Abstract
Background
The Nellix endovascular aneurysm sealing (EVAS) system is a novel approach for the treatment of abdominal aortic aneurysm (AAA). We aimed to evaluate the efficacy of EVAS in the management of patients with AAA.
Materials and methods
We searched PubMed/MEDLINE, CINAHL, and bibliographic reference lists to identify studies reporting clinical outcomes in patients with asymptomatic, non-ruptured AAA treated with EVAS with the Nellix device. We pooled dichotomous outcome data using random-effects models.
Results
We identified 14 single-arm observational studies, reporting a total of 1,510 patients. The pooled estimate of technical success was 99% (95% CI =98–100; heterogeneity: P=0.869, I2=0%). Adjunctive procedures were carried out in 39% (95% CI =19–63; heterogeneity: P<0.0001, I2=88%). Two cases of aneurysm rupture were reported within 30 days of treatment (0.7%, 95% CI =0.3–1.6; heterogeneity: P=0.923, I2=0%) and another five cases of rupture occurred during follow-up (0.8%, 95% CI =0.4–1.6; heterogeneity: P=0.958, I2=0%). The pooled estimates of early (within 30 days) and late (during follow-up) type I endoleak were 2.8 % (95% CI =1.8–4.2; heterogeneity: P=0.254, I2=18%) and 1.9% (95% CI =1.3–2.8; heterogeneity: P=0.887, I2=0%), respectively. Sac enlargement was noted in 3.1% (95% CI =1.8–5.4; heterogeneity: P=0.419, I2=0%) and device migration in 2.1% (95% CI =0.8–5.3; heterogeneity: P=0.004, I2=65%). The early and late reintervention rates were 2.7% (95% CI =1.7–4.2; heterogeneity: P=0.183, I2=27%) and 3.5% (95% CI =2.3–5.5; heterogeneity: P=0.061, I2=42%), respectively. The pooled estimate of 30-day mortality was 1.5% (95% CI =0.9–2.6; heterogeneity: P=0.559, I2=0%) and the pooled estimate of aneurysm-related death during follow-up was 1.0% (95% CI =0.6–1.9; heterogeneity: P=0.872, I2=0%).
Conclusion
Reported outcomes of EVAS are acceptable. Type I endoleak, sac enlargement, device migration, and aneurysm rupture are recognized complications. High-level research is required to investigate potential advantages of EVAS over conventional treatments.
Introduction
The Nellix system (Endologix Inc., Irvine, CA, USA) for endovascular aneurysm sealing (EVAS) is a novel approach to treatment of abdominal aortic aneurysm (AAA) and conceptually different from endovascular aneurysm repair (EVAR).Citation1 EVAR was introduced in early 1990s and has now become an established treatment.Citation2,Citation3 The technique and devices have rapidly evolved and their application has expanded significantly.Citation4,Citation5
The EVAR procedure involves a stent-graft which is designed to exclude the aneurysm from the systemic circulation. The stent is made of a metallic skeleton and covered with polytetrafluoroethylene (PTFE) or polyester fabric which keeps the stent impermeable. The device is advanced through the femoral artery using fluoroscopic guidance toward the site of the aneurysm and then deployed. The aneurysm is isolated by sealing the proximal and distal ends of the aneurysm, preventing subsequent rupture.Citation6
The Nellix device is designed to seal and obliterate the aneurysm lumen.Citation1 It consists of two balloon-expandable stents which support the aorta flow channel. The system is introduced into the aorta in a similar way to EVAR; using guidewires, the system is advanced into the aorta through the femoral arteries. The catheter sheaths are then pulled back, deploying the device which expands from the non-aneurysmal aorta proximally to the iliac arteries distally. The non-porous PTFE-based endobags will then be filled using biocompatible polyethyleneglycol polymer, which adjusts the endobag to fit the aneurysm sac lumen. This allows sealing of the aneurysm and resists displacement.Citation1
EVAS aims to overcome the shortcomings of EVAR as well as provide better clinical outcomes. The Nellix device received European CE Mark approval recently and is currently being monitored for efficacy.Citation7 We aimed to conduct a comprehensive literature search and systematic review of published evidence to evaluate the efficacy of EVAS in the management of patients with AAA.
Materials and methods
Design
A prespecified protocol of the objectives and methods of the current systemic review was established. We reported this systematic review according to the PRISMA statement standards.
