Abstract
Background
Patients with chronic pain (CP) are often reported to have deficits in working memory. Pain impairs working memory, but so do depression and sleep problems, which are also common in CP. Depression has been linked to changes in brain activity in CP during working memory tasks, but the effect of sleep problems on working memory performance and brain activity remains to be investigated.
Methods
Fifteen CP patients and 17 age-, sex-, and education-matched controls underwent blood-oxygen-level dependent (BOLD) functional magnetic resonance imaging at 3T while performing block design 0-back, 2-back, and paced visual serial addition test paradigms. Subjects also reported their level of pain (Brief Pain Inventory), depression (Beck Depression Inventory II), and sleep problems (Pittsburgh Sleep Quality Index) and were tested outside the scanner with neuropsychological tests of working memory.
Results
The CP group reported significantly higher levels of pain, depression, and sleep problems. No significant performance difference was found on the neuropsychological tests in or outside the scanner between the two groups. There were no correlations between level of pain, depression, and sleep problems or between these and the neuropsychological test scores. CP patients exhibited significantly less brain activation and deactivation than controls in parietal and frontal lobes, which are the brain areas that normally show activation and deactivation during working memory tasks. Sleep problems independently and significantly modulated the BOLD response to the complex working memory tasks and were associated with decreased brain activation in task-positive regions and decreased deactivation in the default mode network in the CP group compared to the control group. The pain and depression scores covaried with working memory activation.
Discussion
Sleep problems in CP patients had a significant impact on the BOLD response during working memory tasks, independent of pain level and depression, even when performance was shown not to be significantly affected.
Introduction
Cognitive complaints are common in patients with chronic pain (CP),Citation1 as well as objectively measured cognitive deficits.Citation2,Citation3 Working memory is often reduced in CP, and the reduction is independent of local analgesia.Citation4 The effect of CP on working memory is moderate and there is considerable discrepancy between studies.Citation5 Furthermore, working memory is affected by depressionCitation6 and sleep problems,Citation7 both of which are common in CP patients. Approximately 70% of CP patients are reported to be moderately or severely depressed,Citation8 and/or experience sleep problems.Citation9,Citation10 It has been shown that pain sensitivity is increased by the induction of sad mood in CPCitation11 and by sleep deprivation.Citation12,Citation13 Moreover, sleep deprivation has negative effects on mood,Citation14 and sleep problems are present in the majority of depressed subjects.Citation15 Several prospective studies have also found that sleep problems increase the risk of later CP,Citation16–Citation20 and that restorative sleep is independently associated with later resolution of widespread pain.Citation21 Thus, CP, depression, and sleep problems are closely entwined, and all may affect working memory.
A number of studies have investigated the effect of experimental pain on brain activity during working memory tasks with T2* weighted, blood-oxygen-level dependent (BOLD) functional magnetic resonance imaging (fMRI),Citation22,Citation23 but only one fMRI study has investigated working memory in a group of CP patients.Citation24 In the latter study, patients with chronic fibromyalgia exhibited reduced brain activation relative to controls, and a significant effect of level of depression on brain activity was reported. Since sleep deprivation is also known to reduce BOLD activation in brain regions during working memory tasks in healthy controls (HC),Citation25–Citation31 sleep problems may impact working memory related brain activity in CP patients, but this remains to be studied. Indeed, fMRI studies on working memory in CP patients that simultaneously take into account level of pain, depression, and sleep problems are lacking.
The aim of the current study was to investigate BOLD activation in CP patients compared with HC during different working memory tasks, and to study the relationship between BOLD activation and level of pain, depression, and sleep problems to verify the contribution of each of these to BOLD signal differences.
Methods
The study was approved by the Regional Committee for Medical Research Ethics and the Norwegian Social Sciences Data Service. Written informed consent was obtained from all participants. In addition, all participants were informed personally and in writing that they could withdraw their consent at any time without any consequences. All participants were offered a monetary compensation of 400 NOK and pictures from their morphological brain scan.
Subjects
A total of 20 CP patients (16 females) were recruited from a local university hospital pain clinic. Inclusion criteria for the CP group were ≥6 months with average pain intensity of ≥4 on the Verbal Rating Scale.Citation32,Citation33 An experienced clinician performed the clinical assessment. To minimize external effects on cognition or brain activity, subjects with high consumption of analgesics were excluded (>180 mg codeine or equivalent per 24 hours, 24 hours continuous benzodiazepine treatment, or using carisoprodol). The included subjects were instructed not to consume caffeine and/or nicotine in the hours prior to testing and scanning. No morphological abnormalities were detected in the MRIs of any of the participants.
In addition, a control group of 20 age-, sex-, and education-matched HC (18 females) were recruited from the local community. Exclusion criteria for both CP patients and HC were severe psychiatric disorder and any neurological disorders, including traumatic brain injury (<13 Glasgow Coma Scale at the time of injury) and MRI contraindications. A diagnosis of mild or moderate depression did not warrant exclusion in any of the groups, neither did use of antidepressants. All participants reported being right-handed, and were assessed with the Edinburgh Handedness InventoryCitation34 (CP: 0.82±0.21, range: 0.43–1; HC: 0.91±0.16, range: 0.45–1).
One subject was excluded after previous neurological disease was discovered in the clinical interview. A series of technical problems caused data loss that resulted in the final groups consisting of 15 CP subjects (13 females) and 17 HC subjects (16 females). Of the 15 included patients, ten were classified as having musculoskeletal pain, four idiopathic pain, and one as having visceral pain. None had neuropathic pain.
Pain
Pain intensity was assessed using the validated Norwegian translationCitation35 of the Brief Pain Inventory (BPI).Citation36 Total BPI score was calculated. In BPI, the intensity of pain during the last 24 hours is rated using a numerical rating scale (NRS), where 0 is no pain and 10 is worst imaginable pain. The NRS measure was used as an estimate of individual level of pain at time of the experiment and applied in the fMRI analysis.
