Abstract
Background: Minimally invasive sacroiliac joint (SIJ) fusion (SIJF) has become an increasingly accepted surgical option for chronic SI joint dysfunction, a prevalent cause of chronic low back/buttock pain.
Objective: To report clinical and functional outcomes of SIJF using 3D-printed triangular titanium implants (TTI) for patients with chronic SI joint dysfunction.
Methods: A total of 28 subjects with SIJ dysfunction at 8 centers underwent SIJF with 3D TTI and had scheduled follow-up to 6 months (NCT03122899).
Results: Mean preoperative SIJ pain score was 79.1 and mean preoperative Oswestry Disability Index (ODI) was 49.9. At 6 months, pain scores decreased by 51 points and ODI decreased by 23.6 points (both p<0.0001). The proportion of subjects able to perform various back/pelvis-related physical functions with minimal difficulty improved significantly for nearly all activities. Opioid use decreased and physical function, as assessed with three objective tests, improved.
Conclusion: Early results from this prospective multicenter trial confirm that clinical responses to a 3D triangular titanium implant for SIJF are similar to those from prior trials, with improved physical function and decreased opioid use.
Level of evidence: Level II.
Background
Minimally invasive sacroiliac joint (SIJ) fusion (SIJF) for chronic SIJ dysfunction (ie, disability due to SIJ pain) is increasingly accepted as a safe and effective treatment. The SIJ has been shown to contribute to up to 30% of all cases of chronic low back pain.Citation1–Citation5 Prospective trialsCitation6–Citation8 and case seriesCitation9–Citation14 have shown marked, immediate and sustained improvement in pain, disability and quality of life after minimally invasive surgical treatment with porous triangular titanium implants (TTI, iFuse Implant System, SI-BONE, Santa Clara, California, USA).
To date, all published TTI studies have used solid implants with porous surfaces manufactured through a coating process. Porous surfaces on orthopedic implants are designed to promote osteointegration with host bone, aiming to decrease the incidence of subsidence, pseudoarthrosis and implant failure. Surface technology also needs to address the aging patients with poor bone quality and/or healing related to osteoporosisCitation15 or smoking.Citation16 At the same time, any changes in surface technology or implant manufacturing need to protect overall implant resistance to high daily loads.
Titanium porous sprays, while commonly used on metallic implants for the hip,Citation17 knee and spine,Citation18 typically have low interconnectivity and small pore sizes (100–150 μm). With the advent of 3D metal printing (also known as additive manufacturing), implants with new microstructures can be produced. In 2015, a new version of TTI, manufactured via 3D printing (iFuse-3D), was designed and tested; the implant became commercially available in the USA in 2017. In tissue culture, these implants support growth of human osteoblasts with higher calcium production vs implants with porous spray surfaces.Citation19 In a sheep femur model, this implant showed higher bone ingrowth.Citation20
Herein we report early outcomes from the first human prospective experience with iFuse-3D TTI in patients with SIJ dysfunction.
Methods
Design and site participants
“Study of Bone Growth in the Sacroiliac Joint after Minimally Invasive Surgery with Titanium Implants” (SALLY, NCT03122899) is a prospective multicenter single-arm clinical trial conducted at 11 sites in the United States. Site participation required an orthopedic surgeon or neurosurgeon with experience placing these devices along with a dedicated study coordinator to manage participant assessment and data collection. The target sample size was 50 enrolled/treated subjects. Herein we report 6-month outcomes on the first 28 subjects.
Study goals
Overall study goals were 1) to demonstrate similar clinical responses after iFuse-3D compared to prior studies of iFuse in the same patient population, 2) to further characterize physical function changes after SIJF using TTI, 3) to investigate the efficacy of “opioid contracts” and self-efficacy reminders (see Opioid contract and self-efficacy below) on reduction in pain-related opioid use after SIJF, and 4) to determine whether iFuse-3D accelerates bony fusion of the SIJ compared to the prior version of the device.