Eligibility criteria for study selection and patient inclusion
Inclusion criteria
Patients of any gender and age.
Studies reporting clinical outcomes in series of patients with asymptomatic, non-ruptured AAA treated with EVAS with the Nellix device.
Articles written in English.
Exclusion criteria
Case reports or case studies reporting less than five patients.
Editorials and letters to the editor or vascular images studies.
Review articles or experimental studies.
Articles that report clinical outcomes treated with other vascular devices.
Outcome measures
Outcome parameters were technical success, procedure time, fluoroscopy time, need for adjunctive procedures, mortality, postoperative complications, rupture of AAA, endoleak, device migration, sac enlargement, reintervention, and length of hospital stay.
Search strategy
Studies included in this review were identified through a focused search of the electronic databases PubMed/MED-LINE and CINAHL. The keywords used were “Nellix” and “endovascular aneurysm sealing”. The last search was conducted in April 2018. We also searched the bibliographic lists of relevant articles and reviews for further potentially eligible studies. Finally, we hand-searched the following leading journals in vascular and endovascular surgery: Journal of Vascular Surgery, European Journal of Vascular and Endovascular Surgery, and Journal of Endovascular Therapy.
Data collection
We created an electronic data extraction spreadsheet, pilot-tested it in randomly selected articles, and adjusted it accordingly. Our data extraction spreadsheet included the following information:
Study-related data: prospective or retrospective study design, type of study (case series or cohort study), year of publication, recruitment period, country of corresponding author, case type (single- or multicenter), and inclusion and exclusion criteria.
Baseline demographic and clinical characteristics of the study populations: age, gender, American Society of Anesthesiologists grade, smoking history, hypertension, diabetes, cardiac disease, respiratory disease, cerebrovascular disease, and renal disease.
Aneurysm anatomic data: aneurysm maximum diameter; aortic neck diameter, length and angulation; and whether the device was used within recommended instructions for use (IFU).
Outcome data.
Two authors independently collected and recorded data in the data extraction spreadsheet. Disagreements were resolved by discussion. If no agreement could be reached, a third author was consulted.
Data synthesis
We used simple descriptive statistics to present demographic and clinical data. We used the method of conversion from median to mean that was recommended by Hozo et al.Citation38 We pooled categorical outcome data in the entire review population by meta-analyzing data from individual studies. The pooled proportion was calculated as the back transformation of the weighted mean of the transformed proportions. We anticipated considerable clinical between-study heterogeneity and, therefore, applied random-effects models. We examined heterogeneity with the combination of the Cochran’s Q (χ2) test and the I2 statistic. We used the Comprehensive Meta-Analysis software (Biostat, Englewood, NJ, USA).
Results
Results of literature search
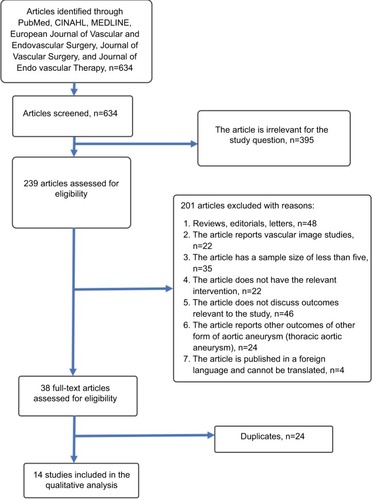
Search of electronic databases identified a total of 634 articles (). Following assessment of titles and abstracts, 395 articles were excluded as they were not relevant to the subject of this study. Following further evaluation, 201 articles were excluded. The full texts of the remaining 38 articles were obtained and assessed for eligibility. Fourteen studies met the inclusion criteria of our study and were included in qualitative and quantitative synthesis.Citation8–Citation21
Study characteristics
presents study-related information. Baseline demographic and clinical characteristics of the study populations are summarized in . All 14 articles were single-arm observational studies and were published after 2011. The recruitment period in all but one study was 1 year or more.Citation17 Four studiesCitation10,Citation12,Citation13,Citation16 reported 1-year outcomes, while the remaining studies reported longer follow-up outcomes.Citation8,Citation9,Citation11,Citation14,Citation15,Citation18–Citation21 The weighted mean follow-up was 11.6±5.4 months. Nine studiesCitation8,Citation9,Citation11,Citation13,Citation16–Citation19,Citation21 were multicenter studies and the remaining five were single-center studies.Citation10,Citation12,Citation14,Citation15,Citation20
Table 1 Study characteristics
Table 2 Baseline demographics and clinical characteristics of the study population
The included studies reported a total of 1,510 patients with asymptomatic, non-ruptured AAA treated with EVAS with the Nellix device. The weighted mean age of the included patients was 74±2 years and 89% of the patients were male. Hypertension was the most common comorbidity (74%), followed by cardiac disease (coronary artery disease 40%, myocardial infarction 28%, arrhythmia 21%, angina 19%, congestive cardiac failure 8%), respiratory disease (32%), renal disease (19%), diabetic mellitus (17%), and cerebrovascular disease (12%). Smoking was present in 58% of the patients.
