (B) and 5 µm in (C).
Nrxn3 Knockdown Reduces the Number of Synaptic Connections Between Central Amygdala Neurons and Excitable Neurons Within the Lateral Parabrachial Nucleus
The number of synaptophysin positive terminals colocalized with CaMKIIa positive neurons was counted within the lateral parabrachial nucleus. Nrxn3 knockdown significantly reduced F(1, 32)=33.5, P < 0.0001 the number of synaptic connections in the group given vehicle (, compare black triangles to green squares) and in rats injected with VZV (, compare blue triangles to red diamonds). VZV injection significantly increased synaptophysin puncta in CaMKIIa positive cells F(1, 32)=12.5, P < 0.01 (, compare black diamonds to blue diamonds). No significant interaction between shRNA treatment and VZV administration was observed F(1, 32)=2.6, P = 0.11. Representative images show the number of synaptophysin positive puncta (arrows) colocalizing with CaMKIIa positive cells (dotted white outline) for the control shRNA/no VZV () or control shRNA/VZV () or Nrxn3 shRNA/no VZV () or Nrxn3 shRNA/VZV () group.
Figure 5 Synaptophysin and CaMKII expression in the lateral parabrachial nucleus. In these rats the central amygdala was infused with virus expressing a scrambled shRNA or a Nrxn3 shRNA. The central amygdala of Gad1-iCre Long Evans rats was infused with AAV1 containing pAAV hSyn FLEx mGFP-2A-Synaptophysin-mRuby. Excitable cells within the lateral parabrachial nucleus were labeled by infusing this nucleus with AAV5 virus containing pAAV-CaMKIIa-EGFP. After four weeks post-surgery the whisker pad of the infused rats were injected with MeWo cells without varicella zoster virus (no VZV) or MeWo cells containing VZV. Two weeks after injection the brain was isolated and imaged. (A) shows the average number of synaptophysin terminals or puncta localized to each CaMKII positive cell within the lateral parabrachial nucleus. Representative images of rats treated with control shRNA/no VZV (B–E) or control shRNA/VZV (F–I) or Nrxn3 shRNA/no VZV (J–M) or Nrxn3 shRNA/VZV (N–Q) are shown. Hoechst 33342 nuclear stain (B, F, J and N) and CaMKII stain (C, G, K and O) and synaptophysin stain (D, H, L and P) is represented in several cells . Individual CaMKII positive cells (green) are outlined with a white dotted line. Arrows point to synaptophysin positive puncta (red, D, H, L and P) colocalizing with CaMKII staining (E, I, M and Q). Bar = 10 µm. Panel R shows the number of CaMKII positive cells in the lateral parabrachial nucleus that colocalized with synaptophysin. Each point is from an individual animal in panels A and R. An asterisk indicates a significant difference of α=0.05. Representative images of rats treated with control shRNA/no VZV (S) or control shRNA/VZV (T) or Nrxn3 shRNA/no VZV (U) or Nrxn3 shRNA/VZV (V) show cells with synaptophysin stain colocalizing with CaMKII stain (yellow, arrows). Bar = 50 µm.
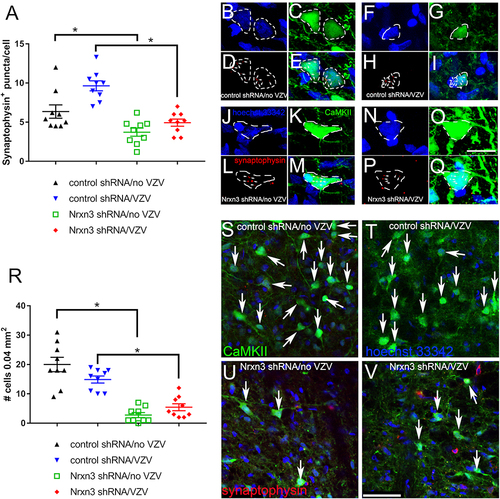
Counting the number of excitable cells (ie, CaMKIIa positive) in the lateral parabrachial that colocalize with synaptophysin indicates that fewer excitable cells F(1, 32)=74.7, P < 0.0001 have synaptic connection (ie, synaptophysin positive) to central amygdala neurons after Nrxn3 shRNA administration. Post-hoc testing indicated a decrease in the number of synaptophysin positive cells colocalizing with CaMKII after Nrxn3 shRNA treatment in both the no VZV (, compare black triangles to green squares) and the VZV group (, compare blue triangles to red diamonds). A significant interaction between shRNA treatment and VZV administration was observed in the number of cells positive for CaMKIIa and synaptophysin F(1, 32)=6.3, P < 0.05. Representative images for the CaMKIIa and synaptophysin staining within the lateral parabrachial nucleus are shown for the control shRNA/no VZV () and control shRNA/VZV () and Nrxn3 shRNA/no VZV () and Nrxn3 shRNA/VZV () group.
