Abstract
Background
Longitudinal data on the impact of treatment on quality of life (QoL) in advanced non-small cell lung cancer (NSCLC) are limited. In this palliative setting, treatment that does not deteriorate QoL is key. Here we report longitudinal QoL in patients with squamous NSCLC, receiving ≤4 cycles of nab-paclitaxel/carboplatin combination chemotherapy.
Methods
Patients received nab-paclitaxel 100 mg/m2 days 1, 8, 15 + carboplatin area under the curve 6 mg•min/mL day 1 (q3w) for four cycles. QoL was assessed by the Lung Cancer Symptom Scale (LCSS) and Euro-QoL-5 Dimensions-5 Levels (EQ-5D-5L) at baseline and each cycle (day 1).
Results
Two-hundred and six lesion-response-evaluable patients completed baseline + ≥1 postbaseline QoL assessment and were QoL evaluable. LCSS average total score and symptom burden index improved from baseline throughout four cycles. In the LCSS pulmonary symptoms score, 46% of patients reported clinically meaningful improvement (≥10 mm visual analog scale) from baseline. Individual EQ-5D-5L dimensions remained stable/improved in ≥83% of patients; ≈33% reported complete resolution of baseline problems at least once during four cycles. Generally, responders (unconfirmed complete/partial response) had higher scores vs nonresponders.
Conclusion
In patients with squamous NSCLC, four cycles of nab-paclitaxel/carboplatin demonstrated clinically meaningful QoL improvements, with greater benefits in responders vs nonresponders.
Introduction
Patients with advanced non-small cell lung cancer (NSCLC) often experience a high symptom burden and a significantly deteriorated quality of life (QoL).Citation1–Citation3 In a real-world cross-sectional analysis of patients receiving treatment for advanced NSCLC (N = 450), all patients reported experiencing fatigue, and most experienced loss of appetite, shortness of breath, cough, hemoptysis, and pain.Citation4 These data indicate that in addition to prolonging survival, identifying a first-line treatment regimen that does not deteriorate physical well-being but instead maintains or improves symptom burden and/or QoL, is important for disease and treatment outcomes.
Despite recent treatment advances, chemotherapy remains the first-line standard of care for the majority of patients with advanced NSCLC (without a high level of PD-L1 expression), including those with squamous histology.Citation5,Citation6 However, there is a paucity of longitudinal QoL data from patients with advanced NSCLC receiving chemotherapy, and the data that do exist are often contradictory. Some studies have found that platinum-based chemotherapy improves QoL,Citation7 while others have found no improvement in QoL when compared with other treatments such as targeted agents.Citation8–Citation10 Prospective QoL data from clinical trials are even more limited in patients with squamous NSCLC, as few trials have reported QoL outcomes by histology.
nab-Paclitaxel in combination with carboplatin is approved for the first-line treatment of locally advanced or metastatic NSCLC in patients who are not candidates for curative surgery or radiation therapy, based on a favorable risk-benefit profile compared with paclitaxel plus carboplatin in a randomized Phase III trial.Citation11,Citation12 Compared with paclitaxel plus carboplatin, nab-paclitaxel plus carboplatin demonstrated a significant increase in the primary endpoint of overall response rate (ORR) (33% vs 25%, P = 0.005) in the intent-to-treat population. A subset analysis of patients with squamous histology demonstrated a 68% improvement in ORR with nab-paclitaxel plus carboplatin compared with paclitaxel plus carboplatin (41% vs 24%; P < 0.001).Citation12 The nab-paclitaxel plus carboplatin regimen was also well tolerated across histologies, as evidenced by the safety profile and patient-reported taxane-related symptom improvements.Citation12–Citation14 Although significant reductions in taxane-related symptoms such as neuropathy and neuropathic pain were reported with nab-paclitaxel plus carboplatin compared with paclitaxel plus carboplatin, it should be noted that improvements in these taxane-related symptoms may not reflect improvements in overall patient QoL.Citation14 This further underscores the need to assess the impact of this regimen on QoL in a prospective clinical trial.
