Abstract
Heart failure with normal ejection fraction (HeFNEF) accounts for ~50% of heart failure admissions. Its pathophysiology and diagnostic criteria are yet to be defined clearly which may hinder the search for effective treatments. The clinical hallmark of HeFNEF is exertional breathlessness, often due to an abnormal increase in left atrial pressure during exercise. Creation of an interatrial communication to offload the left atrium is a possible therapeutic approach. There are two percutaneously delivered devices currently under investigation which are discussed in this review.
Introduction
Heart failure with normal ejection fraction (HeFNEF) accounts for ~50% of heart failure diagnoses,Citation1,Citation2 yet the underlying pathophysiology and diagnostic criteria are poorly defined.Citation3–Citation5 Partly as a consequence, no medical treatment has yet shown convincing outcome benefit for patients with HeFNEF.Citation6–Citation8 There is a clear unmet need for effective treatments for patients with HeFNEF. Implantable devices, such as cardiac resynchronisation therapy (CRT), improve symptoms and life expectancy in a subset of patients with heart failure and reduced ejection fraction (HeFREF).Citation9 Some others allow early adjustment of medical therapy to avoid hospitalizations by monitoring cardiac haemodynamics.Citation10 However, trials exploring the role of implantable devices such as CRT and rate-adaptive pacing in patients with HeFNEF have stalled, mainly due to recruitment failure,Citation11–Citation13 perhaps reflecting a reluctance of older patients with several comorbidities to participate.
The clinical hallmark of HeFNEF is exertional breathlessness, at least in part due to an abnormal increase in left atrial pressure (LAP) during exercise.Citation14,Citation15 In patients with severe pulmonary artery hypertension (PAH), creation of a right to left atrial shunt reduces right atrial and ventricular pressures and improves symptoms,Citation16–Citation19 possibly due to increased systemic oxygen delivery due to increased cardiac output despite increasing cyanosis.Citation20 Decompressing the left atrium might similarly provide symptomatic and haemodynamic improvement in patients with HeFNEF.
Reducing LAP with a percutaneously delivered atrial septal device is a novel potential therapeutic strategy. This review summarizes the potential clinical implications of reducing LAP with an interatrial shunt device in patients with HeFNEFCitation21–Citation23 and discusses the preliminary results of the clinical trials conducted so far.
Consequences of changing LAP
There are many potential mechanisms leading to reduced exercise tolerance in patients with HeFNEF.Citation14,Citation24,Citation25 In normal physiology, increased stroke volume during exercise is accomplished in part by the positive lusitropic consequence of sympathetic activation: left ventricular (LV) relaxation is enhanced with lower LV pressure in early diastole.Citation26,Citation27 Impaired LV relaxation and increased LV stiffness in patients with HeFNEF prevents an increase in end diastolic LV volume during exercise,Citation28,Citation29 thus increasing pressure in the left atrium.Citation3 The excessive increase in LAP, measured by pulmonary capillary wedge pressure (PCWP), during exercise is a common finding in patients with HeFNEFCitation14,Citation15,Citation29 and identifies those with a worse prognosis.Citation30,Citation31
Although patients with HeFNEF reach a lower peak workload (watts per kilogram of body weight) during incremental exercise tests compared with normal controls, both reach similar peak exercise PCWP.Citation14 Increased workload-indexed peak exercise PCWP may be diagnostic of early-stage HeFNEF.Citation14 A higher ratio of peak exercise PCWP to workload is associated with increased risk of 10-year mortality in patients with HeFNEF.Citation31
Surgical and medical interventions that alter LAP might have a significant impact on symptoms and mortality in various cardiac pathologies. One example is that a device making invasive measurement of LAP as a guide for medical therapy in patients with HeFREF (n=40) was associated with reduced LAP, improved symptoms, and reduced rates of worsening symptoms requiring intravenous diuretic therapy.Citation32