Depression
The level of depression was assessed with the validated Norwegian translationCitation37 of the Beck Depression Inventory (BDI) II.Citation38 BDI has been validated in a CP population with BDI Negative Thoughts and BDI Behavior,Citation39 and recommended for use in clinical studies of CP.Citation40 Score on the BDI was used as the level of depression in analyses, and not for diagnosing the presence or absence of clinical depression.
Quality of sleep
The Norwegian validated versionCitation41 of the Pittsburgh Sleep Quality Index (PSQI)Citation42 was used to measure the quality of sleep. PSQI is related to the subjective sleep experience rather than objective measures of sleep quality and sleep problems.Citation43 It has been used in a number of studies in patients with CP.Citation44–Citation46 The cut-off value of five was used to differentiate good sleepers from bad sleepers (sensitivity 89.6%, specificity 86.5%).Citation42
Working memory and fMRI task design
The Wechsler Adult Intelligence Scale (WAIS)-III subtests Digit Span and Letter Number SequencingCitation47 were administered to all subjects. Age-adjusted scores for the groups are reported. While the Digit Span Forward requires basic attention, phonological loop, and short-term memory, the Digit Span Backward, and to a larger extent the Letter Number Sequencing, requires maintaining and updating the information. WAIS-III subtests were performed according to the instructions described by Wechsler.Citation47
For the fMRI experiments, 0- and 2-back (collectively referred to as n-back) plus paced visual serial addition test (PVSAT) paradigms were implemented. The n-back task is one of the more popular paradigms for studying working memory with functional neuroimagingCitation48 and is frequently used.Citation49 The PVSAT is an adapted version of a working memory, attention, and processing speed test used in CP and other patient groups.Citation50 The n-back and PVSAT paradigms test different attention and executive processes: basic attention and the phonological loop (0- and 2-back and PVSAT), updating and maintaining information (2-back and PVSAT), and manipulation of information (PVSAT). The 0-back probes sustained attention and other processes that underlie working memory. The design of the 0/n-back paradigm resembles a Go/No Go-taskCitation51 as subjects respond if the current element is identical to a predefined element, and in 66% of the trials the subject has to withhold the response. Reaction time (RT) variability on Go-elements of a Go/No Go-task has been used as a measure of inhibitory efficiency and is sensitive to sleep deprivation.Citation52,Citation53
The n-back and PVSAT paradigms were all block designs. There were six 30 seconds “off” blocks and five 30 seconds “on” blocks for the n-back paradigms. For the PVSAT paradigms, there were eight 30 seconds “off” blocks and seven 30 seconds “on” blocks. In the “off” blocks, participants were instructed to fixate on a white cross in the center of a black screen. In each “on” block in the n-back tasks, 12 numbers were shown for 500 ms with a fixation asterisk lasting for 2,000 ms between the numbers. In the “on” blocks in the PVSAT, 15 numbers were shown for 500 ms with a fixation asterisk lasting for 2,000 ms between the start of each numbers. The n-back and PVSAT tasks were balanced in such a way that the number of correct responses per block was similar for all three paradigms. This was done to ensure that data from the different conditions would later be comparable. The n-back and the PVSAT tasks were programmed, presented, and the subjects’ performance recorded in E-Prime 1.1 (Psychology Software Tools, Inc., Sharpsburg, PA, USA). The paradigm presentation order was randomized and the stimuli presentation order was pseudorandomized. During fMRI scanning, the tasks were displayed on an LCD screen mounted behind the bore opening, and viewed through a mirror mounted on the head coil. All responses were recorded using response buttons from NordicNeuroLab (NNL) (Bergen, Norway). The participants were familiarized with the fMRI paradigms outside the scanner and performed computer-based test versions of each paradigm until full compliance was obtained.
n-back paradigm
The subject was instructed to press a response button every time the number shown was identical to the number preceding it by n steps.Citation54 Subjects were tested with n=0 and n=2, referred to as 0-back and 2-back, respectively. The numbers shown were between 1 and 13. For the 0-back, subjects were instructed to respond by pressing the button whenever the number shown was 7 or 13. Thus, no manipulation of information in working memory was required. For the 2-back condition, the subjects were instructed to press the button whenever they saw a number identical to the one before the previous. Both n-back trials induced button presses 33% of the time if performed correctly.Citation54 n-back tasks are usually performed with letters. Since there is a small, but significant difference between using numbers and letters in an n-back paradigm,Citation55 we used numbers in our n-back task in order to ensure comparability with the PVSAT paradigm.
PVSAT
All participants completed one PVSAT paradigm. In the PVSAT, subjects were shown a series of numbers between 1 and 12 and asked to add every number to the number before it. When the sum was either 7 or 13, the subject was instructed to press the response button. This was done in order to keep the PVSAT comparable to the n-back paradigms with regard to both the response method and the interstimulus intervals, ie, nonverbal button press responses. To ensure that all subjects did indeed add the numbers as instructed, the approach of Mainero et alCitation56 was modified by asking subjects to press the response button every time the sum equaled 7 or 13. Previous research shows that training has a significant effect on Paced Auditory Serial Addition Test (PASAT) scores, partly because experience with the test alleviates frustration and anxiety, which have negative effects on scores.Citation57 With this in mind, all participants received a standardized and thorough explanation of the task adapted from the Gronwall version of PASAT instructions,Citation58 including an out-of-scanner 8-minute PVSAT training session, a set up identical to the fMRI run, but with 12 blocks of 15 numbers, and resting blocks only lasting 10 seconds. The training session paused at 33% and 66% completion, and started again when subjects decided they were ready to continue. The subjects also trained in the scanner before fMRI scanning commenced.
fMRI
Scanning was performed on a 3T Siemens Trio scanner with a 12-channel head matrix coil (Siemens AG, Erlangen, Germany). Foam pads were used to minimize head motion. T2* weighted, BOLD sensitive images were acquired using an echo-planar imaging pulse sequence (repetition time 3,000 ms, echo time 35 ms, field of view 220 mm, slice thickness =2.8 mm, slice number =41, in-plane resolution 2.8×2.8 mm). Each functional run contained either 111 (n-back) or 152 volumes (PVSAT), with slices positioned parallel to the plane through the anterior and posterior commissures. For anatomical reference, one T1 weighted 3D volume was acquired (2,300 ms repetition time, 2.88 ms echo time, 900 ms inversion time, 9° flip angle, 526 mm field of view, 160 slices, 1.2 mm slice thickness, 1.0×1.0 mm in-plane resolution).