Participants
To participate, patients needed to meet the following criteria (see https://clinicaltrials.gov/ct2/show/NCT03122899 for a complete listing of eligibility criteria): age 21–70 years, suspected SIJ pain due to degeneration or disruption of the joint for at least 6 months inadequately responsive to conservative care, an Oswestry Disability Index (ODI) score of at least 30%, an average SIJ pain score of at least 50 (on 0–100 mm visual analog scale), and agreement to participate through a signed, study-specific informed consent form. Patients were diagnosed with SIJ pain using a standardized algorithm consisting of history (pain at or close to the posterior superior iliac spine with possible radiation into buttocks, posterior thigh or groin, and can point with a single finger to the location of pain [Fortin Finger TestCitation21]), 3 or more physical examination maneuvers stressing the target SIJ that reproduce pain, and a 50% or more decrease in pain at 30 or 60 minutes following image-guided SIJ block with local anesthetic. Patients were excluded for any of the following: bilateral SIJ symptoms with pain scores >50 but refusal to undergo bilateral treatment within the study, pregnant or attempting pregnancy, severe back or hip pain due to other causes, SIJ dysfunction due to inflammatory condition, tumor, infection or unstable/acute fracture, recent major trauma to the pelvis, body habitus that could prevent implant placement, diagnosed osteoporosis or osteomalacia, pathologic fracture, rheumatologic diagnosis (eg, rheumatoid arthritis), allergy to titanium, any condition that contraindicates surgery or could prevent long-term follow-up, uncontrolled psychiatric disease, unwillingness to sign opioid contract (see “Opioid contract and self-efficacy” section below), or involvement in litigation related to low back/SIJ pain. Potential participants were screened for inclusion in the study and consented with a study-specific written informed consent form that was approved by governing institutional review boards (IRBs) at all sites. Consent forms were consistent with the Declaration of Helsinki and relevant items from ISO 14155:2011. Institutional review boards (IRBs) overseeing the study were:
Western IRB, 1019 39th Avenue SE Suite 120, Puyallup, WA 98374, USA
Louisiana State University Health Sciences IRB, Office of Research Services, 433 Bolivar Street, Room 206, New Orleans, LA 70112, USA
Providence Health Services, 5251 NE Glisan, Building A, 3rd Floor, Portland, OR 97213, USA
Interventions
Eligible patients (subjects) underwent preoperative assessment of medical history, including opioid medication use, as well as physical function tests and quality of life assessments (see “Quality of life assessments” section below). All study data were captured on study-specific electronic case report forms (CRFs). Subjects underwent index side surgery within 30 days of baseline assessments; all surgeries were performed with iFuse-3D () with optional use of FDA-cleared allograft (including demineralized bone matrix) or autograft (typically, bone fragments harvested from the drill used to create the channels for TTI placement that were placed on implants prior to insertion). See Polly et alCitation6 for a detailed description of device placement techniques, which were similar to those used in this study. All device deficiencies, complications and adverse events occurring during the procedure were recorded on CRFs. Subjects were discharged postoperatively according to standard local practices. Subjects with qualifying bilateral SIJ pain underwent contralateral SIJF with the same device/technique within 90 days of the first-side procedure.
Follow-up
Subjects returned to clinic at 3, 6, 12, 24, and 60 months after surgery. At each visit, subjects completed questionnaires described below and were queried regarding the occurrence of adverse events, defined using an international standard (ISO14155:2011). Site investigators rated the severity and relatedness of each adverse event to the study device, the SIJF procedure, other procedures used to treat SIJ pain and pre-existing conditions.
Herein we report outcomes of the first 28 subjects with 6-month data; the study is fully enrolled and long-term follow-up is ongoing. On the basis of prior prospective randomized and single-arm trials, responses at 3–6 months predict long-term (2-,Citation6 3-,Citation22 and 4-yearCitation23) responses to SIJF using the prior version of the device, justifying this early report of patient outcomes.
