Abstract
The introduction of intracoronary stents represented a major advance in interventional cardiology. While bare metal stents set the benchmark for improved safety over angioplasty, intimal hyperplasia and subsequent restenosis were important limitations. First-generation drug-eluting stents demonstrated significant improvements in efficacy, but not necessarily safety, and further technologic developments have focused on optimizing both. Current advances and understanding in stent design continue to improve on these concepts. This review summarizes past and present technology with particular emphasis on the principles underlying the efficacy and safety of drug-eluting stents, and offers a glimpse into the next generations of stents aimed at treating symptomatic coronary artery disease.
Background
Balloon angioplasty has been an important treatment modality for symptomatic coronary artery disease since the early 1980s when the earliest registries demonstrated effective angina reduction and acceptable safety.Citation1 Important limitations included acute closure rates up to 6% and restenosis rates up to 30%, and these served to fuel innovation in technique and technology over the ensuing decades.Citation2,Citation3 The advent of bare metal stents significantly reduced periprocedural complications, but bare metal stents did not have an overly profound impact on restenosis rates. Additionally, stent thrombosis emerged as an important and potentially fatal complication.Citation4,Citation5 The addition of antiproliferative agents to metal stents significantly reduced restenosis rates to <5%,Citation6–Citation8 thus leading to a wave of enthusiasm and technologic advances to extend drug-eluting stent (DES) usage into increasingly complex patient and lesion subsets. This enthusiasm was tempered somewhat by the specter of very late stent thrombosis, a rare but significant complication with an incidence of 0.3%–0.6% per year and no definitive plateau.Citation9,Citation10 As a result, each advance in stent technology now encompasses a combined approach of optimal efficacy (deliverability/restenosis) and safety (stent thrombosis). This review focuses on the evolution of stent design and drug delivery platforms comprising our current and potential future armamentarium in percutaneous coronary intervention.
Stent design
The DES has three main components, ie, the scaffold or struts, the drug, and a polymer ( and ). Each serves an important role in the function of the stent. Additionally, iterative advances in each component can have a potential influence on both efficacy and safety. Stents have loosely been defined in generations based on iterative design changes. For the purposes of this paper, we define those generations as listed in .
Figure 1 Components of a drug-eluting stent.
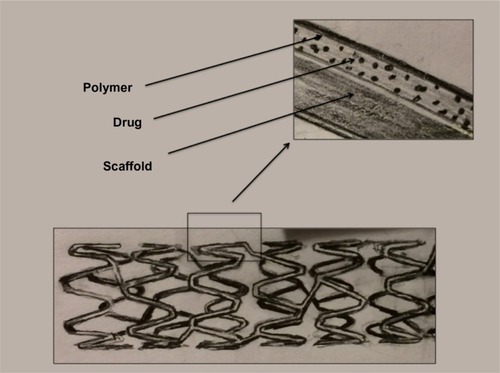
Table 1 Durable polymer stents
Table 2 Stent generations
Scaffold
The original bare metal stents were composed of a 316L stainless steel alloy composed of iron, nickel, and chromium. First-generation DES (Cypher; Cordis Corporation, Miami Lakes, FL, USA, and TAXUS Express, Boston Scientific, Natick, MA, USA) were built on the platforms of each manufacturer’s respective bare metal stent, ie, Bx Velocity and Express. These stents were suboptimal with regard to visibility, potential for allergic response, and deliverability.Citation11 The latter issue was thought to be influenced by the thickness of each stent (132 μm for the TAXUS Express and 140 μm for the Cypher). In the ISAR STEREO (Intracoronary Stenting and Angiographic Results: Strut Thickness Effect on Restenosis Outcome) study, Kastrati et al demonstrated that strut thickness influenced long-term efficacy. A reduction in strut thickness in two similarly designed 316L stainless steel stents (50 μm versus 140 μm) achieved a 42% relative risk reduction in angiographic restenosis and a 38% risk reduction in repeat target vessel revascularization at one year.Citation12 These findings were even more significant when stents of different design were compared.Citation13,Citation14 However, some of the drawbacks of the thinner 316L stainless steel alloy included less successful delivery and reduced angiographic visibility.Citation13
Cobalt chromium (CoCr) was the first of two metal alloys developed to address these issues and defines our second generation of DES. Having been previously used in other medical implants, CoCr had already demonstrated good biocompatibility.Citation15 A modest increase in density from 8.0 g/cm3 for 316L stainless steel to 9.1 g/cm3 for the L605 CoCr (Vision BMS, Abbott Laboratories, Abbott Park, IL, USA) and 8.4 g/cm3 MP35N (Driver BMS, Medtronic Inc., Minneapolis, MN, USA) led to a similarly modest improvement in visibility.Citation16 The higher yield and tensile strength of the CoCr alloy allowed for thinner struts while maintaining adequate radial strength suitable for percutaneous coronary intervention.Citation11,Citation17 However, this alloy is associated with greater stent recoil due to higher elastic properties with increased potential for malapposition and under-expansion of the stent.Citation16,Citation17 CoCr is currently the strut base for the Xience/Promus platforms (strut thickness 81 μm) and the Endeavor platforms (91 μm). The Resolute Integrity (Medtronic Inc.) is the latest 91 μm strut made from a single strand of CoCr alloy on the design idea of continuous sinusoid technology to optimize deliverability and radial strength.Citation18