Functional image analysis
Imaging data preprocessing and analysis were performed with FSL 4 (FMRIB Software Library; Analysis Group, FMRIB, Oxford, UK). Preprocessing involved brain extraction, motion correction (MCFLIRT), interleaved slice time correction, spatial smoothing (FWHM 6.0 mm), intensity normalization, and high-pass temporal filtering (cut-off 90 seconds). Nonlinear coregistration was performed to the 1 mm Montreal Neurological Institute (MNI) template with a warp resolution of 10 mm. For each paradigm, absolute and relative displacements were calculated for all participants.
Individual runs were analyzed with an uncorrected statistical threshold of P<0.05 in the first level. Intra-individual contrasts in the second level (2-back > 0-back, PVSAT > 0-back, PVSAT > 2-back) were analyzed with fixed effects analysis and an uncorrected statistical threshold of P<0.05. Between-subject differences were first investigated with a threshold of P<0.005 uncorrected and cluster size >20 voxels, which is equivalent to a false discovery rate (FDR) of q<0.05 and suggested for use in fMRI studies with smaller samples.Citation59 Group differences were subsequently assessed with a mixed effects analysis (FLAME1) with pain, depression, and sleep scores as regressors (see Group differences on BOLD activations and impact of level of pain, depression, and sleep). These analyses were also subsequently thresholded with a cluster-corrected Z threshold of Z>3.0 and P<0.05. Stricter statistical thresholds were employed to enable better specification of the locations of activation differences between groups for the different contrasts.
It has been shown that CP,Citation60,Citation61 BDI depression score,Citation62 and sleep deprivationCitation25,Citation29,Citation63,Citation64 can affect cerebral blood flow and/or the BOLD response. BOLD activity in the CP group could thus be significantly affected by level of pain, depression, and/or sleep problems, which could mask or increase group differences in brain activation between the CP and HC groups. To unpack the possible independent contributions of pain, depression, and sleep on brain activity during working memory tasks between the CP and HC group, we combined the three self-report measures (NRS rating, BDI score, and PSQI score), which were uncorrelated (“Results” section), as regressors in a common general linear model. Analyses were run one time for each regressor separately, each time with the two other regressors orthogonalized on the regressor of interest. This was done to establish the presence of a unique contribution to BOLD activity for pain, depression, and sleep scores in the CP and HC groups.
Study protocol
The experimental layout was as follows: day one: BDI and BPI, n-back and PVSAT; day two: PSQI and Wechsler Adult Intelligence Test-III. The testing was separated over 2 days to avoid exhausting the participants.
Statistical analysis
Questionnaires and fMRI behavioral data were analyzed using Excel 2004 (Microsoft Corporation, Redmond, WA, USA) and PASW Statistics 18 (SPSS Inc., Chicago, IL, USA). Results are given as mean ± standard deviation and range where normal distribution applied in both groups. Where results from one or both group were not normally distributed, median and range are reported. Normality was assessed with the Shapiro–Wilk test.
For each fMRI paradigm, correct responses and nonresponses were registered as total scores. Likewise, the total number of errors of commissions, ie, a response when a nonresponse was correct, and the total number of errors of omission, ie, a nonresponse when a response was correct, were calculated. RT was measured from the presentation of new stimulus to the time of first subsequent button press.
Sleep deprivation has been found to increase variability in RT.Citation52,Citation53 Since pain is associated with sleep problems we calculated, for each paradigm, the individual variability in RT over all trials where responses were given. RT variability was assessed with Intra-Individual Coefficient of Variation, which is defined as the standard deviation of individual RT divided by the mean individual RT, after removing all trials where subjects did not respond correctly.Citation27 The RT variability was calculated for each fMRI paradigm and compared between the CP and HC groups.
Two-tailed, unpaired Student’s t-tests with P≤0.05 as a statistical threshold for significance were used on the behavioral data with normal distribution to statically evaluate the differences between the CP and HC groups. For measures that were not normally distributed (NRS, BDI, and PSQI among HC, and the majority of n-back and PVSAT behavioral measures), Independent Mann–Whitney U tests were used. To compare proportions in each group, chi-square test was used. Cohen’s d was calculated and classified as small (d=0.15–0.40), medium (d=0.40–0.75), or large (d>0.75). To evaluate potential relationships between the three self-report measures (NRS, BDI, and PSQI) and also with behavior, a correlation matrix with bivariate Spearman correlation was set up in the CP group. The behavioral data obtained from the three fMRI paradigms (total scores) and the scores of pain, depression, and sleep problem questionnaires were entered into the analysis. Similar correlations were not performed in the HC group due to the limited range in scores. Correlations with a P<0.05, two-tailed, were considered significant.
Results
Demographics
Age, sex distribution, and years of education were not significantly different between the groups ().