Quality of life assessments
At baseline and each follow-up study visit, subjects completed the following questionnaires: 1) visual analog pain score in each SIJ and in the lower back using a 100 mm scale indicating 0= no pain and 100= worst imaginable pain; 2) Oswestry Disability Index,Citation24 a validatedCitation25 10-question instrument assessing disability related to low back pain; 3) EuroQOL-5D,Citation26 a commonly used generic quality of life assessment tool that can be used to estimate health state utility (0= death, 1= perfect health); 4) pain maps showing multiple locations on the back, buttocks and legs upon which subjects noted areas that caused pain and area causing the most pain; 5) Likert-scale degree of pain associated with >20 daily activities (eg, sitting, walking, getting out of a car, etc.); and 6) self-efficacy, ie, current level of confidence that subject could cease opioid use. At all but two sites, questionnaires were administered using a handheld computer that communicated directly with the study database; the remaining 2 sites used traditional paper-based methods. Work and ambulatory status were captured with standard paper-based surveys followed by manual data entry into the study database.
Physical function tests
At baseline and each study visit, subjects underwent the following 3 physical examination tests: 1) active straight leg raise test (ASLR), in which the supine subject rates the level of difficulty in actively lifting each leg 20 cm off the examining room table (Video S1) (scale of 0= not difficult to 5= unable to perform),Citation27 2) number of seconds required to complete “five times sit to stand” in which the subject is asked to stand up from an armless chair and sit down five times (Video S2), and 3) number of seconds required to complete “transitional timed up and go,” derived from the standard timed up and go test,Citation28 in which the subject sitting with his/her least-affected side parallel to the wall in an armless chair stands up while simultaneously twisting toward the affected side, walks 3 m, ascends two steps and returns to the chair and sits (Video S3). All sites were provided with both standardized chairs and step stools for this test. All physical function tests were administered by trained study coordinators, and all subjects watched a video of each test prior to performing the test. The study’s assumption was that each physical function test (rating scale for ASLR and number of seconds for sit-to-stand and transitional timed up and go) would improve postoperatively compared to preoperatively.
Opioid contract and self-efficacy
At baseline each subject signed an opioid contract describing opioid-related statements, activities, and behaviors: agreement that opioids can be addicting, opioids used for SIJ pain may be stopped if they are not effective or being misused, opioids will be taken only as prescribed, opioids will be obtained only from the study investigator for at least 2 years, agreeing not to share, trade or sell any opioids, returning unused opioids, and an understanding that, in conjunction with the physician, the goal of SIJF is to provide definitive treatment aimed at ceasing opioid use altogether. The subject completed a self-efficacy questionnaire rating level of confidence that the subject can reduce or stop taking opioids for SIJ pain under the care of the study physician. Self-efficacy theoryCitation29 suggests that confidence in one’s ability to complete a task increases the likelihood of task completion.
CT scans
At either 6 or 12 months after SIJF, subjects underwent high-resolution (<1 mm slice thickness) computed tomography (CT) scan of the pelvis without contrast. Assignment to CT scan timing was determined at random using a site-specific sequence generated by computer and delivered to sites using a password-protected website. An independent radiologist analyzed CT scans for both implant position and signs of SIJ fusion and other joint-related changes; results will be reported separately.
Study monitoring
CRFs were remotely monitored. Study monitors also reviewed subjects’ medical records on site to verify CRF completeness and accuracy as well as complete adverse event reporting during study follow-up.
Subject payment
Subjects were given nominal amounts for time and expense to complete study visit and call requirements, as approved by each site’s governing IRB.
Sponsorship
The study was sponsored by the device manufacturer (SI-BONE, Inc., Santa Clara, CA).
Endpoints
The study’s primary efficacy success endpoint is the improvement in Oswestry Disability Index (ODI) from baseline at 6 months. The observed change is compared with information from prior iFuse studies: the primary endpoint of the study is a non-inferiority comparison to prior studies (mean improvement of 25 points) with a non-inferiority margin of 10 points. With a sample size of 50 and a change score mean similar to prior studies with standard deviation of 20 points, the study has >80% power to conclude non-inferiority. Secondary clinical endpoints include: improvement from baseline in VAS SIJ pain, improvement in ODI at all follow-up visits, improvement in EuroQOL-5D time trade-off (EQ-5D TTO) index, improvement in physical function tests, decrease in opioid use (both proportion using opioids and oral morphine equivalents in opioid users) and proportion using opioids, and rate of serious adverse events related to the procedure or device. Radiographic primary and secondary endpoints will be reported elsewhere.