The Phase III ABOUND.sqm trial is investigating first-line nab-paclitaxel plus carboplatin treatment followed by nab-paclitaxel with or without best supportive care as maintenance treatment in patients with advanced squamous NSCLC. The objective of this analysis was to evaluate the impact of four cycles of nab-paclitaxel plus carboplatin on patient-reported symptoms and health-related QoL outcomes.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Conference on Harmonisation. Informed consent was obtained from all patients prior to study entry. The study received approval from institutional review boards prior to commencement. The trial is registered at ClinicalTrials.gov (NCT02027428).
The approving Institutional Review Boards/Ethics Committees were Bundesinstitut für Arzneimittel und Medizinprodukte, Ethikkommission der Medizinischen Fakultät Heidelberg, Agenzia Italiana del Farmaco, Comitato Etico Campania Nord, Agencia Española de Medicamentos y Productos Sanitarios (AEMPS), CEIC Hospital Clinico San Carlos, Health Research Authority, Medicines and Healthcare Products Regulatory Agency, NRES Committee London Surrey Borders, and Quorum Review IRB.
Materials and methods
Study population
Patients with stage IIIB or IV, histologically or cytologically confirmed squamous NSCLC measurable by “Response Evaluation Criteria In Solid Tumors” (RECIST) version 1.1 were enrolled in this study. Key eligibility requirements included ≥18 years of age, no prior chemotherapy for metastatic disease, Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate hematologic, renal, and liver function. Patients with active brain metastases or preexisting peripheral neuropathy grade ≥2 were excluded.
Study design
The Phase III, randomized, open-label, multicenter ABOUND.sqm study is being conducted at ≈120 sites in the United States and the European Union. In the induction part, patients were treated with nab-paclitaxel (ABRAXANE®, albumin-bound paclitaxel) 100 mg/m2 intravenously on days 1, 8, and 15 plus carboplatin area under the curve 6 mg•min/mL intravenously on day 1, every 21 days. After completion of four cycles, patients without progression could continue to the maintenance part of the study in which they would be randomized 2:1 to receive nab-paclitaxel 100 mg/m2 intravenously on days 1 and 8 every 21 days plus best supportive care or best supportive care alone until disease progression. Randomization was stratified by disease stage before four cycles of treatment (IIIB vs IV), response at randomization (complete response/partial response [CR/PR] vs stable disease), and performance status at the end of the induction part (0 vs 1). The primary endpoint of the study is progression-free survival, which was measured from the time the patient was randomized at the start of the maintenance portion to the point of progression during the maintenance portion. Secondary endpoints include overall survival from randomization through the maintenance part, ORR during the induction and maintenance parts, and disease control rate over the entire study. Change in patient-reported QoL during the induction and maintenance parts was a prespecified exploratory endpoint.
QoL assessments
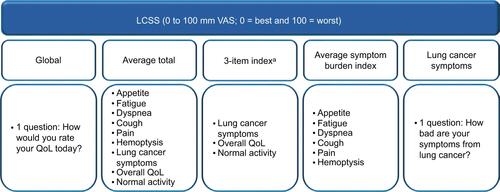
The Lung Cancer Symptom Scale (LCSS) and the Euro-QoL-5 Dimensions-5 Levels (EQ-5D-5L) questionnaires were used to measure QoL (Figure S1). In this analysis, per protocol, patient-reported QoL was assessed using the LCSS and EQ-5D-5L during four cycles of treatment on day 1 of each cycle through the end of the induction phase (cycle 5 day 1; prior to maintenance treatment). Patients completed the QoL self-assessments in the clinic using digital tablets.