The inclusion criteria varied among the studies. The specific inclusion criteria for each of the selected studies are summarized in . van Sterkenburg et al described the outcomes of EVAS using the Nellix device in patients with associated iliac artery occlusive disease.Citation15 Youssef et al investigated the outcomes of EVAS with the Nellix endoprosthesis in patients with AAA and/or common iliac artery aneurysm.Citation18 Zoethout et al reported the outcomes of patients based on the recommended IFU 2013 and IFU 2016.Citation21
Aneurysm anatomic characteristics
presents the aneurysm anatomic characteristics. Most authors reported anatomic data of the aneurysm and whether the aneurysm was treated within the IFU 2013. Eighty percent of the included patients had their aneurysm treated within the IFU for the Nellix device. The weighted mean maximum aneurysm diameter was 60±6 mm. The weighted mean aortic neck diameter and length were 24±2 and 25±4 mm, respectively. The mean angulation of the aortic neck was 32°±9°.
Table 3 Aneurysm anatomic data
Clinical outcomes
Outcome data are presented in –.
Table 4 Surgical data
Table 5 Early clinical outcomes (within 30 days)
Table 6 Late clinical outcomes (during follow-up)
Technical success
Technical success was reported by 13 studies (1,233 patients).Citation8–Citation15,Citation17–Citation21 The technical success rate ranged from 98% to 100%. Technical success was achieved in 1,226 out of 1,233 patients with a pooled estimate of 99% (95% CI =98–100; heterogeneity: P=0.869, I2=0%).
Procedure time
Eleven studies (1,335 patients) reported the procedure time which ranged from 70 to 151 minutes.Citation8–Citation11,Citation13,Citation16–Citation21 The weighted mean procedure time was 106±24 minutes.
Fluoroscopy time
The fluoroscopy time ranged from 8 to 33 minutes across 6 studies (717 patients).Citation8,Citation9,Citation11,Citation13,Citation16,Citation17 The weighted mean was 17±12 minutes.
Adjunctive procedures
Five studies reported data on adjunctive procedures.Citation12,Citation14,Citation15,Citation18,Citation20 Adjunctive procedures were carried out in 105 out of 240 patients (pooled estimate 39%, 95% CI =19–63; heterogeneity: P<0.0001, I2=88%), with a rate ranging from 7.7% to 60%. These included 97 cases of adjunctive iliac stenting, 4 cases of femoral endarterectomy, 2 cases of chimney grafts, 1 case of coil embolization of internal iliac artery, and 1 case of additional proximal stenting.
Postoperative complications
Nine studies (902 patients) reported data on postoperative complications.Citation10,Citation13–Citation15,Citation17–Citation21 The incidence of postoperative complications ranged from 0% to 60% across the nine studies and the pooled estimate was 5.6% (95% CI =1.9–15.2; heterogeneity: P<0.0001, I2=86%). Complications included endoleak,Citation10,Citation21 wound infection,Citation18 thrombus formation in the endograft,Citation10 groin hematoma,Citation14,Citation15,Citation18 occlusion of the femoral arteryCitation15 or the hypogastric arteries,Citation19 embolus formation,Citation14 duodenal bleeding,Citation15 and respiratory failure.Citation14,Citation18,Citation20 Karouki et al reported one case of paraparesis.Citation14 Jeffrey Hing et al reported five cases with post-implantation syndrome.Citation20
Aneurysm rupture
Rupture of AAA within 30 days of the procedure was reported in eight studies (916 patients), with a rate ranging from 0% to 2% and a pooled estimate of 0.7% (95% CI =0.3–1.6; heterogeneity: P=0.923, I2=0%).Citation8,Citation10–Citation14,Citation16,Citation17 Only two ruptures occurring within 30 days were noted in two studies, one case in each.Citation10,Citation16 Over a follow-up ranging from 1 to 23 months, 12 studies reported 5 cases of ruptured AAA (out of 1,455 patients), with an incidence ranging from 0% to 1.3% and a pooled estimate of 0.8% (95% CI =0.4–1.6; heterogeneity: P=0.958, I2=0%).Citation8–Citation14,Citation16,Citation17,Citation19–Citation21
Length of hospital stay
Eight studies (616 patients) reported data on the length of hospital stay, which ranged from 1 to 9 days.Citation8–Citation10,Citation12,Citation16–Citation18,Citation20 The weighted mean length of hospital stay was 5±3 days.