Table 1 Demographics, level of pain, depression, and sleep quality and working memory performance in 15 chronic pain patients and matched healthy controls
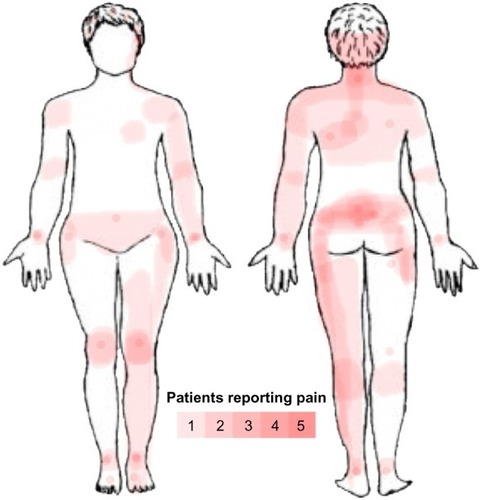
Subjects reported pain in a nonspecific pattern, both with regard to the localization of the painful areas and areas of maximal pain (). Total BPI score was significantly higher in the CP group (45.0, range: 28–81) compared to that in the HC group (2.7, range: 0–16) (P<0.001), as was the average level of pain during the last 24 hours, in the CP group (6.0, range: 3–8) compared to that in the HC group (0.0, range: 0–2) (P<0.0001) ().
Figure 1 Body map over pain location in CP group.
Abbreviation: CP, chronic pain.
The CP group scored significantly higher on BDI with 12.0 (range: 0–33), compared to the HC group scoring 1.0 (range: 0–8) (P<0.0001) (). According to a CP-specific BDI cut-off, only two patients had a BDI score that indicated they were likely clinically depressed.Citation8 Three CP patients were on selective serotonin reuptake inhibitors.
The CP group had a significantly higher PSQI score of 11.0 (range: 2–16) compared to 2.0 (range: 0–6) in the HC group (P<0.0001). Indeed, the CP group differed significantly from the HC group on all the sleep problem subscales (P- values between P<0.02 and P<0.001) (). Furthermore, 86.7% in the CP group were poor sleepers, compared to 5.9% in the HC group (χ2[1] =21.13, P<0.001).
Working memory testing and fMRI task behavior
Analysis of motion correction data showed that there were no significant group differences in maximum absolute or relative displacement during scanning between the CP and HC groups, and also no large effect sizes.
There were no significant group differences on the neuropsychological working memory tests Letter Number Sequencing, Digit Forward or Digit Backward, but there was a medium effect size (Cohen’s d=0.61) for Letter Number Sequencing with lower scores in the CP group ().
Working memory performance during fMRI did not differ with regard to number of correct responses, errors of commission, errors of omission, average RT or RT variability on any of the fMRI paradigms between the CP and HC groups, although a large effect size was evident for RT variability on the 0-back ().
Table 2 Performance on the fMRI paradigms for chronic pain patients and healthy controls
There were no significant correlations between pain, depression, sleep, PVSAT-, and n-back scores in either group (CP group results shown in ).
Table 3 Correlations between working memory test, pain (NRS), depression (BDI), and sleep problems (PSQI) scores in chronic pain patients
Group differences on BOLD activations and impact of level of pain, depression, and sleep
With FDR q<0.05, significant group differences were present for the 2-back > 0-back, PVSAT > 0-back, PVSAT > 2-back contrast without the three self-report measures as regressors. Differences in activations were found in all brain lobes for both HC > CP and HC < CP. In general, the HC groups had higher Z values and more extensive activations compared with the CP group for the 2-back and PVSAT versus 0-back (). When including pain, depression, and sleep problem scores as regressors, the number of significantly different voxels was reduced for pain and depression, but markedly increased for sleep problems. Since the areas of increased activation were quite extensive, a stricter statistical threshold (Z.3.0, cluster P≤0.05) was applied to enable better differentiation of the activations resulting from the different analyses. Again, significant group differences were demonstrated for all three contrasts (2-back > 0-back, PVSAT > 0-back, PVSAT > 2-back) for HC > CP and to a limited extent in CP > HC. As expected, the regions with activation differences were similar, but the activations were more confined. Moreover, only sleep scores remained a significant contributor to working memory related differences in brain activity between the CP and HC groups with the stricter statistical threshold. With sleep scores as the main regressor, the HC group had significantly increased activation compared with the CP group, both for the 2-back > 0-back (bilateral lateral occipital cortex, bilateral middle frontal gyrus, right superior frontal gyrus, bilateral paracingulate gyrus, frontal pole, inferior temporal gyrus, and the thalamus) and the PVSAT > 0-back (bilateral lateral occipital cortex, right middle frontal gyrus, bilateral paracingulate gyrus, left precentral gyrus, left supramarginal gyrus, and right inferior frontal gyrus). The HC group also had increased activation in the frontal poles, bilaterally, in the 2-back > PVSAT condition. In addition, PVSAT > 0-back elicited higher activation bilaterally in the medial frontal lobe, in the CP group compared to the HC group. Detailed information on activation differences between the groups for the different contrasts is given in and . The sleep score related reductions in brain activation in the CP group compared with that in the HC group were found in all regions of the dorsal attention and the frontoparietal control networks for the 2-back > 0-back contrast.Citation65 Several areas in the dorsal attention and frontoparietal control networks also showed reduced activation in the PVSAT > 0-back contrast in the CP group. The regions with decreased activity in the CP compared with the HC group, resulted from less activation, not lack of activation. The increased activation in the CP > HC group for PVSAT > 0-back in the bilateral medial prefrontal gyrus, part of the default mode network,Citation66,Citation67 had a different origin. It stemmed from less deactivation in the CP group compared to the HC group (). The CP group thus showed both significantly reduced activation in the dorsal attention and frontoparietal control networks and significantly reduced deactivation in the default mode network compared to controls during more complex working memory tasks that were performed similarly at the behavioral level in the two groups.
Figure 2 Between-group differences in working memory activation.
Abbreviations: HC, healthy controls; CP, chronic pain; PVSAT, paced visual serial addition test; MNI, Montreal Neurological Institute.