Platinum chromium (PtCr) is another advance in strut alloys, and it defines our third generation of stents. The yield and tensile strengths are higher than for 316L stainless steel and comparable with CoCr, which allows for thinner stent struts. Additionally, it has elastic properties similar to stainless steel, so is associated with less recoil during stent placement.Citation16,Citation17 With a density of 9.9 g/cm3, a 33% PtCr alloy confers improved visibility.Citation16 Early studies have demonstrated the efficacy of PtCr platforms compared with historical controls, and additional clinical trials have demonstrated noninferiority of PtCr alloys compared with current second-generation CoCr DES.Citation19,Citation20
In contrast with its competitors, the PtCr platform has been associated with an increased risk of longitudinal deformity resulting in longitudinal stretching or shrinking and possible malapposition. This may be a result of efforts to make stents thinner, flexible, and more deliverable, and therefore be more related to stent design rather than the alloys.Citation21 Bench testing suggests that the PtCr Element platform may be at higher risk, although this complication has been seen with all newer stents.Citation22 To address this concern, the Promus Element was modified to include additional connectors between rows near the proximal end of the stent (Promus Premier), and early results suggest reduced rates of longitudinal deformity.Citation23
Bioabsorbable stent platforms potentially represent the next generation of technology, and the idea is intuitively attractive for many reasons. Poly-L-lactic acid (PLLA) has previously been used in numerous medical devices and demonstrated predictable degradation kinetics and good safety in previous coronary applications.Citation24,Citation25 It is the basis for the BVS (bioresorbable vascular scaffold) stent (Abbott Vascular, Abbott Park, IL, USA) and will be discussed later. Other absorbable platforms in clinical trials include a tyrosine-derived polycarbonate polymer and magnesium-based stents, and they have been reviewed elsewhere.Citation26
Polymers
Polymers serve a dual purpose of providing a barrier between the tissue and stent struts, and timing release of an antiproliferative drug. Once the drug is fully eluted, the original, durable polymers remain behind and continue to interact with the vascular endothelium. Studies have shown that polymers may contribute to late stent thrombosis, neointimal hyperplasia, hypersensitivity, and restenosis.
Polymers can be divided into two categories, ie, passive and active. Passive polymers provide a barrier-like coating to enhance the biocompatibility of stents. Historically, these polymers were composed of inorganic inert components, such as heparin, gold, carbon, and silicon carbide, and they failed to show a significant improvement over bare metal stents with regard to restenosis.Citation27
On the other hand, active coatings are capable of eluting a localized drug effect over a finite period of time. The coatings themselves can be permanent or temporary. Early-generation DES included permanent types of active polymers in their stent design, and elution kinetics were one of the key drivers for polymer selection.Citation28 The Cypher stent incorporated a three-layer polymer starting with parylene C, a hydrophobic and biocompatible layer attached to the metallic surface. This was followed by polyethylene-co-vinyl acetate (PEVA) and poly-n-butyl methacrylate (PBMA) mixed with sirolimus, and a final third layer of PEVA/PBMA to slow early elution, resulting in a total elution period of 60 days.Citation11,Citation28 The TAXUS Express stent utilized a polystyrene-b-isobutylene-b-styrene copolymer to elute paclitaxel with no top layer leading to a 30-day elution period.Citation11,Citation28
Histologic studies performed at autopsy of first-generation DES showed evidence of hypersensitivity (immunoglobulin E-mediated reactions), which had not been previously seen in bare metal stents studies or known to occur with the antiproliferative agents being used.Citation29 Although inconclusive from this type of study, the polymers were implicated as a contributing factor in stent thrombosis. In an autopsy study, inflammatory cells found around fragments of polymers on pathology suggested that the polymer was a possible mediator of stent thrombosis.Citation30 Additionally, a larger autopsy series showed that DES with late stent thrombosis had evidence of delayed arterial wall healing, again with the presence of inflammatory cells.Citation31
Safety concerns and biocompatibility became more important drivers of polymer selection with next-generation products. As a result, efforts were geared to reducing polymer-induced hypersensitivity. An early porcine model looking at five biodegradable and three permanent polymers in the absence of an antiproliferative drug showed that both polymer types can cause significant vessel inflammation and neointimal thickening.Citation32 Of note, the polylactic acid/polyglycolic acid copolymer showed the least amount of fibrocellular proliferation,Citation32 and its subunits polyglycolic acid (PGA) and polylactic acid (PLA, also known as PLLA), are some of the bioabsorbable polymers being utilized in bioabsorbable products.