Endoleak
All included studies reported endoleak as an outcome. Forty-nine out of 1,510 patients were reported with endoleak within 30 days of the procedure, with a rate ranging from 0% to 9.6% across the studies.Citation8–Citation13,Citation16,Citation19,Citation21 Around half of the endoleaks noted (29, 59%) were type I and the remaining were type II. The pooled estimate of early (within 30 days) type I endoleak was 2.8% (95% CI =1.8–4.2; heterogeneity: P=0.254, I2=18%) and that of early type II endoleak was 1.9% (95% CI =1.2–3.0; heterogeneity: P=0.266, I2=17%). During a follow-up ranging from 1 to 23 months, all 14 studies (1,510 patients) reported cases of endoleak, with an incidence ranging from 0% to 3.1%. Six studies found no endoleaks during follow-up.Citation9,Citation10,Citation12,Citation14,Citation15,Citation18 The remaining eight studies found a total of 31 endoleaks.Citation8,Citation11,Citation13,Citation16,Citation17,Citation19–Citation21 The most common type of endoleak was type I (22 counts), followed by type II (8 counts) and type III (1 count). The pooled estimate of type I endoleak was 1.9% (95% CI =1.3–2.8; heterogeneity: P=0.887, I2=0%), that of type II endoleak was 1.1% (95% CI =0.7–2.0; heterogeneity: P=0.871, I2=0%), and the pooled estimate of type III endoleak was 0.7 (95% CI =0.4–1.5; heterogeneity: P=0.847, I2=0%).
Sac enlargement
Five studies (302 patients) reported sac enlargement within 30 days.Citation10,Citation15–Citation17,Citation20 None of the studies found sac enlargement occurring within 30 days of the procedure. During follow-up ranging from 12 to 23 months, six studies evaluated aneurysm sac enlargement, with an incidence ranging from 0% to 5%.Citation8,Citation10,Citation13,Citation17,Citation20,Citation21 It was noted that 10 patients out of a total of 481 had aneurysm sac enlargement shared between two studies, with one study reporting 2 cases of sac enlargement and the other reporting 8 cases.Citation13,Citation21 The four remaining studies did not find any sac enlargement during follow-up.Citation8,Citation10,Citation17,Citation20 The pooled estimate of the incidence of sac enlargement was 3.1% (95% CI =1.8–5.4; heterogeneity: P=0.419, I2=0%).