The LCSS is a validated, disease-specific instrument completed by both patients and observers to measure QoL in patients with lung cancer.Citation15 The patient version of the LCSS consists of eight individual questions about lung cancer symptoms (appetite, fatigue, dyspnea, cough, pain, hemoptysis, overall symptom severity, and normal activities) all measured during the past 24 hours, one global question on QoL, one average total score of the nine questions, and one mean score of the six questions on major lung cancer symptoms. Patients answer each question using a visual analog scale (VAS) of 0 to 100 mm, where 0 = best and 100 = worst to indicate the intensity of a symptom. Figure S1 shows the components of the LCSS scores used in this analysis.
The EQ-5D is a generic instrument designed for self-completion that measures the patient’s health today and comprises the EQ-5D and a VAS for overall QoL.Citation16,Citation17 The EQ-5D includes questions on five dimensions of health (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). The answers to each EQ-5D-5L question are based on five qualitative levels (no problems, slight problems, moderate problems, severe problems, or unable to perform the activity). The EQ-5D-5L global index combines each of the five dimensions as a measure of the patient’s global health state. The VAS records the patient’s self-rated status for overall QoL based on a scale of 0 to 100 where 0 = worst and 100 = best.
Lesion response evaluation
Patients had a radiographic evaluation of lesions at the end of cycles 2 and 4. Patients with a CR or PR (by RECIST version 1.1 during the induction phase per the investigator), or those who experienced progressive disease in either evaluation or stable disease in both evaluations, or those who discontinued before or by the end of cycle 4, were considered lesion-response-evaluable.
Statistical analysis
This analysis included patients who were lesion-response-evaluable during the induction part of the study with available QoL data from baseline and ≥1 postbaseline visit. A clinically meaningful improvement in LCSS or EQ-5D VAS-scaled questions was defined as ≥1 postbaseline score for a given time point that was ≥10 mm higher than the baseline score.Citation3 For statistical analysis purposes, all scales were aligned so that a positive change from baseline indicated improvement. Changes from baseline LCSS and EQ-5D VAS-scaled items were described by descriptive statistics. The percentages of patients who had a clinically meaningful response, of patients who maintained or improved from baseline in the 5 EQ-5D-5L dimensions, and of patients who had complete resolution of a baseline problem at least once during four cycles of treatment are summarized.
Results
Patients
Of the 540 patients planned for enrollment into the induction part of the study, 284 patients had been treated as of September 6, 2016. Of these 284 patients, 246 patients were lesion-response-evaluable. Of the lesion-response-evaluable patients, the median age was 68 years, and 41% of patients were aged ≥70 years. The majority (67%) of patients had a baseline Eastern Cooperative Oncology Group performance status of 1 ().
Table 1 Selected baseline characteristics
QoL compliance
Of the 284 patients treated in the induction part, 251 were treated in cycle ≥2 and were eligible to complete ≥1 post-baseline QoL assessment. Of these, 223 (89%) completed baseline and ≥1 postbaseline assessments.
QoL results
LCSS
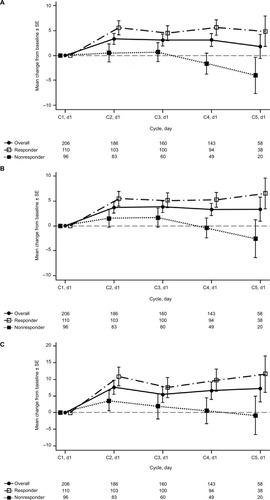
In total, 206 patients were lesion-response-evaluable with available QoL data from baseline and ≥1 postbaseline visit and were included in the LCSS analysis. In general, symptom burden decreased and QoL improved during four cycles of treatment with nab-paclitaxel plus carboplatin. Positive changes from baseline were observed in several LCSS symptom scores, including cough, appetite, and pain. For the LCSS average total score, improvement from baseline peaked on cycle 2 day 1 and remained stable throughout the rest of the induction phase (). A similar trend was noted for the average symptom burden index (). For the lung cancer symptom score that rated the severity of disease-specific symptoms, improvements from baseline were observed during each of the four cycles of nab-paclitaxel plus carboplatin treatment (). The changes in global score remained generally stable with respect to baseline scores throughout the induction phase, as did the changes in the 3-item index scores (). Overall, 46% of patients achieved a clinically meaningful improvement (≥10 mm) from baseline in the composite score of pulmonary symptoms during the induction phase. Clinically meaningful improvements in the individual items of cough, shortness of breath, and hemoptysis were also reported in 58%, 49%, and 16% of patients, respectively.