Excitable Neurons in the Lateral Parabrachial Nucleus Express Neurotransmitters Associated with Pain
Expression of CaMKII in the lateral parabrachial nucleus ( and , green). Immunofluorescent staining using a prodynorphin antibody ( and , red). CaMKII and prodynorphin colocalized in the lateral parabrachial nucleus (, yellow). A cross-sectional z plane image was acquired using confocal microscopy ( insert). This optical sectioning allowed for counting the number of synaptophysin terminals ( insert, yellow) colocalizing with prodynorphin ( insert, red) and CaMKII EGFP positive cells ( insert, green).
Figure 6 Prodynorphin cells within the lateral parabrachial nucleus. The central amygdala of Gad1-iCre Long Evans rats was infused with AAV1 containing pAAV hSyn FLEx mGFP-2A-Synaptophysin-mRuby. Excitable cells within the lateral parabrachial nucleus were labeled by infusing this nucleus with AAV5 virus containing pAAV-CaMKIIa-EGFP. Four weeks after infusion the whisker pad was injected with either no VZV or VZV. Six weeks after infusion brain sections of the treated rats were immunostained for prodynorphin. In (A and B) multiple prodynorphin positive (red) cells were imaged in the lateral parabrachial nucleus (LPB). Prodynorphin is a marker for neurons involved in pain. Cell nuclei are labeled blue with Hoechst 33342 in (A–C). (B) shows only the prodynorphin cells (red) from the same region and (C) shows only the EGFP positive cells (green). A higher magnification image of the LPB is shown in (D) and cells that colocalize prodynorphin and EGFP are yellow. Insert in (D) is an image through the z plane of the lateral parabrachial nucleus after staining for prodynorphin. Prodynorphin is red, EGFP is green and synaptophysin terminals are in yellow for the insert image in (D). Images are from a representative rat that was treated with Nrxn3 shRNA and VZV. scp = superior cerebellar peduncle. Bar= 20 µm. The histogram in (E) shows the number of EGFP/prodynorphin positive cells that colocalized with synaptophysin in the lateral parabrachial nucleus after knockdown of Nrxn3α in the central amygdala. Each point is from an individual animal. An asterisk indicates a significant difference of α=0.05. Representative images of rats treated with control shRNA/no VZV (F) or control shRNA/VZV (G) or Nrxn3 shRNA/no VZV (H) or Nrxn3 shRNA/VZV (I) show cells with synaptophysin stain colocalizing with prodynorphin stain (yellow, arrows). Bar = 50 µm.
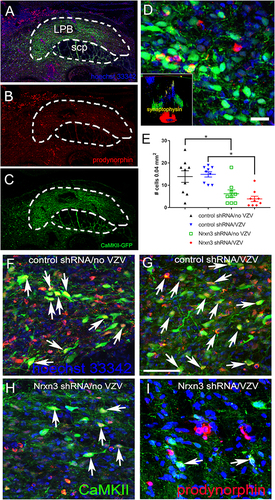
Knockdown of Nrxn3 Expression Reduced the Synaptic Connections to Neurons Expressing Prodynorphin
Using optical sections, the synaptophysin terminals colocalizing with prodynorphin and EGFP positive cells were counted (). Rats that received Nrxn3 shRNA had significantly lower F(1, 32)=27.8, P < 0.0001 levels of prodynorphin/EGFP positive neurons colocalizing with synaptophysin versus the group receiving scrambled shRNA (ie, control shRNA) in both the no VZV group ( compare back triangles to green squares) and in the VZV group ( compare blue triangles to red diamonds). Comparing the no VZV group and the VZV group in rats treated with Nrxn3 shRNA there was no significant difference F(1, 32)=0.14, P = 0.7 in the number of prodynorphin positive cells colocalized with synaptophysin. No significant interaction between shRNA treatment and VZV administration was observed F(1, 32)=0.88, P = 0.35. There was no significant difference in the number of prodynorphin cells colocalizing with synaptophysin if the cell was negative for EGFP and CaMKIIa (data not shown). Representative images indicate a decrease in the number of cells colocalized for prodynorphin and CaMKII staining (arrows) in the Nrxn3 shRNA/no VZV (panel H) or Nrxn3 shRNA/VZV (panel I) as compared to the control shRNA/no VZV (panel F) or control shRNA/VZV (panel G) group.