Statistical analysis
A standard approach to statistical analysis was employed to calculate standard aspects of change scores and binary outcomes. Non-inferiority testing was performed using a student’s T test. Repeated measures analysis of variance, which simultaneously takes into account multiple measurements per subject, was used to determine statistical significance of changes from baseline. Where relevant, binary outcomes were measured with a Fisher’s test or a McNemar test.
Results
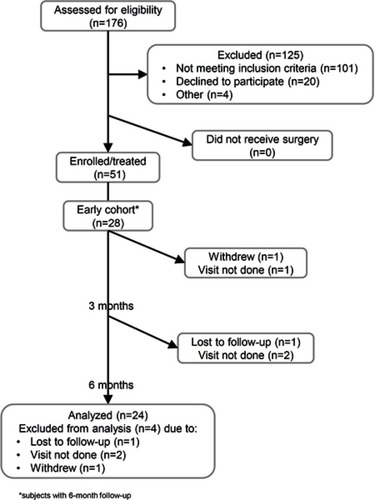
Of 176 screened patients, 51 met eligibility criteria and underwent treatment between October 2017 and January 2019. All subjects met study eligibility criteria except for the following, all of which were pre-approved by the study sponsor: one subject was included despite not having a documented 50% or more decrease in SIJ pain with image-guided SIJ block; in one subject, block was not performed, but the site believed the subject had SIJ pain; in one subject patient age was slightly above the upper age criteria (70 years old). Because they were enrolled and treated, patients not meeting all eligibility criteria were included in all analyses. Herein we report baseline status and clinical outcomes in the first 28 subjects with 6-month follow-up.
Mean subject age was 52 years and mean pain duration was 7.5 years (range 0.6–44, ). 82% were women.
Table 1 Baseline and surgical characteristics of SALLY participants (n=28). Data from first procedure (if more than one provided) are reported
In affected right-sided SIJs, diagnosed conditions were disruption (7 sides) and degeneration (9 sides); in left-sided SIJs, diagnosed conditions were disruption (7 sides) and degeneration (7 sides). Mean pain reduction on SIJ block was 83% and 80% at 30 and 60 minutes after SIJ block.
Twenty-seven (96%) subjects had had prior physical therapy and 27 (96%) had undergone prior SIJ corticosteroid injections; 4 (14%) had prior radiofrequency ablation of lateral branches of the sacral nerve roots. Nine (32%) had a history of prior lumbar fusion and 4 (14%) had prior SIJ fusion contralateral to the affected side. In 6 of 23 (21%) female participants, SIJ or back pain started prior to first pregnancy.
One subject underwent staged bilateral SIJ fusion for bilateral SIJ dysfunction; all other subjects underwent unilateral SIJF. Mean procedure time was 54 minutes and graft materials (autograft, derived from drills used, or allograft) were used in 39% of the cases. Mean estimated blood loss was 46 cc. No technical complications, device malfunctions or adverse events during the procedure were reported. Mean and median length of stay were 1 day each; 13 (46%) of subjects were discharged from the hospital on the day of surgery.
6-month follow-up was available in 24 cases. One subject was lost to follow-up, one withdrew from the study and two 6-month visits were not completed ().
Pain, disability (ODI) and quality of life (EQ5D-TTO index) scores improved rapidly and statistically significantly (p<0.0001 for each endpoint at each postoperative time point) following SIJF (); the changes in the current study (green lines) were very similar to those in prior studies, including INSITE (randomized trial of SIJF vs non-surgical managementCitation6), iMIA (randomized trial of SIJF vs conservative managementCitation8) and SIFI (single-arm prospective trial of SIJFCitation7), all of which used the prior version of the study device. The 6-month mean ODI change score (an improvement of 23.6 points) met the study’s primary endpoint non-inferiority hypothesis (p=0.0061). By month 6, 92% and 67% of the subjects, respectively, reported improvements of 20 or more points in VAS SIJ pain on the target side and 15 or more points in ODI, thresholds that are commonly accepted as clinically important responses.Citation30,Citation31 63% had both a 20-point improvement in pain score and a 15-point improvement in ODI. These response rates are similar to those from prior studies of iFuse.