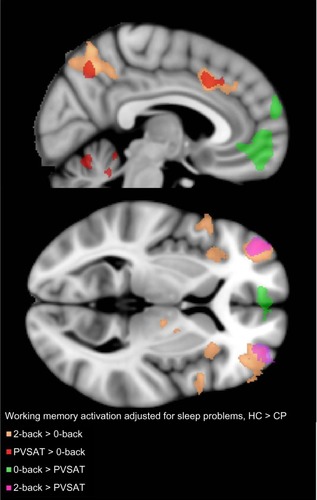
Figure 3 Brain regions with decreased activation at the whole brain level for contrast 0-back > 2-back with sleep problem score (PSQI), and scores for depression (BDI) and pain (NRS) as orthogonalized covariates in the CP group alone (blue), HC group alone (yellow), and the significant difference between them (HC > CP; green).
Abbreviations: HC, healthy controls; CP, chronic pain; PSQI, Pittsburgh Sleep Quality Index score; BDI, Beck Depression Inventory II score; NRS, average pain last 24 hours, rated on a numerical rating scale before scanning; MNI, Montreal Neurological Institute.
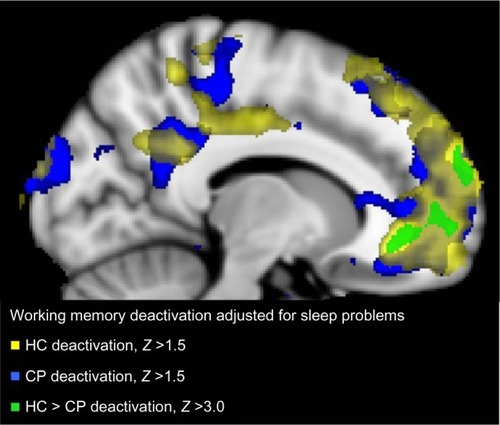
Table 4 Clusters of significantly increased or decreased activity in the CP versus HC groups during working memory fMRI
Table 5 Localization of maxima of increased and decreased BOLD signal in patients with CP versus HC for working memory tasks with sleep problems as main regressor and pain and depression scores orthogonalized
Discussion
The current study demonstrated that working memory performance was similar in the CP group and the matched HC group both for the traditional working memory tests and during fMRI. However, this similar performance was accompanied by areas of both reduced brain activation in the dorsal attention and frontoparietal control networks and deactivation in the default mode network in the CP group. Importantly, the difference in brain activity was explained by sleep problems in the CP group.
The CP and HC groups performed similarly on the working memory tests from WAIS-III and on the fMRI tasks. A lack of significant group differences on cognitive measures is not uncommon in CP studies.Citation5 There was a large effect size for RT variability for the simplest task, 0-back, but not for the 2-back and PVSAT in the CP group. Increased RT variability is often seen in sleep deprivation, and simple rather than more complex tasks are most affected at the behavioral level.Citation68 It should be noted that the CP group was not comparable to controls with total sleep deprivation. The CPs most likely suffered from partial sleep deprivation. In partial sleep deprivation in HC, the behavioral effects increase with time and the degree of deprivation, and significant performance effects are not observed before sleep deprivation reaches 50% of recommended sleep duration.Citation69 The lack of significant effects or correlations between sleep scores and test scores are therefore not unexpected.
Importantly, despite similar performance, there were significant group differences in brain activation during the more complex working memory tests. The between-group differences in the current study are quite similar to those reported in the only other fMRI study of working memory in chronic fibromyalgia patients using an n-back task.Citation24 Furthermore, the increased activity in the HC compared with that in the CP group during the 2-back and PVSAT tasks was located to areas where healthy subjects generally activate on the two tasks.Citation48,Citation70–Citation72
The main finding in this study is that sleep problems contribute independently to the differences in brain activation between the CP and HC group. When using pain or depression scores as primary regressors, the difference in BOLD activations between the CP and HC groups during performance of working memory tasks became smaller (significant impact seen only using the less strict statistical threshold) and not present (with the stricter threshold). This is in line with the Seo et alCitation24 study that reported a negative correlation between pain and depression scores and BOLD activity in frontoparietal regions in chronic fibromyalgia patients. Seo et alCitation24 specifically noted that pain and depression could not fully explain the differences in brain activity between the CP patients and controls. The current study adds to their findings by demonstrating the importance of sleep for differences in brain activity between the CP and HC groups. Sleep problems are as frequent in CP groups referred to specialist pain services as depression, and are found in ∼70%.Citation8,Citation9 Still, controlling for sleep in studies in CP is not common. In a meta-analysis of 23 behavioral working memory studies in CP, most of the studies did not control for sleep, which was described as a risk of bias.Citation5 Specifically, the present results demonstrated that sleep problems had an effect on brain activity in the CP group during complex working memory tasks since brain activity differences were increased for 2-back > 0-back and PVSAT > 0-back with sleep scores included in the model and pain and depression scores orthogonalized. Depression and pain scores, on the other hand, covaried similarly with brain activity for 2-back, PVSAT, and 0-back conditions, and with these as main regressors, the differences in brain activity between the HC and CP groups were reduced (for the sensitive statistical threshold) or had no additional impact (with the stricter statistical threshold). Increasing sleep problems were associated primarily with decreased BOLD response in the CP group in the same areas that the HC group activated. Sleep deprivation has previously been demonstrated to reduce working memory related BOLD signal in parietalCitation25–Citation31 and frontalCitation26,Citation27,Citation31 regions in HC, the same regions in which the CP group had lower activation compared with the HC group in the current study. Reduced activation in the frontoparietal areas in the CP group could be explained by reduced cerebral blood flow and glucose metabolism described in previous studies on sleep deprivation in HC.Citation73,Citation74 It is suggested that sleep deprivation causes local populations of neurons to collectively enter a nonrapid eye movement-sleep-like state and stop firing in wake subjects.Citation75 Such “local sleep” could explain reduced cerebral blood flow, glucose metabolism, and BOLD signal. The lower activation implies a reduced ability in the CP group to recruit more neural resources within the task-positive networks with increasing sleep problems. The CP group also displayed lack of deactivation during working memory task performance in medial frontal lobe, part of the default mode network. With increasing sleep problems, an increasing impairment in de-engaging the default mode activity was detected in the CP group. This is in line with previous reports in HC,Citation28,Citation29,Citation76–Citation78 and in chronic back pain patients during a simple attention task.Citation79 Taken together, sleep problems were shown to be connected to both reduced activation of task-positive networks and reduced deactivation of the default mode network during more complex working memory tasks in the CP group.