In an observational study of 5 patients with high LAP and lower right atrial pressure (RAP) as a result of congenital obstructive left heart defects, the creation of an interatrial communication alleviated left atrial hypertension and improved symptoms.Citation33 Conversely, pulmonary edema can develop in some patients secondary to dramatic increases in LAP following closure of an atrial septal defect (ASD).Citation34,Citation35
Two devices, the V-Wave® (V-Wave Ltd, Or Akiva, Israel) and IASD® (DC Devices Inc., Tewksbury, MA, USA) have been tested in patients with heart failure ().Citation22,Citation23,Citation36–Citation41
Table 1 Summary of trials of interatrial shunt devices
V-Wave device
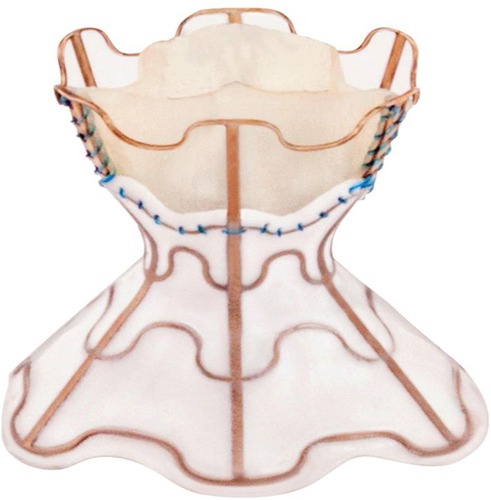
The V-Wave device is a tri-leaflet porcine tissue valve on an hourglass-shaped nickel-titanium frame ().Citation36 The device is implanted via a femoral venous approach under general anesthetic with fluoroscopic and trans-esophageal echocardiography (TOE) guidance.Citation22 Following radiofrequency trans-septal puncture, the center of the hourglass (5 mm diameter) is placed across the fossa ovalis with the ends of the hourglass sitting in left and right atria securing the device in place.Citation22 The left atrial orifice is lined with expanded polytetrafluoroethylene (ePTFE) designed to improve blood flow and restrict new tissue growth over the device.Citation22 Blood flows from the left to right via the porcine valve which is designed to close when RAP exceeds 2 mmHg, thus preventing right to left shunting.Citation22 After device implantation, patients require anticoagulation with warfarin or direct-acting oral anticoagulant (DOAC) for 3 months and with low-dose aspirin indefinitely.Citation22
Figure 1 The V-Wave® interatrial shunt device.
A proof-of-principle study was conducted in a single center in Canada, in patients with heart failure and reduced ejection fraction. They had raised PCWP (≥15 mmHg) without substantial right ventricular dysfunction (n=10; average age 62 years; average left ventricular ejection fraction (LVEF) 25%; average PCWP 23 mmHg; and average NTproBNP 2712 pg/mL).Citation36 After 3 months, insertion of the device was associated with a reduction in New York Heart Association (NYHA) class, increased 6-minute walk test distance, and improved quality of life (QoL) and physical function assessed by Kansas City cardiomyopathy questionnaire and Duke activity status index.Citation36 Device implantation was associated with a modest decrease in LV volumes and PCWP (23 mmHg pre-procedure to 17 mmHg at 3 months; p=0.035); however, natriuretic peptides (NPs), RAP (9 vs 8 mmHg at 3 months; p=0.18), and mean pulmonary artery pressure (29 vs 26 mmHg at 3 months, p=0.37) were unchanged.Citation36
A preliminary case report demonstrated the feasibility and safety of the V-Wave device in a patient severely symptomatic with HeFNEF and a history of ischemic heart disease and atrial fibrillation (AF) (PCWP 22 mmHg, LVEF 50%, taking 240 mg of furosemide per day).Citation39 Device insertion was associated with improved symptoms (NYHA III at baseline vs II at 6 months), functional capacity (6-minute walk test distance 281 m at baseline vs 617 m at 6 months), and a substantial drop in NTproBNP (2983 pg/mL at baseline vs 1334 pg/mL at 6 months).Citation39
A further prospective, nonrandomized, open label, multicenter study of the device is planned in patients with both HeFREF and HeFNEF (NCT02511912) ().Citation42
Table 2 Summary of future trials of interatrial shunt devices
IASD device
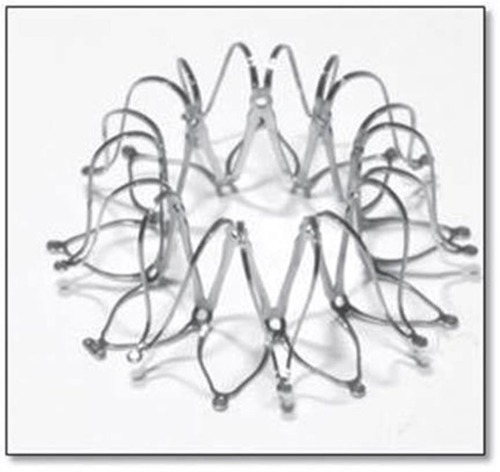
The interatrial shunt device (IASD) differs from the V-Wave device in three ways: first, the device does not incorporate valve tissue; second, the inter-atrial communication is larger (8 mm diameter compared with 5 mm with the V-Wave device); third, the device is a bare metal and not coated with ePTFE. Instead, the left atrial side of the device is flush with the atrial tissue to reduce the risk of thrombus formation ().Citation23
Figure 2 Expanded IASD® device.