Figure 3 Improvement in visual analog scale SIJ pain (top) and Oswestry Disability Index (middle) and EuroQOL-5D (bottom) by time and treatment. Figure shows results for the current study in green. Figure also includes data from prior studies: INSITE (black, randomized trial of SIJF vs non-surgical management),Citation6 iMIA (orange, randomized trial of SIJF vs conservative management)Citation8 and SIFI (light blue, single-arm prospective trial of SIJF).Citation7

The proportion of subjects who reported minimal difficulty with a variety of activities improved markedly from baseline to 3- and 6-month follow-up (, ); improvements were statistically significant (McNemar test p<0.05) in 20 of 22 activities.
Figure 4 Proportion of subjects reporting ability to perform activity with minimal difficulty by activity and months after SIJF.
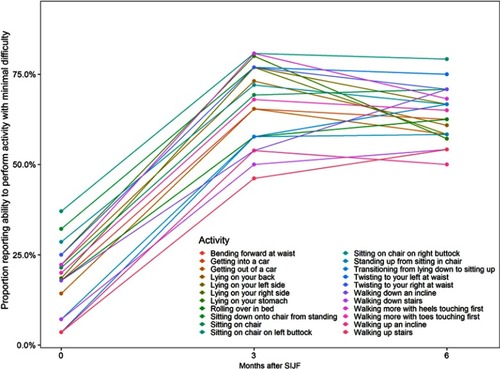
Table 2 Proportion of subjects reporting ability to perform activity with minimal difficulty
At 3 and 6 months, 82% and 92% of the subjects reported being satisfied or very satisfied with outcomes of the procedure. At 3 and 6 months, 69% and 71% stated they would have the procedure again. Satisfaction rates correlated strongly with improvements in both VAS SIJ pain and ODI.
The proportion of subjects taking opioids decreased from 57% at baseline to 35% at 3 months and 21% at 6 months (McNemar p=0.0077). Including all subjects, daily mean oral morphine equivalents decreased from 27 mg at baseline to 8.0 mg at 6 months (Wilcoxon p=0.0222).
At 6 months, physical function tests improved as follows (). Mean ASLR (0–5 scale) on the most painful side improved from 2.7 at baseline to 0.9 at 6 months (improvement of 1.8 points, p<0.0001). Five times sit-to-stand mean times improved from 26 s at baseline to 21 s at 6 months, an improvement of 7 s (p=0.0298). Mean scores for timed transitional up and go improved from 24 s at baseline to 18 s at 6 months, an improvement of 7 s (p=0.0076).
Figure 5 Change in functional test performance from prior to SIJF to 3 and 6 months after SIJF. Sit-to-stand and transitional timed up and go show seconds required to complete the task. Active straight leg raise test shows number of categories of improvement on 0–5 scale.
Abbreviation: SIJF, sacroiliac joint fusion.

At baseline, 89% were fully ambulatory; at 6 months 83% were fully ambulatory (McNemar p=1 for 6-month change in ambulatory status). Two subjects who were fully ambulatory at baseline were not so at 6 months (one due to a flare-up of knee pain related to a motor vehicle accident and one due to trochanteric bursitis and overexertion related to L5-S1 degeneration). One subject who required an assistive device for ambulation at baseline was fully ambulatory at 6 months.
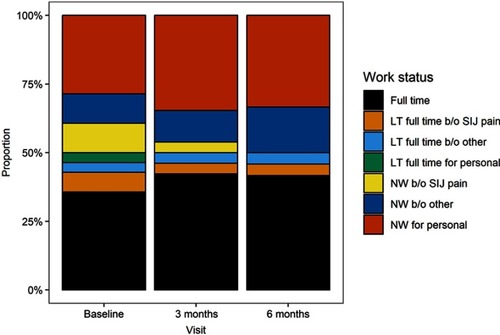
At baseline, 36% were working full time, 14% were working part-time and 50% were not working. By month 6, the proportion working full time increased to 42%, with a balanced reduction in those working part-time (8.3%); the proportion not working was unchanged at 50% (). The proportion reporting not working full time due to SIJ pain decreased from 11% to 0%. These changes suggested improvement in overall work status, but no change was statistically significant.