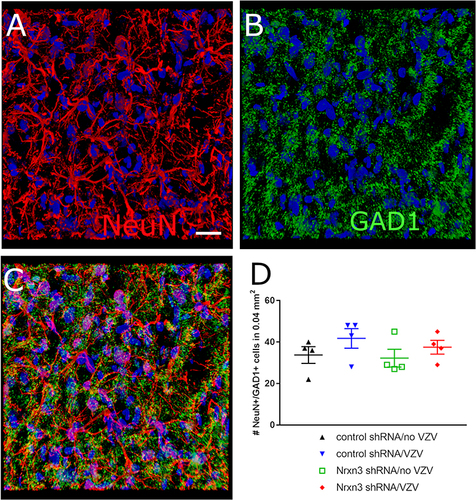
In the Central Amygdala GABAergic Cell Numbers Did Not Decrease After Nrxn3 shRNA Treatment
Cells positive for the neuronal marker NeuN (, red) and the GABAergic marker GAD1 (, green) were counted in the central amygdala after shRNA and VZV treatment (). Counts of the number of GABAergic neurons indicated that Nrxn3 shRNA treatment had no significant effect (). Most of the neurons within the central amygdala were GABAergic (data not shown) consistent with previous studies.Citation15,Citation16
Figure 7 GAD1 expression in the central amygdala. The central amygdala of Gad1-iCre Long Evans rats was infused with a mix of the shRNA viruses and the CaMKIIa EGFP virus. Four weeks after surgery the whisker pad was injected with either no VZV or VZV. Six weeks after infusion brain sections were immunostained for the neuronal marker NeuN. (A) indicates cells with immunofluorescent signal for NeuN (red) in the central amygdala. Hoechst 33342 nuclear stain is blue. (B) shows the same region of cells but the GAD1 (ie, GAD67) immunofluorescent signal is green. (C) has the immunofluorescent signal for both NeuN (red) and GAD1 (green) with colocalization in yellow. Bar = 20 micrometers. (D) is a histogram for the number of NeuN positive neurons colocalizing with GAD1 within the central amygdala of the treated rats. Each point is from an individual animal.
Discussion
Knockdown of Nrxn3 in the central amygdala significantly increased VZV associated orofacial pain. In this study, knockdown of Nrxn3 expression in the central amygdala also reduced GABAergic connections to excitatory neurons in the lateral parabrachial nucleus that express the pain marker prodynorphin. Because dynorphin positive neurons in the lateral parabrachial nucleus induce aversive behaviors and are considered critical in the pain pathway with the central amygdala,Citation23 these results suggest Nrxn3 controls pain signaling, in part, by regulating synaptic plasticity within the lateral parabrachial nucleus.