The first in man experience of IASD was a nonrandomized, unblinded, feasibility study in patients with HeFNEF and baseline PCWP ≥15 mmHg at rest or ≥25 mmHg during exercise (n=11, average age 71 years; average LVEF 57%; median NTproBNP 148 pg/mL; average PCWP 19 mmHg).Citation23 Device implantation was associated with reduction in mean PCWP (by 28%, from 19+5 to 14+3 mmHg, p=0.005) after 30 days alongside symptomatic, functional, and QoL improvements.Citation23 NTproBNP plasma levels did not significantly change.Citation23 Devices were successfully implanted in all but one patient in whom device malpostion was corrected by the insertion of a new device. There were no reports of device-related complications such as migration or loss of patency (although in one patient the direction of flow could not be detected) after 30 days follow up.Citation23 During the same period, one patient was admitted with acute heart failure.Citation23 At 1 year, all patients survived and symptomatic improvement (measured by NYHA class) was sustained, although some patients required an increase in their daily dose of loop diuretics.Citation37
REDUCE LAP-HF trial
The REDUCE LAP-HF trial was an open-label, nonrandomized phase 1 study of the IASD in patients with HeFNEF and raised PCWP (≥15 mm at rest or ≥25 mmHg during exercise).Citation38 A total of 68 patients were enrolled in 21 centers, with an average age of 69 years. Average LVEF was 47% and mean PCWP at rest was 17 mmHg; median NT-pro-BNP was 377 pg/mL (interquartile range 222–925 pg/mL).Citation40 The co-primary endpoints were the proportion of patients with successful device implantation, reduction in PCWP at rest and during exercise after 6 months, and evidence of a left– right shunt on echocardiography at the same time point.Citation40
Procedure and short-term safety outcomes
Procedural complication rate was 5 in 66 initial procedures: 3 patients required removal of the initial device (misplacement n=2; suspected right atrial thrombus n=1). All the 3 patients subsequently underwent uncomplicated insertion of a second device. Two procedures had to be abandoned and were not repeated (one due to complications following trans-septal puncture and another due to unsuitable atrial anatomy). Overall, the device was successfully implanted in 64 patients (94%).Citation40
All the patients received dual antiplatelet therapy with aspirin and clopidogrel post procedure. In the first 6 months, there were no major adverse events, such as stroke, myocardial infarction, pulmonary or systemic embolism, or surgical intervention for device-related complications.Citation40 Left to right blood flow through patent devices was confirmed at 6 months in all patients who had adequate images (n=50).Citation40
Hemodynamic outcomes
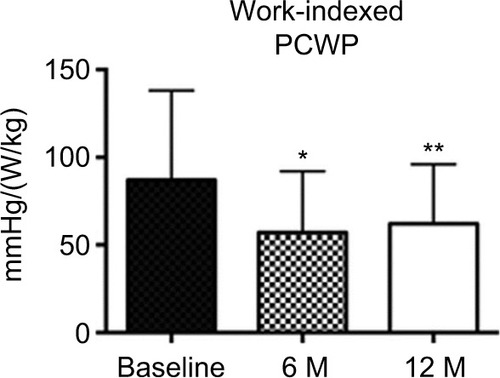
After 6 months, IASD implantation was associated with reduced PCWP at rest in 52% of patients (n=32) and reduced PCWP measured by right heart catheterization during supine bicycle exercise in 58% of patients (n=34). The device was associated with reduced rest or exercise PCWP in 71% of patients (n=42) and reduced rest and exercise PCWP in 39% of patients (n=23).Citation40 However, hemodynamic testing in a subset of patients at 12 months (n=18) showed no difference in average rest or exercise PCWP or RAP between baseline, 6 months, and 12 months.Citation41 Despite this, IASD insertion was associated with reduction in workload-indexed PCWP at peak exercise from baseline to 6 months (p≤0.05) which was sustained at 12 months (p≤0.01) ().Citation41
Figure 3 Bar graph showing workload-indexed peak exertion wedge pressure before and after interatrial shunt device placement (n=16).