Phosphorylcholine is a unique passive biomimicry polymer that also has active characteristics, making it an attractive option for drug elution.Citation28 Phosphorylcholine is a naturally occurring component of cell membrane structure and demonstrates excellent biocompatibility. A key benefit of phosphorylcholine is its ability to reduce adhesion of platelets and inflammatory cells;Citation33 as well as its compatibility with other chemical compounds. Phosphorylcholine was the polymer utilized in the second-generation CoCr zotarolimus-eluting Endeavor stent (Medtronic Inc.),Citation34 but the rapid elution kinetics of the phosphorylcholine polymer and resultant late lumen loss proved to be a significant limitation and led to a redesign of the Endeavor platform. This fact illustrated how characteristics such as the kinetics of polymer degradation still play an important role in polymer selection and stent design.Citation35
Drugs
There was no arguing that first-generation DES were associated with significant reductions in neointimal hyperplasia and in-stent restenosis;Citation7,Citation8,Citation36 however, the possibility of the drugs contributing to delayed arterial healing and late stent thrombosis could not be ignored. In an animal model, paclitaxel was shown to have an inverse and dose-dependent relationship with late lumen loss, which was also associated with increased medial wall necrosis.Citation37 Sirolimus was associated with increased inflammation and fibrin deposits, as well as delayed endothelialization.Citation38
It has also been noted that the more lipophilic the drug, the less drug lost upon exposure to blood and the easier it will transmit through the hydrophobic vessel wall.Citation37 Newer agents such as everolimus, zotarolimus, and Biolimus (Biosensors International Pte Ltd, Singapore) are sirolimus analogs that may benefit from being more lipophilic.Citation39,Citation40 Everolimus and zotarolimus have also been shown to inhibit proliferation of coronary arterial smooth muscle cells, thereby possibly reducing neointimal formation.Citation39,Citation41 Again, long-term studies will show if these agents can outperform currently approved drugs.
Stent evolution in clinical trials
Despite some limitations, first-generation DES demonstrated excellent efficacy and safety profiles when compared with bare metal stents. It therefore makes sense that each successive advancement or improvement in stent technology would incorporate the conceptual advances of the previous generation. As a result, second-generation and third-generation stents represent more of an iterative advance than a complete paradigm shift. Comparative studies of established platforms with novel designs therefore do not necessarily demonstrate the overwhelming benefit seen with DES when they were first studied. We will review the iterative stent advancements focusing on the determinants that drove the clinical benefit.
Second-generation cobalt chromium DES
Zotarolimus-eluting stent
Endeavor (Medtronic Inc.), a 91 μm CoCr strut with a phosphorylcholine biocompatible polymer that eluted zotarolimus over a 2-week period, was the first commercially available zotarolimus-eluting stent (ZES). and summarize some of the pivotal clinical trials evaluating the Endeavor stent. ENDEAVOR II (Randomized, Double-Blind, Multicenter Study of the Endeavor Zotarolimus-Eluting Phosphorylcholine-Encapsulated Stent for Treatment of Native Coronary Artery Lesions) compared the Endeavor ZES platform with a bare metal stent and showed a significant improvement in target vessel failure (target vessel revascularization, myocardial infarction [MI], target vessel-related cardiac death) driven primarily by a 55% relative risk reduction in target vessel revascularization at 9 months.Citation34 A 61% relative risk reduction in target lesion revascularization (4.6% versus 11.8%, P=0.0001) was also seen at 9 months, and improvements in target vessel failure and target lesion revascularization persisted at 5 years.Citation42 ENDEAVOR IV (Randomized Comparison of Zotarolimus and Paclitaxel-Eluting Stents in Patient with Coronary Artery Disease) showed noninferiority of the ZES to the paclitaxel-eluting stent (PES) for target vessel failure (cardiac death, MI, target vessel revascularization) at 9-month follow-up.Citation43 Long-term follow-up found similar rates of target lesion revascularization (6.5% versus 6.1%, P=0.662) with an improvement in rates of cardiac death, MI, and very late stent thrombosis, (0.1% versus 1.6%, P=0.004), favoring the Endeavor.Citation44 However, when the ZES was compared with the sirolimus-eluting stent (SES) in ENDEAVOR III (Comparison of Zotarolimus-Eluting and Sirolimus-Eluting stents in Patients With Native Coronary Artery Disease), the results were not as convincing regarding its improvement over first-generation DES.Citation45 ENDEAVOR III favored the SES platform for both late lumen loss and binary restenosis (11.7% versus 4.3%, P=0.04). SORT OUT III (Scandinavian Organization for Randomized Trials with Clinical Outcome III) was a larger trial comparing the ZES and SES, and this also demonstrated superior major adverse cardiac events (MACE, ie, cardiac death, MI, target vessel revascularization), (6% versus 3%, P=0.0002), driven by both MI and target vessel revascularization.Citation46 These results persisted at 3 years, with MACE (12.9% versus 10.1%, P=0.022) driven by 40% higher target vessel revascularization in the Endeavor platform and no improvement in definite stent thrombosis (1.1% versus 1.4%, P=0.61).Citation47 Interestingly, the 5-year follow-up to ENDEAVOR III found that MACE rates favored the thin strut CoCr ZES over SES (14% versus 22%, P=0.05) and that secondary endpoints such as target lesion revascularization, target vessel revascularization, and stent thrombosis was similar between the two groups.Citation48
Figure 2 Trials of the ZES stent (Endeavor II, IV, SORT OUT III).Citation34,Citation43,Citation46
Abbreviations: BMS, bare metal stent; SES, sirolimus-eluting stent; PES, paclitaxel-eluting stent; ZES, zotarolimus-eluting stent; TLR, target lesion revascularization; vs, versus.