Nrxn3α can function in presynaptic GABA release in certain neuronal cell types.Citation19 It is likely that GABA release inhibits neuronal signals ascending from the trigeminal nucleus and trigeminal ganglia.Citation13,Citation15 Thus, an increase in Nrxn3α expression and GABA release in the parabrachial would control the pain response in this VZV pain model. VZV injection decreased GABA release in the lateral parabrachial and knockdown of Nrxn3α expression further decreased GABA release.Citation21 Nrxn3 shRNA treatment reduced Nrxn3 expression in the central amygdala but there was no significant reduction in the number of GABAergic cells.Citation21 In contrast, GABA release from the neurons was decreased by Nrxn3 knockdown.Citation21 Opsin stimulation of the lateral parabrachial reversed the effect of Nrxn3 knockdown by increasing GABA release.Citation21 This increase in GABA release reduced the orofacial pain response. The lateral parabrachial reduces the activity of ascending excitatory pain neurons.Citation15 Our results are consistent with this result in that GABAergic neurons within the central amygdala inhibit ascending pain signals from the whisker pad through release of GABA in the lateral parabrachial.
VZV infection of rats produces the same symptoms observed in humans having herpes zoster “shingles” and post-herpetic neuralgia.Citation6,Citation8–11 Recently, our lab reported that Nrxn3 is expressed in the central amygdala and parabrachialCitation18 and that attenuating Nrxn3 expression within the central amygdala increases VZV associated orofacial pain.Citation21 Nrxn3 is a member of the neurexin family, and neurexins are presynaptic cell-adhesion molecules that are essential for synapse formation, synaptic transmission, and neurotransmitter release.Citation19,Citation20 Recent evidence suggests Nrxn3 can enhance the connection between GABAergic neurons post-synaptically.Citation19 In this study Nrxn3 expression was shown to enhance GABAergic activity and reduce VZV associated pain. Based on this work, we suggest a Nrxn3 agonist should decrease VZV associated pain. Unfortunately, there are no Nrxn3 agonists, and the Nrxn3 gene is too large for a viral expression vector but a conditional knock-in mouse for Nrxn3 could be made. This mouse could test if activating the Nrxn3 pathway would reduce pain. This result would indicate that the Nrxn3 pathway is a good therapeutic target for pain treatment.
A potential weakness of these studies was utilizing a single pain test that can be influenced by stress or fear. The central amygdala alters the fear responseCitation16,Citation29 and the parabrachial has been shown to effect the fear response.Citation30 Thus, some of the changes observed in the PEAP assay values may be the result of stress/fear and not pain. All tests were completed in male rats but VZV associate orofacial pain differs between males and femalesCitation12 and to address this gap future studies will test synaptic plasticity in females.
In summary, Nrxn3 regulates VZV induced pain not only by controlling GABA releaseCitation21 but also by controlling the synaptic strength between GABA cells and cells that signal pain from orofacial region. Nrxn3 is, in effect, activating descending inhibitory pathways to control the pain.Citation31 Using this model Nrxn3 is likely a potential target for the control of VZV induced orofacial pain.
Contributions to the Field
Attenuating Nrxn3 decreases GABAergic synaptic connections in lateral parabrachial nucleus.
GABAergic synaptic connections decrease on dynorphin cells after Nrxn3 knockdown.
Decreasing GABAergic synaptic connections increases VZV induced orofacial pain.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016.
- Johnson RW, Bouhassira D, Kassianos G, Leplege A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8:37.
- Pevenstein SR, Williams RK, McChesney D, Mont EK, Smialek JE, Straus SE. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J Virol. 1999;73(12):10514–10518.
- Pavan-Langston D. Herpes zoster ophthalmicus. Neurology. 1995;45(12 Suppl 8):S50–1.
- Ragozzino MW, Melton LJ, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine. 1982;61(5):310–316.
- Guedon JM, Yee MB, Zhang M, Harvey SA, Goins WF, Kinchington PR. Neuronal changes induced by Varicella Zoster Virus in a rat model of postherpetic neuralgia. Virology. 2015;482:167–180.
- Kinchington PR, Goins WF. Varicella zoster virus-induced pain and post-herpetic neuralgia in the human host and in rodent animal models. J Neurovirol. 2011;17(6):590–599.
- Hasnie FS, Breuer J, Parker S, et al. Further characterization of a rat model of varicella zoster virus-associated pain: relationship between mechanical hypersensitivity and anxiety-related behavior, and the influence of analgesic drugs. Neuroscience. 2007;144(4):1495–1508.
- Dalziel RG, Bingham S, Sutton D, et al. Allodynia in rats infected with varicella zoster virus--a small animal model for post-herpetic neuralgia. Brain Res Brain Res Rev. 2004;46(2):234–242.