The areas involved in pain processing, sometimes referred to as the pain neuromatrix, include the primary and secondary somatosensory cortex, insula, anterior cingulate cortex, prefrontal cortex, and thalamus.Citation80 One hypothesis for cognitive impairments in CP is the limited resource hypothesis.Citation3,Citation81 Here, brain activity caused by pain interferes with concurrent cognitive processing relying on the same brain regions. There was overlap between the regions where differences in working memory activations where detected between the CP and HC groups and areas in the pain neuromatrix. Both prefrontal cortex and thalamus had significantly lower activity levels in the CP compared with the HC groups both in the analysis with sleep as main regressor and in the between-group analysis without regressors. However, current pain did not increase activation differences between the CP and HC groups in this study. This may be due to spontaneous pain fluctuations occurring during fMRI scanning in the CP group being more important for brain activity than average pain reported prior to scanning.Citation82 Nevertheless, these results indicated that CP per se affected brain activation rather than the current level of pain. Furthermore, CP may induce changes in the pain neuromatrix, which in turn influences cognitive processing capabilities. However, since the brain activity differences between the CP and HC groups without and with regressors were mostly outside the neuromatrix, other mechanisms appear to be more important for the altered BOLD response in CP than the limited resource hypothesis.
This study has several limitations. First, the CP group had CP of mixed etiology, which reduces the study’s sensitivity to any etiology-specific effects. This design does, however, increase the ecological validity and generalizability of the study’s results to CP patients in general. Moreover, most participants in the CP group were on analgesics and some on opioids, although high-dose users were excluded to avoid strong confounding effects, as opioids increase cerebral blood flow in HC.Citation83 Opioids are known to affect sleep patterns in both healthy subjects and CPCitation84,Citation85 and could therefore influence the results. Similarly, three patients were on antidepressants, which might be a confounder. Exclusion of all patients on opioids or antidepressants would have made it impossible to study the effect of depression, pain, and sleep in the same group of patients, and reduced the ecological validity of the results, while stopping medication would have introduced confounding withdrawal effects and be ethically questionable. Moreover, the small sample size makes it sensitive to type I and type II errors. Relatively strict statistical thresholds were used in the fMRI analysis, while all other statistical analyses were uncorrected for multiple testing. This limits the general-izability of the results before more research is done. Another issue is PSQI as a measure of sleep. PSQI measures subjective sleep quality and habitual patterns of sleep over time, ie, aspects of the sleep–wake experience distinct from objective measures like actigraphy or polysomnography.Citation43 The use of nonobjective measure of sleep problems makes it difficult to pinpoint the exact aspect(s) of the CPs’ sleep cycle, which is disturbed and possibly linked to the observed changes in brain activation. An objective measurement of habitual sleep behavior is very resource-intensive. For a first study of the impact of sleep on working memory performance and brain activity, PSQI is a reasonable compromise.
In conclusion, the current study demonstrated that sleep problems independently and significantly contributed to differences in BOLD activity in the CP group compared with the HC group during complex working memory tasks. The degree of sleep problems was associated with both decreased activation and deactivation in the CP group. These results suggest that working memory problems in CP stem from impaired recruitment of task-positive networks, which normally override the effects of lack of sleep as task complexity increases. This could have implications for future treatment of CP.
Disclosure
The authors report no conflicts of interest in this work.