Figure 6 Change in work status over time from prior to SIJF to 6 months after SIJF.
Abbreviations: NW, not working; LT, less than; b/o, because of; SIJ, sacroiliac joint; SIJF, sacroiliac joint fusion.
Forty-six adverse events were reported in 19 subjects. Of these, none were device-related and 4 were probably or definitely related to the study procedure. The 4 events were: 1 cm wound dehiscence at day 12, trochanteric bursitis at day 26, hip muscle dysfunction at day 83, and postoperative surgical site pain at day 1 (resolved). None of these events were deemed severe. One serious adverse event occurred: a subject with a history of gastroesophageal reflux disease had aspiration pneumonia soon after the study procedure resulting in an emergency room visit for evaluation.
Discussion
Previous prospective randomized trialsCitation6,Citation8 and other prospectiveCitation7,Citation22,Citation23 and retrospectiveCitation9–Citation14,Citation32 studies have demonstrated that SIJF with iFuse Implant System, a milled solid triangular titanium implant with a spray-coated surface, is safe and effective for the treatment of chronic SIJ dysfunction related to degeneration or disruption of the SIJ. The current study confirms that the newer 3D-printed version of the device provides similar improvements in pain, disability and quality of life along with similarly high satisfaction rates in the same patient population compared to studies of the prior device version. Along with these standard measures, subjects reported decreased difficulty with performing a wide variety of activities associated with SIJ pain (), improvement in functional tests and a reduction in opioid use. Though not statistically significant, changes in work status trended toward improved ability to work.
The new device we studied is identical to the previous device (for which substantial clinical evidence, cited above, is available) in terms of device shape, materials, placement techniques and mechanism of action (acute stabilization, long-term joint fusion). Compared to the solid iFuse device, iFuse-3D has interstices through which bone can grow more rapidly (demonstrated in vitro for the current deviceCitation19 as well as other surfacesCitation33) as well as high control over pore sizes (approximately 300 microns) that may more closely mimic cancellous bone.Citation34 While animal studies show improved bone ingrowth compared to the iFuse device,Citation20 the current trial cannot yet assess whether these properties improve bone binding to the sacrum and ilium and/or bone bridging in humans, thereby affecting the time course to joint fusion. These endpoints will be evaluated separately.
Our study had several distinguishing features. First, the trial used objective physical function tests that have either been shown to be altered in SIJ pain (active straight leg raise test [ASLR]Citation27) or were predicted to be challenging to patients with SIJ pain (5 times sit to stand and transitional timed up and go). At baseline, ASLR difficulty was elevated; similarly, at baseline, the mean number of seconds required to complete the latter two tasks was prolonged compared to expectations for patients without pain. Postoperatively, all tests showed statistically significant improvement. In the absence of clinical improvement benchmarks for these tests, the clinical significance of these improvements is somewhat difficult to interpret. However, all three tests were correlated with Oswestry Disability Index, a validated measure of disability due to back pain, at each time point (not shown), suggesting that these functional tests are valid. Combined, our results suggest that improvements in pain, disability and quality of life (ie, standard measures in spine surgery trials) were accompanied by improvements in objective physical function tests.
Second, in contrast to prior trials, the current trial implemented active opioid management, including use of an opioid contract. Though opioid contracts remain relatively unproven, in our trial these contracts, combined with counseling from the care team as well as definitive surgical treatment of the subjects’ underlying pathology, resulted in marked short-term decreases in opioid use from 57% at baseline to 21% at 6 months, reductions that were larger than those observed in prior studies of the same device.Citation6–Citation8,Citation23, Although long-term persistence of opioid cessation is of great interest in our study, current results suggest that “active management” of opioids combined with definitive surgical procedures that successfully address the underlying source of pain may reduce opioid use, potentially decreasing the likelihood of chronic opioid use and its associated negative social consequences.
Early results from this trial also show an excellent safety profile of SIJF with iFuse-3D, with no unanticipated events and no revisions.