EQ-5D-5L
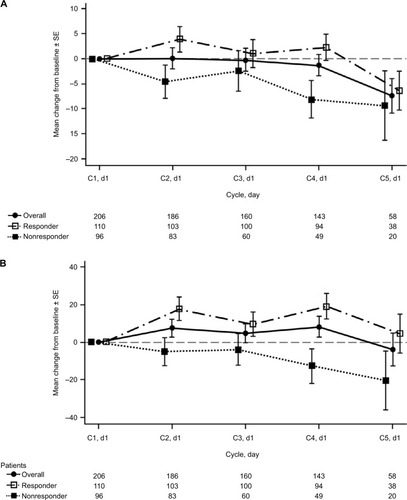
Two-hundred and six patients who were lesion-response-evaluable with available QoL data from baseline and ≥1 postbaseline visit were also included in the EQ-5D-5L analysis. The majority of patients experienced stability or improvement from baseline in individual EQ-5D-5L items, including self-care (91%), mobility (88%), anxiety/depression (88%), pain/discomfort (85%), and usual activities (83%) (). In patients reporting problems at baseline, complete resolution from baseline at least once during four cycles of treatment was reported in 30% to 50% of patients in individual EQ-5D-5L dimensions.
Table 2 EQ-5D-5L maintenance/improvement and complete resolution of problems in each dimension
LCSS and EQ-5D-5L by lesion response
Overall, of lesion-evaluable patients who completed baseline and ≥1 postbaseline QoL assessment, 110 were responders (CR/PR), and 96 were nonresponders. Responders had higher QoL scores compared with the overall population and nonresponders. LCSS average total score, average symptom burden index, and lung cancer symptom scores were improved from baseline in responders and were generally stable with respect to baseline in nonresponders (). Overall, 56% of responders and 33% of nonresponders achieved a clinically meaningful improvement in the composite score of pulmonary symptoms.
Across all EQ-5D-5L dimensions, stabilization or improvements in QoL scores were more pronounced in responders vs nonresponders and vs the overall population (). The percentage of responders reporting complete resolution at least once was nearly double that of nonresponders in the individual dimensions of usual activities (39% vs 21%), pain/discomfort (44% vs 27%), and anxiety/depression (59% vs 31%).
Discussion
These are the first published data of QoL outcomes with nab-paclitaxel plus carboplatin treatment in patients with advanced NSCLC. In this interim analysis of the Phase III ABOUND.sqm trial, treatment with four cycles of first-line nab-paclitaxel plus carboplatin resulted in maintenance of or improvement in QoL in patients with advanced squamous NSCLC. Clinically meaningful improvements from baseline were observed in composite LCSS lung cancer pulmonary symptoms in 46% of patients. In addition, several EQ-5D-5L components stabilized or improved in the majority of patients, and approximately one third of patients reported complete resolution of a problem reported at baseline at least once during four cycles of nab-paclitaxel plus carboplatin. Improvement in QoL was more pronounced in responders compared with the overall population and nonresponders.