Device migration
Five studies reported data on device migration that occurred within 30 days of surgery, with a rate ranging from 0% to 6.7% across the studies and a pooled estimate of 0.9% (95% CI =0.3–3.3; heterogeneity: P=0.211, I2=32%).Citation10,Citation11,Citation17,Citation19,Citation20 Only 2 out of 635 patients were noted with device migration within 30 days. During a follow-up ranging from 5 to 23 months, nine studies reported the incidence of device migration which ranged from 0% to 13% and the pooled esti mate was 2.1% (95% CI =0.8–5.3; heterogeneity: P=0.004, I2=65%).Citation8–Citation11,Citation13,Citation17,Citation19–Citation21 Nineteen out of a total of 1,008 patients were found to have migration of the Nellix device during follow-up.Citation13,Citation19–Citation21
Reintervention
Reintervention within 30 days of the procedure was reported in 29 out of 1,260 patients, with a rate ranging from 0% to 6.4% across eleven studies and a pooled estimate of 2.7% (95% CI =1.7–4.2; heterogeneity: P=0.183, I2=27%).Citation8,Citation9,Citation11,Citation12,Citation14–Citation17,Citation19–Citation21 During a follow-up period of 5–23 months, 47 out of 1,355 patients had reintervention, with a rate ranging from 0% to 9.5% across 12 studies and a pooled estimate of 3.5% (95% CI =2.3–5.5; heterogeneity: P=0.061, I2=42%).Citation8,Citation9,Citation11,Citation13–Citation21
Mortality
Mortality within 30 days of surgery was reported by 13 studies, with a rate ranging from 0% to 4.8% across the studies and a pooled estimate of 1.5% (95% CI =0.9–2.6; heterogeneity: P=0.559, I2=0%).Citation8–Citation20 Ten deaths out of 1,342 patients occurred within 30 days. Seven out of ten deaths (70%) were non-aneurysm/device related. Seven out of 13 studies reported zero 30-day mortality.Citation11,Citation14,Citation15,Citation17–Citation20 Mortality during follow-up was reported by all 14 studies.Citation8–Citation21 The follow-up period ranged from 1 to 23 months. Overall, 67 deaths (out of 1,510 patients) were reported during follow-up, with a mortality rate ranging from 0% to 20% across the studies. The pooled estimate for mortality during follow-up was 5.2% (95% CI =3.7–7.3; heterogeneity: P=0.076, I2=38%). Six of the 67 deaths (9%) were found to be aneurysm related. The pooled estimate of aneurysm-related death during follow-up was 1.0% (95% CI =0.6–1.9; heterogeneity: P=0.872, I2=0%).
Discussion
We conducted a systematic review and identified 14 single-arm observational studies, reporting a total of 1,510 patients who underwent repair for an asymptomatic, non-ruptured AAA with EVAS using the Nellix device. Our review demonstrated that, despite the wide range of aneurysm morphologies among and within the included studies, treatment with the Nellix device was associated with a high a technical success rate ranging from 98% to 100%. Most authors defined technical success as successful deployment of the device to exclude the aneurysmal flow and absence of endoleak or stent thrombosis on completion of angiography.Citation8–Citation15,Citation17–Citation19,Citation21 Interestingly, technical success of an aneurysm with a proximal neck angulation of 80° was reported in a patient treated outside the IFU for the Nellix device.Citation20 The rate of postoperative complications ranged widely from 0% to 60% across nine studies, reflecting the variability in reporting perioperative morbidity among the studies.Citation10,Citation13–Citation15,Citation17–Citation21 We found that the weighted mean procedure time and length of hospital stay were 106±24 minutes and 5±3 days, respectively. These values are comparable with the procedure time and length of hospital stay reported in EVAR trials.Citation22
EVAS with the Nellix device was designed to reduce complications, particularly endoleaks, and subsequent reinterventions during follow-up.Citation23 We found that the use of the Nellix device was associated with a low rate of endoleak that is comparable to the reported rates of endoleak after EVAR.Citation24 Type I endoleak was the most common type of endoleak reported by the included studies. In our study, the reported reintervention rate was low, ranging from 0% to 9.5% across 12 studies over a follow-up period of 5–23 months.
The impact of EVAS with the Nellix device on prevention of aneurysm sac enlargement has been promising based on the available evidence. Sac enlargement was reported in 10 out of 481 patients (with an incidence ranging from 0% to 5%) over a follow-up ranging from 12 to 23 months. The rates of aneurysm sac enlargement are generally lower than those reported following EVAR; aneurysm sac enlargement has been observed in 21%–42% of patients at 5 years following EVAR.Citation25,Citation26 However, differences in incidence of sac enlargement between studies reporting EVAS and those reporting EVAR may be related to differences in follow-up. Further studies are necessary to make direct comparison of outcomes between EVAS and EVAR.
Device migration is one of the notable complications that can occur post-EVAS.Citation27 England et al reported a migration rate of up to 28%, none of which had associated clinical implications.Citation27 In addition, Antoniou et al described a case of Nellix endograft migration with increasing sac diameter.Citation28 In our study, we found a rate of migration ranging from 0% to 13% over a follow-up ranging from 5 to 23 months, highlighting the importance of surveillance after EVAS.