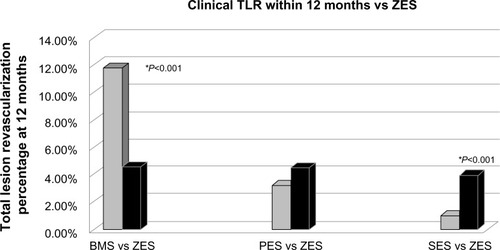
Table 3 ZES outcomes in large trials
One of the reasons for the decreased effectiveness of the Endeavor stent within the first year was thought to be due to the early release kinetics of phosphorylcholine.Citation49 A second version of the Endeavor, the Endeavor Resolute (Medtronic Inc.), was engineered to have a longer drug elution time by utilizing a new biocompatible polymer named BioLinx. BioLinx consists of three polymers, ie, a hydrophobic C10 polymer to help control drug release, a polyvinyl pyrrolidone to enhance initial drug delivery, and a C19 hydrophilic layer for biocompatibility. The polymer elutes 85% of the zotarolimus in 60 days and the rest by 180 days.Citation49 A third iteration, Resolute Integrity (Medtronic Inc.) also uses zotarolimus and the BioLinx polymer with a new CoCr strut design as noted earlier in an attempt to improve flexibility and deliverability without compromising radial strength.Citation50,Citation51 Clinical trials regarding these new platforms will be reviewed later in this paper.
Everolimus-eluting stent
Everolimus, another of the engineered lipophilic sirolimus analogs, has been incorporated into second-generation and third-generation DES. Everolimus binds to FKBP12 and interferes with a regulatory protein that controls cell metabolism and proliferation through inhibition of protein translation.Citation52 It has a direct effect on vascular endothelium and has been shown to significantly reduce neointimal proliferation.Citation41 Additionally, everolimus leads to selective clearing via autophagy of macrophages which are implicated in plaque destabilization, while only arresting proliferation of smooth muscle cells which helps to stabilize plaques.Citation53 The larger pivotal trials involving the everolimus-eluting stent (EES) are summarized in and .
Figure 3 Trials of the everolimus-eluting stent (Spirit IV, SORT OUT IV, RESOLUTE ALL COMERS).
Abbreviations: EES, everolimus-eluting stent; SES, sirolimus-eluting stent; PES, paclitaxel-eluting stent; ZES, zotarolimus-eluting stent; TLR, target lesion revascularization; vs, versus.
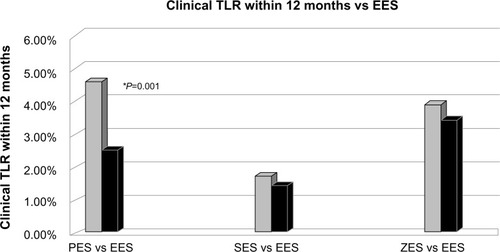
Table 4 EES outcomes in large trials
EES versus bare metal stent and PES
The first EES introduced into clinical practice was the Xience V (Abbott Vascular). Consisting of an 81 μm CoCr strut and PBMA primer layer followed by a biocompatible copolymer of polyvinylidene fluoride-co-hexafluoropropylene, it eluted approximately 80% of its everolimus in the first 30 days and 100% by 4 months.Citation54 The SPIRIT FIRST (A Clinical Evaluation of an Investigational Device. The Abbott XIENCE V® Everolimus Eluting Coronary Stent System in the Treatment of Patients With de Novo Native Coronary Artery Lesions) was the initial study of the Xience V platform comparing it against bare metal stents, and showed a greater than eight-fold increase in late lumen loss versus the EES (0.87 mm versus 0.1 mm, P<0.001) at 6 months.Citation55 The EXAMINATION (Everolimus-eluting stent versus bare metal stent in ST-elevation MI) trial demonstrated the improved safety and efficacy of the newer limus analogs in patients with ST-segment elevation MI. At one year, rates for the combined primary endpoint (all-cause death, any MI, any revascularization) were similar. Target lesion and vessel revascularization significantly favored the EES, as did definite and probable stent thrombosis (0.9% versus 2.5%, P=0.019).Citation56
When compared with the PES in small trials (SPIRIT II and III), the EES showed a significant improvement in late lumen loss (0.14 mm versus 0.28 mm, P≤0.004) at 8 months and was noninferior for target vessel failure (cardiac death, MI, target vessel revascularization) at 9-month clinical follow-up, with improved MACE (cardiac death, MI, target lesion revascularization) driven by target lesion revascularization.Citation57,Citation58 The MACE reduction remained significant at 2 years (10.7% versus 15.4%, P=0.04), with no significant difference in stent thrombosis rates.Citation59 The superiority and improved long-term safety was demonstrated in SPIRIT IV, a large-scale comparison of EES (n=2,458) and PES (n=1,229). SPIRIT IV showed superiority of the EES platform at one year, with a 38% relative risk reduction (P=0.001) for target lesion failure (cardiac death, target vessel MI, target lesion revascularization).Citation60 These results remained significant at 2 years, with decreased stent thrombosis (0.4% versus 1.2%, P=0.008).Citation61 A second large-scale real-world use trial, COMPARE (A Randomized Controlled Trial of Everolimus-eluting Stents and Paclitaxel-eluting Stents for Coronary Revascularization in Daily Practice), also showed superiority of the Xience V stent over the TAXUS Liberté PES platform for the composite primary endpoint (target vessel revascularization, all-cause death, and MI; 6% versus 9%, P=0.02) at one year.Citation62 Individual secondary endpoints of target lesion revascularization, MI, and cardiac death were all superior as well. Definite and probable stent thrombosis at 12 and 24 months (0.9% versus 3.9%, P<0.001) were also significantly reduced with XIENCE V, as was very late stent thrombosis (more than one year; 0.3% versus 1.4%, P=0.01).Citation62,Citation63 From these data, it seemed clear that the EES platform had achieved a significant clinical improvement over the PES.