nab-Paclitaxel plus carboplatin is a recommended regimen for the first-line treatment of patients with both squamous and nonsquamous advanced NSCLC, based on results of the registrational Phase III trial vs paclitaxel plus carboplatin.Citation5,Citation6,Citation12 In the Phase III registrational trial, significant improvements in ORR were reported for nab-paclitaxel plus carboplatin vs paclitaxel plus carboplatin for the intent-to-treat population, as well for the subset of patients with squamous histology. The regimen was well tolerated with significantly less grade ≥3 neuropathy and arthralgia but more thrombocytopenia and anemia observed compared with paclitaxel plus carboplatin.Citation13 Additionally, significant improvements in taxane-related symptoms, such as neuropathy and neuropathic pain, were reported with nab-paclitaxel plus carboplatin compared with paclitaxel plus carboplatin, regardless of histology.Citation14 The improvements in QoL reported during four cycles of nab-paclitaxel plus carboplatin treatment from the ABOUND. sqm study corroborate these findings and further expand the understanding of the overall clinical benefit of the nab-paclitaxel plus carboplatin regimen in patients with advanced NSCLC.Citation12,Citation14,Citation18
Given the substantial symptom distress (cough, pain, loss of appetite, and hemoptysis) associated with both lung cancer and its treatment, QoL data are becoming an important tool for chemotherapy decision making.Citation19–Citation23 The high tumor burden in many patients with advanced NSCLC can result in a negative impact on QoL and worsen disease-related symptoms.Citation24,Citation25 Patients with squamous histology represent nearly one third of all patients with NSCLC; thus, analyses of QoL are of great significance, as the location, size, and cavitating nature of many squamous NSCLC tumors may increase the symptomatic burden compared with other histologies.Citation25–Citation27 However, there remains a lack of prospective clinical trial data of the impact of chemotherapy on symptoms/QoL, particularly for platinum-doublet therapy in patient subsets with unmet needs, such as squamous NSCLC. The current analysis adds to the limited body of knowledge on QoL data in patients with squamous NSCLC treated with first-line platinum doublets, and indicates that four cycles of nab-paclitaxel plus carboplatin treatment maintain or improve QoL in this patient population.
In addition to symptoms, patient health status (i.e., mobility, anxiety/depression, and usual activities) can be impacted by disease and treatment. For example, in a cross-sectional study of 126 newly diagnosed patients with advanced NSCLC treated with docetaxel plus cisplatin, 38% reported depression at baseline.Citation28 QoL significantly declined with treatment in these patients, relative to those who did not report depression at baseline. In addition, depression was associated with worse survival. Another study assessed QoL changes in patients with advanced NSCLC (N = 39) and found significant declines from baseline in physical and social functioning, among other QoL areas, during treatment with platinum-doublet chemotherapy.Citation29 In the current study evaluating nab-paclitaxel plus carboplatin treatment, some health status issues (individual EQ-5D-5L dimensions of mobility, anxiety/depression, usual activities, pain/discomfort, and self-care) were maintained or improved in the majority of patients, with ≈30% reporting complete resolution from baseline at least once during treatment.
Understanding the impact of response on QoL is also important, as tumor shrinkage associated with tumor response could alleviate symptoms that are related to tumor size.Citation30,Citation31 In the current study, QoL improvements measured by both the LCSS and the EQ-5D-5L were more pronounced in patients responding (CR/PR) to nab-paclitaxel plus carboplatin during the induction phase of the study vs those not responding. In line with these findings, in a retrospective analysis of patient-reported health-related QoL data from a large Phase III study in advanced NSCLC (N = 488), patients with a CR/PR or stable disease after treatment with pemetrexed or docetaxel demonstrated statistically significant mean maximum improvements from baseline in all LCSS items except for hemoptysis.Citation30 In contrast, mean LCSS scores for each individual item worsened for patients with disease progression. Although the response data in this analysis are preliminary, they provide some insight on the clinical benefit of nab-paclitaxel plus carboplatin in patients with advanced squamous NSCLC.
EQ-5D and LCSS are validated tools for QoL measurements, yet they both have limitations that have been discussed at length elsewhere.Citation15,Citation16,Citation32 Limitations of this analysis include QoL assessment only during the induction phase, the homogeneous patient subset with respect to race (mostly white), and the limited patient number in some cycles. In addition, differences in use of supportive care for the treatment of pain may have diluted the results of the EQ-5D-5L pain/discomfort category. Further analyses including data from the maintenance setting are forthcoming and may help strengthen future QoL analyses from this study.