- Fleetwood-Walker SM, Quinn JP, Wallace C, et al. Behavioural changes in the rat following infection with varicella-zoster virus. J Gen Virol. 1999;80(Pt 9):2433–2436.
- Kennedy PG, Grinfeld E, Bontems S, Sadzot-Delvaux C. Varicella-Zoster virus gene expression in latently infected rat dorsal root ganglia. Virology. 2001;289(2):218–223.
- Stinson C, Deng M, Yee MB, Bellinger LL, Kinchington PR, Kramer PR. Sex differences underlying orofacial varicella zoster associated pain in rats. BMC Neurol. 2017;17(1):95.
- Rodriguez E, Sakurai K, Xu J, et al. A craniofacial-specific monosynaptic circuit enables heightened affective pain. Nat Neurosci. 2017;20(12):1734–1743.
- Brudvik P, Rygh P. The initial phase of orthodontic root resorption incident to local compression of the periodontal ligament. Eur j Orthodontics. 1993;15(4):249–263.
- Raver C, Uddin O, Ji Y, et al. An Amygdalo-Parabrachial Pathway Regulates Pain Perception and Chronic Pain. J Neurosci. 2020;40(17):3424–3442.
- Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82(5):966–980.
- Liu J, Hu T, Zhang MQ, Xu CY, Yuan MY, Li RX. Differential efferent projections of GABAergic neurons in the basolateral and central nucleus of amygdala in mice. Neurosci Lett. 2021;745:135621.
- Hornung R, Pritchard A, Kinchington PR, Kramer PR. Comparing Gene Expression in the Parabrachial and Amygdala of Diestrus and Proestrus Female Rats after Orofacial Varicella Zoster Injection. Int J Mol Sci. 2020;21(16).
- Aoto J, Földy C, Ilcus SM, Tabuchi K, Südhof TC. Distinct circuit-dependent functions of presynaptic neurexin-3 at GABAergic and glutamatergic synapses. Nat Neurosci. 2015;18(7):997–1007.
- Aoto J, Martinelli DC, Malenka RC, Tabuchi K, Südhof TC. Presynaptic neurexin-3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell. 2013;154(1):75–88.
- Kramer PR, Umorin M, Hornung R, Kinchington PR. Neurexin 3α in the Central Amygdala has a Role in Orofacial Varicella Zoster Pain. Neuroscience. 2022;496:16–26.
- Boxer EE, Seng C, Lukacsovich D, et al. Neurexin-3 defines synapse- and sex-dependent diversity of GABAergic inhibition in ventral subiculum. Cell Rep. 2021;37(10):110098.
- Chiang MC, Nguyen EK, Canto-Bustos M, Papale AE, Oswald AM, Ross SE. Divergent Neural Pathways Emanating from the Lateral Parabrachial Nucleus Mediate Distinct Components of the Pain Response. Neuron. 2020;106(6):927–39.e5.
- Calhoun ME, Jucker M, Martin LJ, Thinakaran G, Price DL, Mouton PR. Comparative evaluation of synaptophysin-based methods for quantification of synapses. J Neurocytol. 1996;25(12):821–828.
- Eisfeld AJ, Yee MB, Erazo A, Abendroth A, Kinchington PR. Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J Virol. 2007;81(17):9034–9049.
- LaBuda CJ, Fuchs PN. A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol. 2000;163(2):490–494.
- Baastrup C, Jensen TS, Finnerup NB. Pregabalin attenuates place escape/avoidance behavior in a rat model of spinal cord injury. Brain Res. 2011;1370:129–135.
- Liu XB, Murray KD. Neuronal excitability and calcium/calmodulin-dependent protein kinase type II: location, location, location. Epilepsia. 2012;53(Suppl 1):45–52.
- Frye CA, Walf AA. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behav Neurosci. 2004;118(2):306–313.
- Sato M, Ito M, Nagase M, et al. The lateral parabrachial nucleus is actively involved in the acquisition of fear memory in mice. Molecular Brain. 2015;8:22.
- Millan MJ. Descending control of pain. Progress Neurobiol. 2002;66(6):355–474.