Abbreviations: PCWP, pulmonary capillary wedge pressure; M, months.
QoL and symptom outcomes
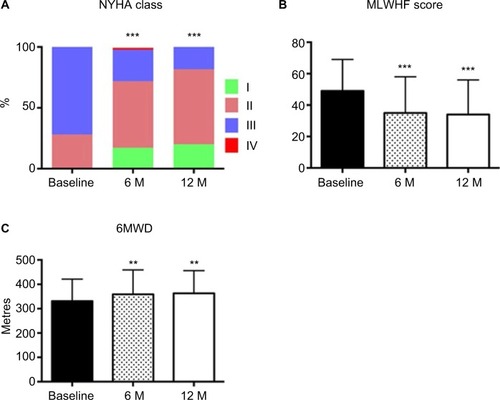
Insertion of the IASD was associated with significant improvements in symptoms, QoL, and functional status at 6 months which were sustained at 12 month follow-up: NYHA class (p≤0.001 at 6 and 12 months compared to baseline), QoL score (p≤0.001), and 6-minute walk distance (p≤0.01) all improved ().Citation41
Figure 4 Bar graph represents the effects of interatrial septal shunt device implantation on (A) NYHA class (n=60); (B) MLWHF (n=60); (C) 6MWD (n=55).
Abbreviations: NYHA, New York Heart Association; MLWHF, Minnesota Living with Heart Failure; 6MWD, 6-minute walk distance; M, months.
Long-term safety of IASD
Of the 64 patients who had the device implanted, 3 patients (5%) died between 6- and 12-month follow-up; 1 death was due to stroke, 1 due to pneumonia, and in 1 the cause was undetermined.Citation41 There were 17 HF hospitalizations among 13 patients in the year post implantation, 10 of which occurred in 10 patients in the first 6 months.Citation41 Left–right shunt through a patent device was confirmed on echocardiography at 12 months in all patients with good-quality images (n=48).Citation41
Introduction of a left–right atrial shunt with the IASD was associated with an increase in the right ventricular volume and ejection fraction, but not RAP compared to baseline.Citation41 There was no effect on LVEF and left atrial volume, although LV diastolic volume decreased.Citation41
Pulmonary artery oxygenation increased from 69% to 75% at 6 month follow-up (p≤0.0001) confirming the presence of a left–right shunt, but was not reported at 12 months.Citation40,Citation41 Although oximetry was used to measure LV output (which remained unchanged at 6 and 12 months), measures of systemic oxygenation were not reported at 6 or 12 months.Citation40,Citation41
Limitations of REDUCE LAP-HF
Creation of a left–right atrial shunt with either the V-Wave or IASD seems feasible and safe in the mid-term and may cause symptomatic improvement in some carefully selected patients with heart failure and raised LAP. However, caution is required when interpreting the available data.
Around one third of the patients had AF,Citation40 in which case PWCP might vary beat to beat. It is not clear whether one-off readings can be considered accurate or whether an average reading from a specific number of cardiac cycles was used instead.
The change in PCWP (a designated primary outcome) was not significant unless indexed for a subjective, patient-dependent variable such as exercise workload.
The investigators reported a decrease in the difference between LAPs and RAPs (reduced PCWP-RAP gradient) which, in fact, would have meant an increased RAP/PWCP ratio.Citation40,Citation41 Increased RAP/PCWP ratio is associated with poorer long-term outcome in hospitalized patients with advanced HeFREF (LVEF <30%),Citation43 but the clinical importance of this in HeFNEF is not yet known.
Many of the other measured outcomes were obtained through subjective tests in nonrandomized and unblinded participants and investigators. Outcomes such as QoL score, exercise time, and workload are subject to bias. Inevitably, there must be at least some placebo effect from undergoing a novel procedure involving general anesthesia.