Conclusion
These results from the Phase III ABOUND.sqm trial demonstrate that four cycles of first-line nab-paclitaxel plus carboplatin improved or maintained QoL in patients with advanced squamous NSCLC. In addition, patients with a tumor response (CR/PR) appeared to have better QoL outcomes than those without a response. These findings in conjunction with those reported from the Phase III registrational trial support nab-paclitaxel plus carboplatin as a treatment choice for this difficult-to-treat patient population. Moreover, it appears that declining QoL observed in nonresponders may be related to symptoms of disease progression. Therefore, given the importance of response during induction therapy, the impact of four cycles of nab-paclitaxel combination treatment on QoL in the squamous population is promising.
Acknowledgments
The authors thank Lipu Tan at Celgene for his programming work. Medical writing assistance was provided by Dena Jacob, PhD, MediTech Media, Ltd, funded by Celgene Corporation. The authors were fully responsible for all content and editorial decisions for this manuscript. This work was supported by Celgene Corporation.
Supplementary material
Disclosure
MT: honoraria, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Lilly Novartis, MSD, Roche. DRS: consulting/advisory role, Celgene; research funding, Celgene; travel, accommodations, expenses, Celgene. RMJ: nothing to disclose. MM: nothing to disclose. MAS: honoraria and speaker’s bureau, Celgene. RDP: nothing to disclose. LG: nothing to disclose. JK: consulting/advisory role: Cardinal Health; speaker’s bureau, Alexion, Celgene, Novartis. OJ: advisory or speaker, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Lilly, MSD, Pierre-Fabre, Pfizer, Roche. DM: advisory board, Celgene and Bristol-Myers Squibb, speaker’s bureau, Genentech. DI: nothing to disclose. ESK: grant/research support, Celgene. HW: consultant/advisor and honoraria, AstraZeneca, Boehringer-Ingelheim, BristolMyersSquibb, Celgene, Genentech/Roche; Merck, Spectrum, Takeda; Speaker: BMS, Genentech/Roche. AK, NT, TJO: employment or leadership position and stock ownership, Celgene Corporation. CG: nothing to disclose.
References
- AkinSCanGAydinerAOzdilliKDurnaZQuality of life, symptom experience and distress of lung cancer patients undergoing chemotherapyEur J Oncol Nurs201014540040920149733
- LarssonMLjungLJohanssonBBHealth-related quality of life in advanced non-small cell lung cancer: Correlates and comparisons to normative dataEur J Cancer Care (Engl)201221564264922519874
- HirshVAre the data on quality of life and patient reported outcomes from clinical trials of metastatic non-small-cell lung cancer important?World J Clin Oncol201344828424926427
- IyerSRoughleyARiderATaylor-StokesGThe symptom burden of non-small cell lung cancer in the USA: A real-world cross-sectional studySupport Care Cancer201422118118724026981
- National Comprehensive Cancer Network Clinical Practice Guidelines in OncologyNon-Small Cell Lung Cancer. V3Fort Washington, PA, USANational Comprehensive Cancer Network2017
- NovelloSBarlesiFCalifanoRMetastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-upAnn Oncol201627Suppl 5v1V2727664245
- AvelinoCUCardosoRMAguiarSSSilvaMJAssessment of quality of life in patients with advanced non-small cell lung carcinoma treated with a combination of carboplatin and paclitaxelJ Bras Pneumol201541213314225972967
- GeaterSLXuCRZhouCSymptom and quality of life improvement in LUX-lung 6: an open-label phase III study of afatinib versus cisplatin/gemcitabine in Asian patients with EGFR mutation-positive advanced non-small-cell lung cancerJ Thorac Oncol201510688388925933111
- BoyeMWangXSrimuninnimitVFirst-line pemetrexed plus cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in East Asian never-smoker patients with locally advanced or metastatic nonsquamous non-small-cell lung cancer: quality of life results from a randomized phase III trialClin Lung Cancer201617215016026809984