Most of the included patients had their aneurysm treated within the IFU 2013 of the Nellix device. Some studies reported clinical outcomes of aneurysm treatment within and beyond IFU 2013. Zerwes et al found no significant difference in technical success between patients treated within IFU and outside the IFU.Citation10 Gossetti et al reported that patients treated outside the IFU had a statistically higher incidence of device-related complications.Citation19 Comparative evidence is non-adherence to required to robustly evaluate complications associated with the recommended IFU of EVAS with the Nellix device.
The refined version of recommended IFU for the Nellix device was introduced in 2016.Citation39 Zoethout et al compared 2-year clinical outcomes of patients treated within IFU 2013 and IFU 2016;Citation21 they found less complications in the IFU 2016 group as compared to IFU 2013 group, although the difference was not significant.Citation21 The authors suggested that the refined IFU 2016 did not clearly show better outcomes of the EVAS procedure as compared to IFU 2013. Furthermore, the applicability of Nellix has significantly reduced with IFU 2016. As the refined IFU are relatively new, further analysis and follow-up would be helpful to determine the impact of the new IFU on clinical outcomes.
Radiation exposure during EVAR poses a potential hazard toward patient safety.Citation29,Citation30 EVAS may have a benefit by exposing patients to less radiation compared to EVAR. The studies included in our review reported the fluoroscopy time; however, data on radiation exposure were not available. Ockert et alCitation31 and Antoniou et alCitation32 compared radiation exposure during EVAR and EVAS. The studies reported similar outcomes with reduced radiation exposure in EVAS compared to EVAR. This is beneficial to the patient as well as the theater team, in view of the well-recognized carcinogenic risk with radiation exposure; hence, it is worth further analysis.Citation31
Aneurysm rupture after EVAS is a well-described complication. Antoniou et al reviewed late aneurysm ruptures after EVAR and noted that graft-related endoleaks are the predominant cause of rupture.Citation33 In our study, a total of seven AAA ruptures were reported. Zerwes et al reported an early rupture of the aneurysm sac due to iatrogenic reason.Citation10 The filling of the endobags had apparently caused the aortic rupture. This was not perceived during surgery. Computed tomography a week later showed retroperitoneal hematoma and a type Ia endoleak which was treated by implanting two additional Nellix endografts along with chimney grafts into both renal arteries. This allowed the endoleak to be successfully treated.Citation10 Carpenter et al reported two late aneurysm ruptures, one iatrogenic and another one related to a type Ia endoleak.Citation13 The first patient experienced multiple infections and rectal bleedings post-procedure. The Clinical Events Committee adjudicated the incident as a device-related bowel ischemia.Citation13 The latter patient developed a type Ia endoleak 7 months after the procedure.Citation13 This patient had the contained aneurysm rupture identified during open conversion and unfortunately died a month later.Citation13 Thompson et al reported three aortic ruptures; one early rupture due to a type Ib endoleak and two late ruptures due to an untreated type Ia endoleak.Citation16 These ruptures were treated with a distal extension and two conversions, respectively.Citation16 Zoethout et al reported a rupture in a patient who previously had an unsuccessful Nellix-in-Nellix procedure.Citation21 Aneurysm ruptures noted in our study were iatrogenic and endoleak related, in line with the findings of Antoniou et al.Citation33 This further highlights the importance of surveillance and follow-up after EVAR.
Chimney EVAS (Ch-EVAS) is a newly described technique which was mentioned as an adjunctive procedure in two of the included studies.Citation15,Citation20 In both cases, Ch-EVAS was used to extend the proximal landing zone. Several studies have reported cases of patients treated with Ch-EVAS and analyzed clinical outcomes with encouraging results. Torella et al reported two cases where Ch-EVAS was successfully used to treat a failed EVAR and a juxtarenal aneurysm.Citation34 Their study was further enhanced by Youssef et al reporting Ch-EVAS as a sensible treatment for failed EVAR due to endoleak.Citation35 de Bruin et al also reported Ch-EVAS as a feasible solution for juxta and suprarenal aneurysms with adverse morphology, noting a low rate of endoleak over a short-term follow-up of a median 123 days.Citation36 In a study with a larger cohort of patients, Thompson et al reported Ch-EVAS results from the ASCEND Registry and supported the use of Ch-EVAS in patients with complex aortic disease.Citation37 Even though recent studies suggest encouraging results with Ch-EVAS to handle complex aneurysm morphology and persistent endoleaks from previous EVAR, further studies with larger patient cohorts and longer follow-up are essential to contribute to the knowledge regarding the durability and possible complications of Ch-EVAS.