Advantages to our study include the following: the study was prospective and multicenter, with scheduled visits and assessments. Assessments included both standard measures (pain, disability, and quality of life scores, assessed using a standardized computer interface) as well as functional tests specifically designed to assess limitations related to SIJ pain. At each visit, opioid use was carefully assessed. All study data were monitored and source verified and, in most cases, patient-reported outcomes were collected directly from study subjects using a handheld computer. Finally, 6-month follow-up was reasonably high. Further confirmation of these positive outcomes through long-term trial follow-up is in process.
Conclusion
In a prospective study, improvements in pain and SIJF after surgery utilizing 3D-printed triangular titanium implants were substantial and accompanied by both functional improvement and substantial opioid use reduction.
Data sharing statement
Study data are available to qualified requestors through Yale University’s Yale Open Data Access (YODA) platform; provided data include de-identified study data, study protocol, and case report forms.
Disclosure
All authors conduct clinical research as part of prospective trials sponsored by SI-BONE. S Craig Meyer, Harry Lockstadt, Andy Kranenburg, Abhineet Chowdhary and James Billys are consultants to SI-BONE and are involved in teaching about SIJ pain/surgery and/or product development. Dr Vikas Patel reports grants from SI-BONE, during the conduct of the study as well as grants from Medicrea, Globus, Mainstay, Premia, Orthofix, Aesculap, Springer, and ZimmerBiomet, outside the submitted work. Dr Fernando Techy reports personal fees from Spine Way, Grafton Medical, and Spine Frontier. He also received grants from Orthofix, M6, and personal fees from Spine Wave, outside the submitted work. Dr Daniel Cher is employed by SI-BONE. The authors report no other conflicts of interest in this work.
References
- Bernard TN, Kirkaldy-Willis WH. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop. 1987;217:266–280.
- Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine. 1995;20(1):31–37.7709277
- Maigne JY, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine. 1996;21(16):1889–1892.8875721
- Irwin RW, Watson T, Minick RP, Ambrosius WT. Age, body mass index, and gender differences in sacroiliac joint pathology. Am J Phys Med Rehabil. 2007;86(1):37–44. doi:10.1097/PHM.0b013e31802b855417304687
- Sembrano JN, Polly DW. How often is low back pain not coming from the back? Spine. 2009;34(1):E27–E32. doi:10.1097/BRS.0b013e31818b888219127145
- Polly DW, Swofford J, Whang PG, et al. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016;10: Article 28. doi:10.14444/302827652199
- Duhon BS, Bitan F, Lockstadt H, Kovalsky D, Cher D, Hillen T. Triangular titanium implants for minimally invasive sacroiliac joint fusion: 2-year follow-up from a prospective multicenter trial. Int J Spine Surg. 2016;10: Article 13. doi:10.14444/301327162715
- Dengler J, Kools D, Pflugmacher R, et al. Randomized trial of sacroiliac joint arthrodesis compared with conservative management for chronic low back pain attributed to the sacroiliac joint. J Bone Jt Surg. 2019;101:400–411. doi:10.2106/JBJS.18.00022
- Rudolf L. Sacroiliac joint arthrodesis-MIS technique with titanium implants: report of the first 50 patients and outcomes. Open Orthop J. 2012;6(1):495–502. doi:10.2174/187432500120601049523284593
- Rudolf L. MIS fusion of the SI joint: does prior lumbar spinal fusion affect patient outcomes? Open Orthop J. 2013;7:163–168. doi:10.2174/187432500130701016323730380
- Sachs D, Capobianco R. One year successful outcomes for novel sacroiliac joint arthrodesis system. Ann Surg Innov Res. 2012;6(1):13. doi:10.1186/1750-1164-6-1323270468