- YangJCHirshVSchulerMSymptom control and quality of life in LUX-lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutationsJ Clin Oncol201331273342335023816967
- Abraxane for injectable suspension (paclitaxel protein-bound particles for injectable suspension) (albumin-bound) [prescribing information]Summit, NJCelgene Corporation2015
- SocinskiMABondarenkoIKarasevaNAWeekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trialJ Clin Oncol201230172055206222547591
- SocinskiMALangerCJOkamotoISafety and efficacy of weekly nab®-paclitaxel in combination with carboplatin as first-line therapy in elderly patients with advanced non-small-cell lung cancerAnn Oncol201324231432123123509
- HirshVOkamotoIHonJKPatient-reported neuropathy and taxane-associated symptoms in a phase 3 trial of nab-paclitaxel plus carboplatin versus solvent-based paclitaxel plus carboplatin for advanced non-small-cell lung cancerJ Thorac Oncol201491839024346096
- HollenPJGrallaRJKrisMGMeasurement of quality of life in patients with lung cancer in multicenter trials of new therapies. Psychometric assessment of the Lung Cancer Symptom ScaleCancer1994738208720988156514
- HerdmanMGudexCLloydADevelopment and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L)Qual Life Res201120101727173621479777
- euroqol.org [homepage on the Internet]EuroQol. EQ-5DEuroQol2016 Available from: www.euroqol.orgAccessed: August 31, 2017
- BraunDPGuptaDStarenEDQuality of life assessment as a predictor of survival in non-small cell lung cancerBMC Cancer20111135321843358
- HollenPJGrallaRJKrisMGEberlySWCoxCNormative data and trends in quality of life from the Lung Cancer Symptom Scale (LCSS)Support Care Cancer19997314014810335932
- TemelJSGreerJAMuzikanskyAEarly palliative care for patients with metastatic non-small-cell lung cancerN Engl J Med2010363873374220818875
- TemelJSPirlWFLynchTJComprehensive symptom management in patients with advanced-stage non-small-cell lung cancerClin Lung Cancer20067424124916512977
- SmithELHannDMAhlesTADyspnea, anxiety, body consciousness, and quality of life in patients with lung cancerJ Pain Symptom Manage200121432332911312047
- US Department of Health and Human Services, US Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and ResearchGuidance for industry: clinical trial endpoints for the approval of cancer drugs and biologicsFDA2007 Available from: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm071590.pdfAccessed: August 31, 2017
- GeigerCChenZZhangCInvestigating the correlation between disease burden and symptoms in patients with advanced stage lung cancerJ Clin Oncol201533Suppl abstract e19057
- NicholsLSaundersRKnollmannFDCauses of death of patients with lung cancerArch Pathol Lab Med2012136121552155723194048
- DrilonARekhtmanNLadanyiMPaikPSquamous-cell carcinomas of the lung: emerging biology, controversies, and the promise of targeted therapyLancet Oncol20121310e418e42623026827
- ScagliottiGVNovelloSRapettiSPapottiMCurrent state-of-the-art therapy for advanced squamous cell lung cancerAm Soc Clin Oncol Educ Book201335435823714545
- ChenJLiWCuiLChemotherapeutic response and prognosis among lung cancer patients with and without depressionJ Cancer20156111121112926516360
- ArrarasJIHernandezBMartinezMQuality of life in Spanish advanced non-small-cell lung cancer patients: determinants of global QL and survival analysesSpringerplus20165183627386285
- de MarinisFPereiraJRFossellaFLung Cancer Symptom Scale outcomes in relation to standard efficacy measures: an analysis of the phase III study of pemetrexed versus docetaxel in advanced non-small cell lung cancerJ Thorac Oncol200831303618166838
- WuYLFukuokaMMokTSTumor response and health-related quality of life in clinically selected patients from asia with advanced non-small-cell lung cancer treated with first-line gefitinib: post hoc analyses from the IPASS studyLung Cancer201381228028723540718
- OppeMDevlinNJvan HoutBKrabbePFde CharroFA program of methodological research to arrive at the new international EQ-5D-5L valuation protocolValue Health201417444545324969006