Conclusion
Outcomes of EVAS are acceptable. Type I endoleak, sac enlargement, device migration, and aneurysm ruptured are the recognized complications. High-level research is required to investigate potential advantages of EVAS over conventional treatments.
Disclosure
The authors report no conflicts of interest in this work.
References
- FilardoGPowellJTMartinezMABallardDJSurgery for small asymptomatic abdominal aortic aneurysmsCochrane Database Syst Rev20152CD001835
- SchwarzeMLShenYHemmerichJDaleWAge-related trends in utilization and outcome of open and endovascular repair for abdominal aortic aneurysm in the United States, 2001–2006J Vasc Surg200950472272919560313
- JacksonAYeohSEClarkeMTotally percutaneous versus standard femoral artery access for elective bifurcated abdominal endovascular aneurysm repairCochrane Database Syst Rev20142CD010185
- JaffanAAPrinceEAHampsonCOMurphyTPThe preclose technique in percutaneous endovascular aortic repair: a systematic literature review and meta-analysisCardiovasc Intervent Radiol201336356757723483284
- ManiKBjörckMWanhainenAChanges in the management of infrarenal abdominal aortic aneurysm disease in SwedenBr J Surg2013100563864423334950
- WellerAShahAMSeyedARTouskaPSayerCVlahosINellix endovascular aneurysm sealing system (EVAS): a new concept in endovascular repair – what the radiologist needs to knowJ Vasc Med Surg20160402258
- Endologix Announces CE Mark for Next-Generation Nellix® EndoVascular Aneurysm Sealing System | Endologix, inc. [Internet]Endologix, inc2016 [cited July 21, 2018 Available from: http://investor.endologix.com/news-releases/news-release-details/endologix-announces-ce-mark-next-generation-nellixr-endovascularAccessed July 10, 2018
- KrievinsDKHoldenASavlovskisJEVAR using the Nellix Sac-anchoring endoprosthesis: treatment of favourable and adverse anatomyEur J Vasc Endovasc Surg2011421384621497521
- DonayreCEZarinsCKKrievinsDKInitial clinical experience with a sac-anchoring endoprosthesis for aortic aneurysm repairJ Vasc Surg201153357458221211931
- ZerwesSNurzaiZLeissnerGEarly experience with the new endovascular aneurysm sealing system Nellix: first clinical results after 50 implantationsVascular201624433934726486377
- BöcklerDHoldenAThompsonMMulticenter Nellix endovascular aneurysm sealing system experience in aneurysm sac sealingJ Vasc Surg201562229029825953017
- BrownriggJRde BruinJLRossiLEndovascular aneurysm sealing for infrarenal abdominal aortic aneurysms: 30-day outcomes of 105 patients in a single centreEur J Vasc Endovasc Surg201550215716425892319
- CarpenterJPCuffRBuckleyCNellix InvestigatorsOne-year pivotal trial outcomes of the Nellix system for endovascular aneurysm sealingJ Vasc Surg201765233033627986486
- KaroukiMSwaelensCIazzolinoLClinical outcome after endovascular sealing of abdominal aortic aneurysms: a retrospective cohort studyAnn Vasc Surg20174012813527908817
- van SterkenburgSMvan den HamLHSmeetsLLardenoijeJWReijnenMMThe Nellix™ endovascular sealing system in patients with abdominal aortic aneurysms in conjunction with iliac artery occlusive diseaseVascular201725219019527586091
- ThompsonMMHeyligersJMHayesPDEVAS FORWARD Global Registry InvestigatorsEndovascular aneurysm sealing: early and midterm results from the EVAS forward global registryJ Endovasc Ther201623568569227555430
- SilingardiRCoppiGFerreroEMidterm outcomes of the Nellix endovascular aneurysm sealing system: a dual-center experienceJ Endovasc Ther201623569570027371944
- YoussefMNurzaiZZerwesSInitial experience in the treatment of extensive iliac artery aneurysms with the Nellix aneurysm sealing systemJ Endovasc Ther201623229029626802611
- GossettiBMartinelliOFerriMSilingardiRVerziniFIRENE Group InvestigatorsPreliminary results of endovascular aneurysm sealing from the multicenter Italian research on Nellix endoprosthesis (IRENE) studyJ Vasc Surg20186751397140329242065