References
- McCrackenLMIversonGLPredicting complaints of impaired cognitive functioning in patients with chronic painJ Pain Symptom Manage200121539239611369160
- LandrøNIForsEAVåpenstadLLHoltheØStilesTCBorchgrevinkPCThe extent of neurocognitive dysfunction in a multidisciplinary pain centre population. Is there a relation between reported and tested neu-ropsychological functioning?Pain2013154797297723473784
- MoriartyOMcGuireBEFinnDPThe effect of pain on cognitive function: a review of clinical and preclinical researchProg Neurobiol201193338540421216272
- DickBDRashiqSDisruption of attention and working memory traces in individuals with chronic painAnesth Analg2007104512231229 tables of contents17456678
- BerrymanCStantonTRJane BoweringKTaborAMcFarlaneALorimer MoseleyGEvidence for working memory deficits in chronic pain: a systematic review and meta-analysisPain201315481181119623707355
- LandrøNDepressive symptoms account for deficient information processing speed but not for impaired working memory in early phase multiple sclerosis (MS)J Neurol Sci2004217221121614706226
- NebesRDBuysseDJHalliganEMHouckPRMonkTHSelf-reported sleep quality predicts poor cognitive performance in healthy older adultsJ Gerontol B Psychol Sci Soc Sci200964218018719204069
- PooleHWhiteSBlakeCMurphyPBramwellRDepression in chronic pain patients: prevalence and measurementPain Pract20099317318019298363
- PilowskyICrettendenITownleyMSleep disturbance in pain clinic patientsPain198523127334058926
- MorinCMGibsonDWadeJSelf-reported sleep and mood disturbance in chronic pain patientsClin J Pain19981443113149874009
- TangNKYSalkovskisPMHodgesAWrightKJHannaMHesterJEffects of mood on pain responses and pain tolerance: an experimental study in chronic back pain patientsPain2008138239240118325674
- LeeJKimJShinHEffects of sleep deprivation on pain sensitivity in healthy subjectsSleep Med2013142013e180
- ØdegårdSOmlandPNilsenKGravdahlGStjernMSandTThe effect of two nights of partial sleep restriction on objective and subjective pain measurementsSleep Med2013142013e256e257
- DurmerJSDingesDFNeurocognitive consequences of sleep deprivationSemin Neurol200525111712915798944
- OhayonMMShapiroCMKennedySHDifferentiating DSM-IV anxiety and depressive disorders in the general population: comorbidity and treatment consequencesCan J Psychiatry200045216617210742876
- GuptaASilmanAJRayDThe role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population-based studyRheumatology (Oxford)200746466667117085772
- MirandaHViikari-JunturaEPunnettLRiihimäkiHOccupational loading, health behavior and sleep disturbance as predictors of low-back painScand J Work Environ Health200834641141919137202
- SaloPOksanenTSivertsenBHallMPenttiJVirtanenMSleep disturbances as a predictor of cause-specific work disability and delayed return to workSleep2010331020120613
- NitterAKPrippAHForsethKØAre sleep problems and nonspecific health complaints risk factors for chronic pain? A prospective population-based study with 17 year follow-upScand J Pain201234210217
- LusaSMirandaHLuukkonenRPunakallioASleep disturbances predict long-term changes in low back pain among Finnish firefighters: 13-year follow-up studyInt Arch Occup Environ Health201488336937925085527
- DaviesKAMacfarlaneGJNichollBIRestorative sleep predicts the resolution of chronic widespread pain: results from the EPIFUND studyRheumatology (Oxford)200847121809181318842606
- BingelURoseMGläscherJBüchelCfMRI reveals how pain modulates visual object processing in the ventral visual streamNeuron200755115716717610824
- CoenSJAzizQYágüezLBrammerMWilliamsSCRGregoryLJEffects of attention on visceral stimulus intensity encoding in the male human brainGastroenterology20081356206520742074. e118848558
- SeoJKimSHKimYTWorking memory impairment in fibro-myalgia patients associated with altered frontoparietal memory networkPLoS One201276e3780822715371
- MuQNahasZJohnsonKADecreased cortical response to verbal working memory following sleep deprivationSleep2005281556715700721
- MuQMishoryAJohnsonKADecreased brain activation during a working memory task at rested baseline is associated with vulnerability to sleep deprivationSleep200528443344616171288
- LimJChooW-CCheeMWLReproducibility of changes in behaviour and fMRI activation associated with sleep deprivation in a working memory taskSleep2007301617017310866
- ChooW-CLeeW-WVenkatramanVSheuF-SCheeMWLDissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation aloneNeuroimage200525257958715784437
- CheeMWLChooWCFunctional imaging of working memory after 24 hr of total sleep deprivationJ Neurosci200424194560456715140927
- Bell-McGintySHabeckCHiltonHJIdentification and differential vulnerability of a neural network in sleep deprivationCereb Cortex200414549650215054065
- LytheKEWilliamsSCRAndersonCLibriVMehtaMAFrontal and parietal activity after sleep deprivation is dependent on task difficulty and can be predicted by the fMRI response after normal sleepBehav Brain Res20122331627022565029
- ApkarianAVBalikiMNGehaPYTowards a theory of chronic painProg Neurobiol2009872819718952143
- WilliamsonAHoggartBPain: a review of three commonly used pain rating scalesJ Clin Nurs200514779880416000093
- OldfieldRThe assessment and analysis of handedness: the Edinburgh inventoryNeuropsychologia197191971135146491
- KlepstadPLogeJHBorchgrevinkPCMendozaTRCleelandCSKaasaSThe Norwegian brief pain inventory questionnaire: translation and validation in cancer pain patientsJ Pain Symptom Manage200224551752512547051
- CleelandCSPain assessment in cancerOsobaDEffect of Cancer on Quality of LifeBoca Raton, FLCRC Press1991293305
- AasenHAn empirical investigation of depression symptoms: norms, psychometric characteristics and factor structure of the Beck Depression Inventory-II [master’s thesis]BergenUniversity of Bergen2001
- BeckATSteerRABrownGKBeck Depression Inventory Manual2nd edSan Antonio, TXPsychological Corporation1996
- PooleHBramwellRMurphyPFactor Structure of the Beck Depression Inventory-II in patients With chronic painClin J Pain200622979079817057561
- DworkinRHTurkDCWyrwichKWInterpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendationsJ Pain20089210512118055266
- PallesenSNordhusIHSivertsenBMBjorvatnBPittsburgh sleep quality indexTidsskr Nor Psykol200542714717
- BuysseDJReynoldsCFMonkTHBermanSRKupferDJThe Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and researchPsychiatry Res19892821932132748771