Improvement in NYHA class was mirrored by an increase in average 6-minute walk distance (331 m at baseline vs 363 m after 12 months, p=0.001).Citation41 However, inter- and intra-observer NYHA class ascriptions are variable with low validity and reproducibility.Citation44,Citation45 Therefore, an improvement in exercise distance of an average 32 m may not be compatible with an improvement in NYHA class and may be due, instead, to a learning effect intrinsic to the test.Citation46
HeFNEF was defined as the presence of symptoms and signs compatible with the diagnosis, a LVEF >40%, and PCWP >15 mmHg at rest or >25 mmHg during supine bicycle exercise on right heart catheterization. Although there are no universally accepted criteria to diagnose HeFNEF, some evidence suggests that there is no difference in resting and peak exercise PCWP between healthy controls, patients with hypertension, and patients with HeFNEF.Citation14,Citation47
There were no data on the average or median systolic blood pressure of the participants in REDUCE LAP-HF or, indeed, any trial of V-Wave or IASD. A reduction in LAP can be achieved with commonly used heart failure medications, such as angiotensin-converting enzyme inhibitors (ACEi) or beta-blockers.Citation32 The use of diuretics, alone or in combination with an ACEi or angiotensin receptor blocker, improves symptoms and QoL in patients with HeFNEF.Citation48 It cannot be known from the published data whether patients in whom the V-Wave or IASD was implanted were eligible for up-titration of their antihypertensive medications as an alternative means to reduce LAP, although 70%–90% were hypertensive.Citation23,Citation36,Citation40 Indeed, 11 patients in REDUCE LAP-HF (17%) required an increased dose of diuretics during 6-month follow-up.Citation40
The signs and symptoms of heart failure are notoriously nonspecific, and under-diagnosis and misdiagnosis of HeFNEF may be common.Citation49–Citation51 Serum NPs are the most powerful prognostic marker in HeFNEF,Citation52,Citation53 and raised levels are a diagnostic requirement.Citation54 However, raised serum NPs were not an inclusion criteria for REDUCE LAP-HF.Citation38 Patients with HeFNEF and low NP levels probably have a good prognosis and thus are unlikely to benefit from invasive intervention. Of those with higher NTproBNP plasma levels, the proportion who had echocardiographic evidence to support the diagnosis of HeFNEF (raised E/E’ ratio or augmented LV mass or left atrial volume),Citation55 is not reported. Importantly, NP levels were not affected by device implantation.Citation40,Citation41
Future perspective
The long-term effects of the IASD are unknown. A chronic left–right shunt increases pulmonary blood flow and may be well tolerated at younger ages: patients with ASD are at increased risk of PAH and atrial arrhythmias, but clinical problems do not usually emerge until the fourth or fifth decade.Citation56–Citation65 However, in elderly individuals (the average age of patients in REDUCE LAP-HF was 69 years)Citation40 with HeFNEF and altered ventricular compliance, left–right atrial shunts may cause increased pulmonary arterial pressure and subsequent right ventricular dysfunction. Such hemodynamic changes might affect other organs already compromised in HeFNEF, such as the kidneys,Citation3 with a subsequent negative impact on long-term outcome.
Another factor that may influence long-term outcome is the use of anticoagulation: IASD implantation required 1-year dual antiplatelet therapy,Citation23 whereas V-Wave implantation required warfarin or DOAC for 3 months following implantation.Citation22 Both the devices require indefinite, low-dose aspirin.Citation22,Citation23 A large proportion of patients with HeFNEF have AF and require anticoagulation anyway,Citation3 which, in combination with aspirin, might increase bleeding risk. It is unclear whether, in patients with sinus rhythm, the anticoagulation required for either device is sufficient thrombo-prophylaxis or increases the risk of adverse events such as GI bleeding: one patient had a stroke during 12-month follow-up post IASD implantation,Citation41 and one patient had a nonfatal gastrointestinal bleed during 3-month follow-up post V-Wave implantation.Citation36 Vigilance and caution will be required with future trials.
Finally, changing patient behavior due to study involvement, known as the “Hawthorne effect,”Citation66 may have influenced the results; possibly by increasing medication compliance during follow-up. In the absence of a control group with a sham procedure or blind readers, trials of V-Wave and IASD may be subject to the same bias that has been observed in trials of other invasive treatments, such as renal nerve denervation.Citation67
Conclusion
HeFNEF remains an ill-defined clinical entity, which has lead, in part, to the failure to find effective treatment for the condition. Devices that can directly reduce LAP seem to be safe in the short- and mid-term, but there are no data on their long-term safety and efficacy.