- Sachs D, Capobianco R. Minimally invasive sacroiliac joint fusion: one-year outcomes in 40 patients. Adv Orthop. 2013;2013:536128. doi:10.1155/2013/53612823997957
- Cummings J Jr, Capobianco RA. Minimally invasive sacroiliac joint fusion: one-year outcomes in 18 patients. Ann Surg Innov Res. 2013;7(1):12. doi:10.1186/1750-1164-7-1224040944
- Schroeder JE, Cunningham ME, Ross T, Boachie-Adjei O. Early results of sacro–iliac joint fixation following long fusion to the sacrum in adult spine deformity. Hosp Spec Surg J. 2013;10(1):30–35. doi:10.1007/s11420-013-9374-4
- Geusens PP, van Den Bergh JP. Osteoporosis and osteoarthritis: shared mechanisms and epidemiology. Curr Opin Rheumatol. 2016;28(2):97–103. doi:10.1097/BOR.000000000000025626780427
- César-Neto JB, Duarte PM, Sallum EA, Barbieri D, Moreno H, Nociti FH. A comparative study on the effect of nicotine administration and cigarette smoke inhalation on bone healing around titanium implants. J Periodontol. 2003;74(10):1454–1459. doi:10.1902/jop.2003.74.10.145414653391
- Cook SD, Barrack RL, Thomas KA, Haddad RJ Jr. Quantitative analysis of tissue growth into human porous total hip components. J Arthroplasty. 1988;3(3):249–262.3183679
- Yoon BJV, Xavier F, Walker BR, Grinberg S, Cammisa FP, Abjornson C. Optimizing surface characteristics for cell adhesion and proliferation on titanium plasma spray coatings on PEEK. Spine J. 2016. doi:10.1016/j.spinee.2016.05.017
- MacBarb RF, Lindsey DP, Bahney CS, Woods SA, Wolfe ML, Yerby SA. Fortifying the bone-implant interface part 1: an in vitro evaluation of 3D-printed and TPS porous surfaces. Int J Spine Surg. 2017;11:15. doi:10.14444/401528765799
- MacBarb R, Lindsey D, Woods S, Lalor P, Gundanna M, Yerby S. Fortifying the bone-implant interface part 2: an in vivo evaluation of 3D-printed and TPS-coated triangular implants. Int J Spine Surg. 2017;11(3):116–128. doi:10.14444/4016
- Fortin JD, Falco FJ. The fortin finger test: an indicator of sacroiliac pain. Am J Orthop Belle Mead NJ. 1997;26(7):477–480.9247654
- Darr E, Meyer SC, Whang PG, et al. Long-term prospective outcomes after minimally invasive trans-iliac sacroiliac joint fusion using triangular titanium implants. Med Devices Evid Res. 2018;11:113–121. doi:10.2147/MDER.S160989
- Darr E, Cher D. 4-year outcomes after minimally invasive transiliac sacroiliac joint fusion with triangular titanium implants. Med Devices Evid Res. 2018;11:287–289. doi:10.2147/MDER.S179003
- Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940–2952; discussion 2952.11074683
- Copay AG, Cher DJ. Is the Oswestry Disability Index a valid measure of response to sacroiliac joint treatment? Qual Life Res. 2016;25(2):283–292 [Epub 2015 Aug 6] 10.1007/s11136-015-1095-3.26245709
- EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy Amst Neth. 1990;16(3):199–208. doi:10.1016/0168-8510(90)90421-9
- Mens JM, Vleeming A, Snijders CJ, Koes BW, Stam HJ. Reliability and validity of the active straight leg raise test in posterior pelvic pain since pregnancy. Spine. 2001;26(10):1167–1171.11413432
- Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148.1991946
- Bandura A. Human agency in social cognitive theory. Am Psychol. 1989;44(9):1175–1184.2782727
- Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine. 2005;30(11):1331–1334.15928561
- Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J Off J North Am Spine Soc. 2008;8(6):968–974. doi:10.1016/j.spinee.2007.11.006
- Graham Smith A, Capobianco R, Cher D, et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res. 2013;7(1):14. doi:10.1186/1750-1164-7-1424172188
- Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–5491. doi:10.1016/j.biomaterials.2005.02.00215860204
- Doktor T. Pore size distribution of human trabecular bone - comparison of intrusion measurements with image analysis In: Kytýř D, Valach J, Jiroušek O, editors; 2011 http://hdl.handle.net/11104/0197299. Accessed 216, 2019.