- BuysseDJHallMLStrolloPJRelationships between the Pitts-burgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sampleJ Clin Sleep Med20084656357119110886
- MenefeeLACohenMJAndersonWRDoghramjiKFrankEDLeeHSleep disturbance and nonmalignant chronic pain: a comprehensive review of the literaturePain Med20001215617215101904
- MarinRCyhanTMiklosWSleep disturbance in patients with chronic low back painAm J Phys Med Rehabil200685543043516628150
- NaughtonFAshworthPSkevingtonSMDoes sleep quality predict pain-related disability in chronic pain patients? The mediating roles of depression and pain severityPain2007127324325217055168
- WechslerDWechsler Adult Intelligence Scale-III (WAIS-III)New York, NYPsychological Corporation1997
- OwenAMMcMillanKMLairdARBullmoreEN-back working memory paradigm: a meta-analysis of normative functional neuroimaging studiesHum Brain Mapp2005251465915846822
- WagerTDSmithEENeuroimaging studies of working memory: a meta-analysisCogn Affect Behav Neurosci20033425527415040547
- WörzRPain in depression – depression in painPain Clin Updat200311514
- DondersFCOn the speed of mental processingActa Psychol (Amst)1969304124315811531
- ChuahYMLVenkatramanVDingesDFCheeMWLThe neural basis of interindividual variability in inhibitory efficiency after sleep deprivationJ Neurosci200626277156716216822972
- SmithMEMcEvoyLKGevinsAThe impact of moderate sleep loss on neurophysiologic signals during working-memory task performanceSleep200225778479412405615
- BraverTSCohenJDNystromLEJonidesJSmithEENollDCA parametric study of prefrontal cortex involvement in human working memoryNeuroimage19975149629038284
- KnopsANuerkH-CFimmBVohnRWillmesKA special role for numbers in working memory? An fMRI studyNeuroimage200629111416095920
- MaineroCCaramiaFPozzilliCfMRI evidence of brain reor-ganization during attention and memory tasks in multiple sclerosisNeuroimage200421385886715006652
- TombaughTNA comprehensive review of the Paced Auditory Serial Addition Test (PASAT)Arch Clin Neuropsychol2006211537616290063
- StraussEShermanESpreenOA compendium of Neuropsychological Tests: Administration, Norms, and Commentary3rd edNew York, NYOxford University Press20061204
- LiebermanMDCunninghamWAType I and Type II error concerns in fMRI research: re-balancing the scaleSoc Cogn Affect Neurosci20094442342820035017
- WasanADLoggiaMLChenLQNapadowVKongJGollubRLNeural correlates of chronic low back pain measured by arterial spin labelingAnesthesiology2011115236437421720241
- DuschekSMannhartTWinkelmannACerebral blood flow dynamics during pain processing in patients with fibromyalgia syndromePsychosom Med201274880280923006430
- DunnRTKimbrellTAKetterTAPrincipal components of the beck depression inventory and regional cerebral metabolism in unipolar and bipolar depressionBiol Psychiatry200251538739911904133
- MiyataSNodaAOzakiNInsufficient sleep impairs driving performance and cognitive functionNeurosci Lett2010469222923319969042
- ScheiJRectorDEvoked electrical and cerebral vascular responses following sleep deprivationProg Brain Res201119323324421854966
- VincentJLKahnISnyderAZRaichleMEBucknerRLEvidence for a frontoparietal control system revealed by intrinsic functional connectivityJ Neurophysiol200810063328334218799601
- FoxMDSnyderAZVincentJLCorbettaMVan EssenDCRaichleMEThe human brain is intrinsically organized into dynamic, anticorre-lated functional networksProc Natl Acad Sci U S A2005102279673967815976020
- RaichleMEMacLeodAMSnyderAZPowersWJGusnardDAShulmanGLA default mode of brain functionProc Natl Acad Sci U S A200198267668211209064
- HarrisonYHorneJAThe impact of sleep deprivation on decision making: a reviewJ Exp Psychol Appl20006323624911014055
- Van DongenHPMaislinGMullingtonJMDingesDFThe cumulative cost of additional wakefulness: dose-response effects on neurobehav-ioral functions and sleep physiology from chronic sleep restriction and total sleep deprivationSleep200326211712612683469
- NiendamTALairdARRayKLDeanYMGlahnDCCarterCSMeta-analytic evidence for a superordinate cognitive control network subserving diverse executive functionsCogn Affect Behav Neurosci201212224126822282036
- TüdsöZHokPHrdinaLHluštíkPModality effects in paced serial addition task: differential responses to auditory and visual stimuliNeuroscience2014272102024802163
- LazeronRHRomboutsSAde SonnevilleLBarkhofFScheltensPA paced visual serial addition test for fMRIJ Neurol Sci20032131–2293412873752
- ThomasMSingHBelenkyGNeural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activityJ Sleep Res20009433535211123521
- PoudelGInnesCJonesRCerebral perfusion differences between drowsy and nondrowsy individuals after acute sleep restrictionSleep20123581085109622851804
- VyazovskiyVOlceseUHanlonENirYLocal sleep in awake ratsNature2011472734444344721525926
- CheeMWLChuahYMLFunctional neuroimaging and behavioral correlates of capacity decline in visual short-term memory after sleep deprivationProc Natl Acad Sci U S A2007104229487949217517619
- SämannPGTullyCSpoormakerVIIncreased sleep pressure reduces resting state functional connectivityMAGMA2010235–637538920473549
- De HavasJAParimalSSoonCSCheeMWLSleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performanceNeuroimage20125921745175121872664
- BalikiMNGehaPYApkarianAVChialvoDRBeyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamicsJ Neurosci20082861398140318256259
- ApkarianAVBushnellMCTreedeR-DZubietaJ-KHuman brain mechanisms of pain perception and regulation in health and diseaseEur J Pain20059446348415979027
- EcclestonCCrombezGPain demands attention: a cognitive-affective model of the interruptive function of painPsychol Bull1999125335636610349356
- ApkarianAVKraussBRFredricksonBESzeverenyiNMImaging the pain of low back pain: functional magnetic resonance imaging in combination with monitoring subjective pain perception allows the study of clinical pain statesNeurosci Lett20012991–2576011166937
- LorenzIHKolbitschCHörmannCThe influence of nitrous oxide and remifentanil on cerebral hemodynamics in conscious human volunteersNeuroimage20021721056106412377178
- DimsdaleJENormanDDeJardinDWallaceMSThe effect of opioids on sleep architectureJ Clin Sleep Med200731333617557450
- RoseARCatchesidePGMcEvoyRDSleep disordered breathing and chronic respiratory failure in patients with chronic pain on long term opioid therapyJ Clin Sleep Med201410884785225126029