- Jeffrey HingJXCh’ngJKTayKHChongTTGreater compliance within instruction for use for concomitant iliac aneurysms and adverse aneurysm characteristics – initial experience with the Nellix endovascular aneurysm sealing system at a single institutionAnn Vasc Surg20184914415129428533
- ZoethoutACBoersenJTHeyligersJMMTwo-year outcomes of the Nellix endovascular aneurysm sealing system for treatment of abdominal aortic aneurysmsJ Endovasc Ther201825327028129591724
- PrennerSTurnbullISerraoGOutcome of elective endovascular abdominal aortic aneurysm repair in nonagenariansJ Vasc Surg201154228729421367562
- HoldenASavlovskisJWinterbottomAImaging after Nellix endovascular aneurysm sealing: a consensus documentJ Endovasc Ther201623172026564913
- de La MotteLFalkenbergMKoelemayMJLönnLIs EVAR a durable solution: indications for re-interventionsJ Cardiovasc Surg (Torino)2018592201212
- HoggMEMoraschMDParkTFlanneryWDMakarounMSChoJSLong-term sac behavior after endovascular abdominal aortic aneurysm repair with the Excluder low-permeability endoprosthesisJ Vasc Surg20115351178118321276679
- HoltPJKarthikesalingamAPattersonBOAortic rupture and sac expansion after endovascular repair of abdominal aortic aneurysmBr J Surg201299121657166423023521
- EnglandATorellaFFisherRKMcWilliamsRGMigration of the Nellix endoprosthesisJ Vasc Surg201664230631227066946
- AntoniouGABashaebKIbrahimRNellix stent graft migration after endovascular aneurysm sealingVasa201645650550727598051
- MachadoRFerreiraVMLoureiroLGonçalvesJOliveiraPAlmeidaRRadiation exposure in endovascular infra-renal aortic aneurysm repair and factors that influence itBraz J Cardiovasc Surg201631641542128076617
- JonesCBadgerSABoydCSSoongCVThe impact of radiation dose exposure during endovascular aneurysm repair on patient safetyJ Vasc Surg201052229830220670773
- OckertSHeinrichMKaufmannTSyburraTLopezRSeelosREndovascular aortic sealing with Nellix reduces intraoperative radiation dose when compared to endovascular aortic repairJ Vasc Surg20186741068107329032904
- AntoniouGASeniorYIazzolinoLEndovascular aneurysm sealing is associated with reduced radiation exposure and procedure time compared with standard endovascular aneurysm repairJ Endovasc Ther201623228528926850739
- AntoniouGAGeorgiadisGSAntoniouSALate rupture of abdominal aortic aneurysm after previous endovascular repair: a systematic review and meta-analysisJ Endovasc Ther201522573474426286073
- TorellaFChanTYShaikhUEnglandAFisherRKMcWilliamsRGChEVAS: combining suprarenal EVAS with chimney techniqueCardiovasc Intervent Radiol20153851294129826202393
- YoussefMZerwesSJakobREndovascular aneurysm sealing (EVAS) and chimney EVAS in the treatment of failed endovascular aneurysm repairsJ Endovasc Ther201724111512027798381
- de BruinJLBrownriggJRPattersonBOThe endovascular sealing device in combination with parallel grafts for treatment of juxta/suprarenal abdominal aortic aneurysms: short-term results of a novel alternativeEur J Vasc Endovasc Surg201652445846527527570
- ThompsonMYoussefMJacobREarly experience with endovascular aneurysm sealing in combination with parallel grafts for the treatment of complex abdominal aneurysms: the ASCEND registryJ Endovasc Ther201724676477228895447
- HozoSPDjulbegovicBHozoIEstimating the mean and variance from the median, range, and the size of a sampleBMC Med Res Methodol2005511315840177
- Important Update to Field Safety Notice Nellix® EndoVascular Aneurysm Sealing System Updated Instructions for Use (IFU) [Internet]. Hpra.ie2016 [cited September 1, 2018] Available from: https://www.hsa.gov.sg/content/dam/HSA/HPRG/Medical_Devices/Updates_and_Safety_reporting/Field_Safety_Corrective_Action/FSN/2016/November/HSA%206004101-148-16-02_54%20FSN.pdfAccessed July 28, 2018