A pooled analysis of the 2-year follow-up of the SPIRIT and COMPARE trials evaluated differences in patients with acute coronary syndrome versus those with stable CAD. In patients with acute coronary syndrome, the EES demonstrated a significant reduction in death, MI, and clinical target lesion revascularization, and a 75% reduction in stent thrombosis (0.7% versus 2.9%, P=0.0002). Similar significant outcomes were noted in patients with stable CAD, favoring the EES. These results suggested that the EES platform is safe in all types of patients.Citation64
EES versus SES
Fewer randomized studies have compared the EES with the SES. The randomized EXCELLENT (Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting) trial showed 9-month noninferiority for late luminal loss with no difference in target lesion failure or stent thrombosis.Citation65 In the BASKET-PROVE (BAsel Stent Kosten Effektivitäts Trial PROspective Validation Examination) study, SES and EES showed similar composite endpoints and stent thrombosis rates at 24 months.Citation66 The SORT OUT IV trial showed noninferiority of the EES platform with regards to a composite clinical endpoint of safety (cardiac death, MI, and stent thrombosis) and efficacy, ie, target vessel revascularization (hazard ratio 0.94; 95% CI [0.67–1.31], P=0.71).Citation67 At 18 months, the EES continued to show noninferiority, with significantly lower rates of stent thrombosis (0.2% versus 0.9%, P=0.02).Citation67 These data suggest that the EES platform has improved safety and efficacy over the first-generation SES.
EES versus ZES
Several studies have compared the Xience V EES platform with the Endeavor Resolute ZES platform. In an early study, ie, RESOLUTE ALL COMERS (A Randomized Comparison of a Zotarolimus-Eluting Stent With an Everolimus-Eluting Stent for Percutaneous Coronary Intervention), Serruys et al found the Resolute to be noninferior to the EES for target lesion failure (cardiac death, target vessel MI, target lesion revascularization) at 12 months.Citation68 However, the Resolute ZES had higher rates of definite stent thrombosis (1.2% versus 0.3%, P=0.01). The noninferiority persisted after 2 years of follow-up with a trend towards higher definite and probable stent thrombosis (1.9% versus 1.0%, P=0.08) for the ZES platform.Citation69 A second study, TWENTE (A Randomized Controlled Trial in the Second-Generation Zotarolimus-Eluting Resolute Stents Versus Everolimus-Eluting Xience V Stents in Real-World Patients), also directly compared the two stents, although in a smaller patient population, and found noninferiority between the two platforms at one-year and 2-year follow-up.Citation70,Citation71 In this trial, there was no significant difference of definite or probable stent thrombosis at one year or for very late stent thrombosis (at more than one year).Citation70 Several large registries support the findings of a lower rate of target vessel/lesion revascularization in favor of the EES.Citation72,Citation73 This comparison of similar generation stents is consistent with prior comparison studies suggesting that we should not expect iterative advances of the same generation to achieve large or significant improvements in overall MACE rates compared with each other. Therefore, some of these other measures of improved efficacy and safety become more relevant, such as less target lesion revascularization and less stent thrombosis, in head-to-head comparisons.
Stent thrombosis with EES
The incidence of definite and probable stent thrombosis (early, late, and very late) for the EES platform has been lower than with previous stent designs. A meta-analysis evaluated the randomized controlled trials to date comparing EES with other DES (SES, PES, and ZES) to further assess the safety of the EES platform with regard to stent thrombosis. At 2 years, the results favored the EES platform for definite stent thrombosis (0.5% versus 1.3%, P<0.0001) and definite and probable stent thrombosis (P<0.0001).Citation74 The difference was significant for early stent thrombosis (≤30 days) and persisted through late (30 days to one year) and very late (more than one year). Overall, these data suggest the CoCr EES platform offers improved efficacy and long-term safety over first-generation and other second-generation DES platforms.
Third-generation platinum chromium DES
As previously described, PtCr offers improved radial strength and density that allows for a smaller profile stent strut with excellent deliverability and significantly improved visibility. The Element platforms (Boston Scientific) utilize PtCr struts. The TAXUS Element is an 81 μm PES and in PERSEUS (Prospective Evaluation in a Randomized Trial of the Safety and Efficacy of the Use of TAXUS Element Paclitaxel-Eluting Coronary Stent System) was found to be noninferior to the TAXUS Express (316L stainless steel PES) for target lesion revascularization and percent diameter stenosis at one year.Citation19
The Promus Element utilizes everolimus and PtCr. PLATINUM (A Prospective, Randomized, Multicenter Trial to Assess an Everolimus-Eluting Coronary Stent System [PROMUS Element] for the Treatment of up to Two De Novo Coronary Artery Lesions) compared the Promus Element (PtCr EES) with the Xience V (CoCr EES), and found the new Promus Element stent to be noninferior for target lesion failure (target vessel death or MI, or target lesion revascularization) as well as for stent thrombosis (0.4% versus 0.4%, P=1.0) at one year.Citation20 At 2 years, each stent demonstrated comparable efficacy and safety, with stent thrombosis rates of <1% for both platforms with signals toward decreased target lesion failure (1.2% versus 3.0%, P=0.04) and target lesion revascularization (0.7% versus 2.2%, P=0.02) with the PtCr stent.Citation75
The Promus Element was compared with the Resolute Integrity in the DUTCH PEERS (Third-generation zotarolimus-eluting and everolimus-eluting stents in all-comer patients requiring a percutaneous coronary intervention) study. This study showed noninferiority of the Resolute Integrity stent when compared with the Promus Element stent for target vessel failure (death, target vessel MI, and target vessel revascularization) at 12 months (6% versus 5%, P=0.42).Citation76 Stent thrombosis was again < 1% for both platforms.