Sham-controlled randomized trials would blind a patient to treatment, reducing the risk of bias which necessarily affects measures with a large subjective influence such as 6-minute walk distance or exercise workload. However, the practicalities may be difficult: the presence of a device would be easily spotted by an investigator during right heart catheterization or echocardiography. Careful selection of relevant endpoints (such as changes in symptoms) in patients blind to treatment (with a sham procedure) should be assessed by physicians also blinded to treatment.
Such a trial is essential to confirm that the benefits reported from unblinded studies are real. It is also unclear whether similar reductions in LAP sufficient to improve symptoms may be achievable with pharmacotherapy in patients with HeFNEF. Care must be taken with future trials () to ensure that there is no bias leading to misinterpretation of efficacy.
Disclosure
The authors report no conflicts of interest in this work.
References
- YancyCWLopatinMStevensonLWDe MarcoTFonarowGCCommittee ASA InvestigatorsClinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) DatabaseJ Am Coll Cardiol200647768416386668
- FonarowGCStoughWGAbrahamWTCharacteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF RegistryJ Am Coll Cardiol20075076877717707182
- PellicoriPClelandJGHeart failure with preserved ejection fractionClin Med (Lond)201414Suppl 6s22s2825468914
- PellicoriPClelandJGUpdate on management of heart failure with preserved ejection fractionCurr Opin Cardiol2015302173178
- FerrariRBöhmMClelandJGFHeart failure with preserved ejection fraction: uncertainties and dilemmasEur J Heart Fail20151766567126079097
- PatelKFonarowGCEkundayoOJBeta-blockers in older patients with heart failure and preserved ejection fraction: class, dosage, and outcomesInt J Cardiol2014173339340124703206
- ClelandJGTenderaMAdamusJFreemantleNPolonskiLTaylorJPEP-CHF InvestigatorsThe perindopril in elderly people with chronic heart failure (PEP-CHF) studyEur Heart J200627192338234516963472
- PittBPfefferMAAssmannSFSpironolactone for heart failure with preserved ejection fractionN Engl J Med2014370151383139224716680
- ClelandJGFDaubertJCErdmannEThe effect of cardiac resynchronization on morbidity and mortality in heart failureN Engl J Med20053521539154915753115
- HutchinsonKPellicoriPDierckxRClelandJGClarkALRemote telemonitoring for patients with heart failure: might monitoring pulmonary artery pressure become routine?Expert Rev Cardiovasc Ther20141281025103324984847
- LindeCCurtisABFonarowGCCardiac resynchronization therapy in chronic heart failure with moderately reduced left ventricular ejection fraction: Lessons from the Multicenter InSync Randomized Clinical Evaluation MIRACLE EF studyInt J Cardiol201620234935526426276
- KassDAKitzmanDWAlvarezGEThe restoration of chronotropic competence in heart failure patients with preserved ejection fraction (RESET) study: rationale and designJ Card Fail2010161172420123314
- US National Institutes of Health. ClinicalTrials.govRestoration of Chronotropic Competence in Heart Failure Patients with Normal Ejection Fraction (RESET) Available from: https://clinicaltrials.gov/ct2/show/NCT00670111Accessed February 13, 2017
- MaederMTThompsonBRBrunner-La RoccaHPKayeDMHemodynamic basis of exercise limitation in patients with heart failure and normal ejection fractionJ Am Coll Cardiol2010561185586320813283
- BorlaugBANishimuraRASorajjaPLamCSRedfieldMMExercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fractionCirc Heart Fail20103558859520543134
- McLaughlinVVArcherSLBadeschDBACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc. and the Pulmonary Hypertension AssociationJ Am Coll Cardiol2009531573161919389575
- KeoghAMMayerEBenzaRLInterventional and surgical modalities of treatment in pulmonary hypertensionJ Am Coll Cardiol2009541 SupplS67S7719555860
- SandovalJGasparJPeñaHEffect of atrial septostomy on the survival of patients with severe pulmonary arterial hypertensionEur Respir J2011381343134821349914
- ChiuJSZuckermanWATurnerMEBalloon atrial septostomy in pulmonary arterial hypertension: effect on survival and associated outcomesJ Heart Lung Transplant20153437638025813766