Next-generation technologies
Polymer-free DES
Since polymers have been implicated as a cause for late and very late stent thrombosis, stent design has also evaluated methods to eliminate polymers. The increased strength of the newer metal alloys has allowed for abluminal scoring and reservoirs to be made without affecting overall stent strength.Citation28 Several similar designs utilizing different drugs have recently been tested against prior and current generation DES, and in early studies have demonstrated noninferiority with up to one-year follow-up.Citation77–Citation79 Some of the goals of these studies are to potentially reduce the length of use of dual antiplatelet therapy, late and very late stent thrombosis rates, and dose of antirestenosis drug needed, while maintaining current efficacy rates. The BioFreedom™ is a stainless steel strut with abluminal pores that elute most of its biolimus content over 28 days. In the BioFreedom first in man trial, this stent showed noninferiority with regard to late luminal loss (primary outcome) and MACE when compared with a TAXUS Liberté stent at 12 months.Citation79 Cre8 is a CoCr strut with abluminal reservoirs eluting most of its sirolimus within 28 days. The Cre8 study was a prospective randomized trial comparing the Cre8 with the TAXUS Liberté, and demonstrated a significant reduction in late loss at 6 months (0.14±0.36 mm versus 0.34±0.40 mm, P<0.0001).Citation78 The Demonstr8 (Randomized comparison between a DES and a BMS to assess neointimal coverage by OCT evaluation) study evaluated the Cre8 stent with only one month of dual antiplatelet therapy, measuring coverage of the Cre8 stent by optical coherence tomography at 3 months compared with the Vision BMS at one month. The two stents were found to be noninferior in this regard, and there was a 56% reduction in neointima formation (0.08 mm ±0.03 versus 0.18 mm ±0.10, P<0.0001 for superiority).Citation80 These and other similar devices show promise in early studies, but long-term follow-up with studies designed to evaluate for individual endpoints of safety and efficacy will be needed.
Bioabsorbable polymer coatings
In as much as a durable polymer may impact long-term events, by delaying stent healing, contributing to neointimal hyperplasia and stent thrombosis, efforts have logically focused on developing nondurable (bioabsorbable) polymers such as PGA and PLA. Several bioabsorbable polymer stents, in which the polymers are absorbed within 6–12 months, are currently being evaluated for safety and efficacy. There are over a dozen platforms currently, with different base metals and novel polymers utilizing sirolimus, paclitaxel, biolimus, and everolimus as their drug (). Some of the larger clinical trials regarding these stents are reviewed here.
Table 5 Bioabsorbable stent characteristics
The Excel (JW Medical Systems, Weihai, People’s Republic of China) stent design includes 316L stainless steel struts, a PLA polymer, and sirolimus. CREATE (Multi-Center Registry of EXCEL Biodegradable Polymer Drug Eluting Stents) was a nonrandomized, multicenter, prospective study with 5-year follow-up data on 1,982 patients using the Excel platform and found the overall MACE rate (cardiac death, nonfatal MI, target lesion revascularization) to be 7.4%. There was a 1.1% rate of definite or probable stent thrombosis at 5 years, with a rate of definite stent thrombosis from years 1 to 5 (very late stent thrombosis) of 0.3%.Citation81 When patients who stopped clopidogrel after 6 months (n=1,626) were compared with those who continued clopidogrel after 6 months (n=408), there was no difference in MACE (5.8% versus 7.4%, P=0.256) or stent thrombosis (0.6% versus 0.5%, P=1.0) rates.Citation81 With the promise of reduced need for dual antiplatelet therapy, further study is needed to evaluate the effectiveness of this stent design in comparison with current DES.
The BioMatrix stent (Biosensors Inc., Newport Beach, CA, USA) utilizes a 316L stainless steel strut, biolimus, and a biodegradable PLA polymer that is only administered to the abluminal surface of the stent. LEADERS (the Biolimus-eluting stent with a biodegradable polymer versus the SES with a durable polymer for coronary revascularization trial) was a randomized noninferiority trial. At the 9-month follow-up, the composite endpoint of death, MI, or target vessel revascularization demonstrated noninferiority (9% versus 11%, relative risk 0.88, 95% CI 0.64–1.19, P=0.003 for noninferiority).Citation82 The noninferiority persisted at 4 years, with event rates of 18.7% for the biolimus-eluting stent (BES) versus 22.6% for the SES (relative risk 0.81, 95% CI 0.66–1.0, P<0.0001 for noninferiority) and was just short of showing superiority (P=0.05 for superiority).Citation83 Definite very late stent thrombosis rates were improved with the BES (relative risk 0.20, 95% CI 0.06–0.67, P=0.004).Citation83 With long-term follow-up, the biodegradable BioMatrix BES has demonstrated good efficacy with improved safety.
There still remains some concern regarding the higher rates of late stent thrombosis seen with use of the first-generation DES in patients with ST-segment elevation MI. As previously reported in this paper, the data suggest that the new agent, everolimus (second-generation and third-generation) has shown improved rates of stent thrombosis. The BioMatrix stent was studied in the special population of patients with ST-segment elevation MI in the COMFORTABLE-AMI (Effect of Biolimus-Eluting stents with Biodegradable Polymer versus Bare Metal Stents on Cardiovascular Events Among Patients with Acute Myocardial Infarction) trial. MACE (cardiac death, target vessel MI, target lesion revascularization) at 12 months favored the BES (4.3% versus 8.7%, hazards ratio 0.49, 95% CI [0.30–0.80], P=0.004).Citation84 Definite or probable stent thrombosis rates were no different at one year. This study, along with the previously mentioned data, suggests that the newer limus analogs will be safe in patients with acute coronary syndrome. Long-term follow-up will show if biolimus can reduce very late stent thrombosis rates.
The Nobori® stent (Terumo Corporation, Tokyo, Japan) is another BES that uses 316L stainless steel and the abluminal PLA polymer. SORT OUT V compared the Nobori BES with the Cypher SES, but interestingly found the BES to be inferior to the SES at 9 months for composite cardiac death, MI, and definite stent thrombosis (4.1% BES versus 3.1% SES, P=0.06),Citation85 unlike the BioMatrix data reported in the bioabsorbable polymer coatings section. This was driven by acute and subacute (<30 days) stent thrombosis.Citation85 In the COMPARE II (Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent) study, the Nobori was found to be noninferior to the Xience platform at 12 months for the composite endpoint of cardiac death, MI, and target vessel revascularization.Citation86 Use of a nonallocated stent was higher with the Nobori (5.8% versus 2.1%, P<0.0001) due to limited size availability and failure to cross the lesion. Stent thrombosis was similar at one year, and long-term follow-up will be needed. The different endpoints used in the different BES studies make direct comparisons difficult, and larger-scale studies are needed to better understand individual outcomes, as well as long-term safety. Further, the clinical differences between the BioMatrix and Nobori platforms will need to be better understood to help further refine the stent design process for those stents.
The Synergy stent (Boston Scientific) utilizes a PtCr stent strut with an abluminal bioabsorbable polymer (polylactic acid/polyglycolic acid) and everolimus. Polymer absorption should be complete by 4 months. The EVOLVE (A Randomized Evaluation of a Novel Bioabsorbable Polymer-Coated, Everolimus-Eluting Coronary Stent) trial sought to compare the Synergy stent with the Promus Element (PtCr EES) stent at two doses of everolimus (same dose as the Promus Element stent and at half the dose).Citation87 At 30 days, there was no significant difference in target lesion failure (death, target vessel MI, target lesion revascularization); however, the absolute differences of 0% (Element), 1% (Synergy full dose), and 3.1% (Synergy half dose) were entirely driven by target vessel MI.Citation87 When 2-year follow-up data were reviewed, there was again no difference in target lesion failure between the groups, ie, 6.1% (Element), 5.5% (Synergy), and 5.2% (Synergy half dose).Citation88 Interestingly, there was more target lesion revascularization in the Element stent group and more target vessel MI and death in the Synergy stent group, that led to equalization of absolute event rates. Large-scale studies, such as EVOLVE II, are required.
Bioabsorbable struts
The BVS stent (Abbott Vascular) strut scaffold is constructed of the PLLA polymer that is absorbed by 18–24 months and a second polymer coating of poly-D,L-lactide that elutes everolimus over 30 days. In the ABSORB (A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions) study, an initial safety trial of 30 patients showed a MACE rate of 3.3% which was solely one non-Q wave MI at one year,Citation89 and at 4 years remained at 3.4% with no new clinical events.Citation90 The ABSORB II trial will compare the second-generation BVS with the Xience V in approximately 501 patients across Europe and New Zealand, with a planned 3-year follow-up.Citation91 ABSORB III will also compare the BVS with the Xience V, enrolling approximately 2,250 patients, mostly within the USA, with up to 5 years of planned follow-up. Results from these trials are pending.
Conclusion
The introduction of intracoronary stents represented a major breakthrough in interventional cardiology, and numerous advances have dramatically enhanced the deliverability, safety, and efficacy of these stents over the past two decades. Bare metal stents set the benchmark for improved safety over angioplasty, and the first-generation DES was a significant advance in efficacy, but not necessarily safety. Second- generation and third-generation stents represent advances in performance in terms of enhanced delivery and improved radial strength, as well as efficacy and safety via decreased late loss and a lower risk of stent thrombosis. Current advances and understanding of stent design continue to improve on these concepts, and the current standards outperform earlier designs in all facets. The next frontier includes polymer-free stents or platforms with some degree of bioabsorbability, whether merely the polymer or the entire stent. Randomized studies evaluating many of these products are ongoing, and the results of these trials will most definitely advance our understanding and fuel further innovation.