- BarstRJRole of atrial septostomy in the treatment of pulmonary vascular diseaseThorax2000552959610639523
- KayeDShahSJBorlaugBAEffects of an interatrial shunt on rest and exercise hemodynamics: results of a computer simulation in heart failureJ Card Fail201420321222124487087
- Amat-SantosIJBergeronSBernierMLeft atrial decompression through unidirectional left-to-right interatrial shunt for the treatment of left heart failure: first-in-man experience with the V-Wave deviceEuroIntervention20151091127113124832489
- SøndergaardLReddyVKayeDTranscatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel interatrial implant to lower left atrial pressureEur J Heart Fail20141679680124961390
- HaykowskyMJBrubakerPHJohnJMStewartKPMorganTMKitzmanDWDeterminants of exercise intolerance in elderly heart failure patients with preserved ejection fractionJ Am Coll Cardiol201158326527421737017
- AbudiabMMRedfieldMMMelenovskyVCardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fractionEur J Heart Fail20131577678523426022
- ChengCPIgarashiYLittleWCMechanism of augmented rate of left ventricular filling during exerciseCirc Res19927019191345778
- FukutaHLittleWCThe cardiac cycle and the physiological basis of left ventricular contraction, ejection, relaxation, and fillingHeart Fail Clin20084111118313620
- BorlaugBAJaberWAOmmenSRDiastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fractionHeart20119796496921478380
- ZileMRBaicuCFGaaschWHDiastolic heart failure – abnormalities in active relaxation and passive stiffness of the left ventricleN Engl J Med20043501953195915128895
- BourgeRCAbrahamWTAdamsonPBRandomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF studyJ Am Coll Cardiol2008511073107918342224
- DorfsSZehWHochholzerWPulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fractionEur Heart J201435443103311225161181
- RitzemaJTroughtonRMeltonIHemodynamically Guided Home Self-Therapy in Severe Heart Failure Patients (HOMEOSTASIS) Study Group. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failureCirculation20101211086109520176990
- LeonardGTJrJustinoHCarlsonKMAtrial septal stent implant: atrial septal defect creation in the management of complex congenital heart defects in infantsCongenit Heart Dis2006112913518377559
- ThomaiFGaspardoneAPapaMPoliscaPAcute left ventricular failure after transcatheter closure of a secundum atrial septal defect in a patient with coronary artery disease: a critical reappraisalCatheter Cardiovasc Interv200255979911793503
- MasutaniSSenzakiHLeft ventricular function in adult patients with atrial septal defect: implication for development of heart failure after transcatheter closureJ Card Fail20111795796322041334
- Del TrigoMBergeronSBernierMUnidirectional left-to-right interatrial shunting for treatment of patients with heart failure with reduced ejection fraction: a safety and proof-of-principle cohort studyLancet20163871290129727025435
- MalekFNeuzilPGustafssonFClinical outcome of transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel implantInt J Cardiol201518722722825841123
- HasenfussGGustafssonFKayeDMRationale and design of the reduce elevated left atrial pressure in patients with heart failure (Reduce LAP-HF) trialJ Card Fail201521759460026055211
- Amat-SantosIJDel TrigoMBergeronSLeft atrial decompression using unidirectional left-to-right interatrial shunt: initial experience in treating symptomatic heart failure with preserved ejection fraction with the W-Wave deviceJ Am Coll Cardiol Cardiovasc Interv201586870872
- HasenfussGHaywardCBurkhoffDA transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trialLancet20163871298130427025436
- KayeDMHasenfussGNeuzilPOne-year outcomes after transcatheter insertion of an interatrial shunt device for the management of heart failure with preserved ejection fractionCirc Heart Fail2016912e00366227852653
- US National Institutes of Health. ClinicalTrials.govREducing Lung Congestion Symptoms Using thE V-wavE Shunt in Advanced Heart Failure (RELIEVE-HF) Available from: https://clinicaltrials.gov/ct2/show/NCT02511912Accessed February 13, 2017
- DraznerMHVelez-MartinezMAyersCRRelationship of right- to left-sided ventricular filling pressures in advanced heart failure: insights from the ESCAPE trialCirc Heart Fail20136226427023392790
- RaphaelCBriscoeCDaviesJLimitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failureHeart200793476e8217005715
- GoldmanLHashimotoBCookEFLoscalzoAComparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scaleCirculation1981641227e347296795
- WuGSandersonBBittnerVThe 6-minute walk test: how important is the learning effect?Am Heart J2003146112913312851620
- LamCSPRogerVLRodehefferRJBorlaugBAEndersFTRedfieldMMPulmonary hypertension in heart failure with preserved ejection fraction: a community-based studyJ Am Coll Cardiol200953131119112619324256
- YipGWWangMWangTThe Hong Kong diastolic heart failure study: a randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fractionHeart200894557358018208835
- HancockHCCloseHMasonJMHigh prevalence of undetected heart failure in long-term care residents: findings from the Heart Failure in Care Homes (HFinCH) studyEur J Heart Fail20131515816523112002
- RedfieldMMJacobsenSJBurnettJCJrMahoneyDWBaileyKRRodehefferRJBurden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemicJAMA2003289219420212517230
- van RietEEHoesAWLimburgALandmanMAvan der HoevenHRuttenFHPrevalence of unrecognized heart failure in older persons with shortness of breath on exertionEur J Heart Fail201416777277724863953
- KirkVBayMParnerJN-terminal proBNP and mortality in hospitalized patients with heart failure and preserved vs reduced systolic function: data from the prospective Copenhagen Hospital Heart Failure Study (CHHF)Eur J Heart Fail20046335e4114987585
- O’DonoghueMChenABaggishALThe effects of ejection fraction on N terminal ProBNP and BNP levels in patients with acute CHF: analysis from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) StudyJ Card Fail200511S9e1415948094
- PonikowskiPVoorsAAAnkerSD2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failureEur J Heart Fail20161889197527207191
- PaulusWJTschopeCSandersonJEHow to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of CardiologyEur Heart J2007282539255017428822
- BaumgartnerHBonhoefferPDe GrootNMESC guidelines for the management of grown-up congenital heart disease (new version 2010)Eur Heart J201031232915295720801927
- MurphyJGGershBJMcGoonMDLong-term outcome after surgical repair of isolated atrial septal defect. Follow-up at 27 to 32 yearsN Engl J Med199032324164516502233961
- John SuttonMGTajikAJMcGoonDCAtrial septal defect in patients ages 60 years or older: operative results and long-term postoperative follow-upCirculation1981644024096788403
- WebbGGatzoulisMAAtrial septal defects in the adult: recent progress and overviewCirculation2006114151645165317030704
- Roos-HesselinkJWMeijboomFJSpitaelsSEExcellent survival and low incidence of arrhythmias, stroke and heart failure long-term after surgical ASD closure at young age. A prospective follow-up study of 21-33 yearsEur Heart J200324219019712573276
- DuffelsMGEngelfrietPMBergerRMPulmonary arterial hypertension in congenital heart disease: an epidemiologic perspective from a Dutch registryInt J Cardiol2007120219820417182132
- LoweBSTherrienJIonescu-IttuRDiagnosis of pulmonary hypertension in the congenital heart disease adult population impact on outcomesJ Am Coll Cardiol201158553854621777753
- SteelePMFusterVCohenMIsolated atrial septal defect with pulmonary vascular obstructive disease–long-term follow-up and prediction of outcome after surgical correctionCirculation1987765103710423664992
- BrandenburgROJrHolmesDRJrBrandenburgROMcGoonDCClinical follow-up study of paroxysmal supraventricular tachyarrhythmias after operative repair of a secundum type atrial septal defect in adultsAm J Cardiol1983512732766823836
- LeierCVMeachamJASchaalSFProlonged atrial conduction: a major predisposing factor for the development of atrial flutterCirculation197857213216618606
- SedgwickPThe Hawthorne effectBMJ2011344d8262
- MyersMGWijeysunderaHCRenal nerve denervation–a hypertension bubble?J Clin Hypertens2014167472474
- US National Institutes of Health. ClinicalTrials.gov. A Study to Evaluate the Corvia Medical, IncIASD® System II to REDUCE Elevated Left Atrial Pressure in Patients With Heart Failure With Reduced Ejection Fraction. (REDUCE LAP-HFREF) Available from: https://clinicaltrials.gov/ct2/show/NCT03093961Accessed February 13, 2017