Disclosure
DHS has been a consultant to and/or received honoraria from Terumo Interventional Systems, Boston Scientific, St Jude Medical, and Astra Zeneca. The other authors report no conflicts of interest in this work.
References
- KentKMBentivoglioLGBlockPCPercutaneous transluminal coronary angioplasty: report from the Registry of the National Heart, Lung, and Blood InstituteAm J Cardiol1982498201120206211084
- ChamberlainDAFoxKAAHendersonRAJulianDGParkerDJPocockSJCoronary angioplasty versus medical therapy for angina: the second Randomised Intervention Treatment of Angina (RITA-2) trial. RITA-2 trial participantsLancet199735090764614689274581
- ParisiAFFollandEDHartiganPA comparison of angioplasty with medical therapy in the treatment of single-vessel coronary artery disease. Veterans Affairs ACME InvestigatorsN Engl J Med1992326110161345754
- FischmanDLLeonMBBaimDSA randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study InvestigatorsN Engl J Med199433184965018041414
- SerruysPWde JaegerePKiemeneijFA comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study GroupN Engl J Med199433184894958041413
- LemosPASerruysPWvan DomburgRTUnrestricted utilization of sirolimus-eluting stents compared with conventional bare stent implantation in the “real world”: the Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) registryCirculation2004109219019514691037
- MosesJWLeonMBPopmaJJSirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary arteryN Engl J Med2003349141315132314523139
- StoneGWEllisSGCoxDAA polymer-based, paclitaxel-eluting stent in patients with coronary artery diseaseN Engl J Med2004350322123114724301
- LagerqvistBJamesSKStenestrandULindbackJNilssonTWallentinLLong-term outcomes with drug-eluting stents versus bare-metal stents in SwedenN Engl J Med2007356101009101917296822
- StoneGWMosesJWEllisSGSafety and efficacy of sirolimus-and paclitaxel-eluting coronary stentsN Engl J Med200735610998100817296824
- ManiGFeldmanMDPatelDAgrawalCMCoronary stents: a materials perspectiveBiomaterials20072891689171017188349
- KastratiAMehilliJDirschingerJIntracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trialCirculation2001103232816282111401938
- PacheJKastratiAMehilliJIntracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trialJ Am Coll Cardiol20034181283128812706922
- RittersmaSZde WinterRJKochKTImpact of strut thickness on late luminal loss after coronary artery stent placementAm J Cardiol200493447748014969629
- KereiakesDJCoxDAHermillerJBUsefulness of a cobalt chromium coronary stent alloyAm J Cardiol200392446346612914881
- O’BrienBJStinsonJSLarsenSREppihimerMJCarrollWMA platinum-chromium steel for cardiovascular stentsBiomaterials201031143755376120181394
- MenownIBNoadRGarciaEJMeredithIThe platinum chromium element stent platform: from alloy, to design, to clinical practiceAdv Ther201027312914120437213
- TurcoMAThe Integrity bare-metal stent made by continuous sinusoid technologyExpert Rev Med Devices20118330330621542702
- KereiakesDJCannonLAFeldmanRLClinical and angiographic outcomes after treatment of de novo coronary stenoses with a novel platinum chromium thin-strut stent: primary results of the PERSEUS (Prospective Evaluation in a Randomized Trial of the Safety and Efficacy of the Use of the TAXUS Element Paclitaxel-Eluting Coronary Stent System) trialJ Am Coll Cardiol201056426427120493653
- StoneGWTeirsteinPSMeredithITA prospective, randomized evaluation of a novel everolimus-eluting coronary stent: the PLATINUM (a Prospective, Randomized, Multicenter Trial to Assess an Everolimus-Eluting Coronary Stent System [PROMUS Element] for the Treatment of Up to Two de Novo Coronary Artery Lesions) trialJ Am Coll Cardiol201157161700170821470815
- MortierPDe BeuleMStent design back in the picture: an engineering perspective on longitudinal stent compressionEuroIntervention20117777377521971054
- OrmistonJAWebberBWebsterMWStent longitudinal integrity bench insights into a clinical problemJACC Cardiovasc Interv20114121310131722136972
- OrmistonJClinical, angiographic and IVUS outcomes of the NG PROMUS clinical trial evaluating the novel Promus Premier stentPaper presented at the EuroPCR meetingMay 21–24, 2013Paris, France
- NishioSKosugaKIgakiKLong-term (>10 years) clinical outcomes of first-in-human biodegradable poly-l-lactic acid coronary stents: Igaki-Tamai stentsCirculation2012125192343235322508795
- TamaiHIgakiKKyoEInitial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humansCirculation2000102439940410908211
- OrmistonJASerruysPWBioabsorbable coronary stentsCirc Cardiovasc Interv20092325526020031723
- PendyalaLJabaraRRobinsonKChronosNPassive and active polymer coatings for intracoronary stents: novel devices to promote arterial healingJ Interv Cardiol2009221374819281521
- O’BrienBCarrollWThe evolution of cardiovascular stent materials and surfaces in response to clinical drivers: a reviewActa Biomater20095494595819111513
- NebekerJRVirmaniRBennettCLHypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) projectJ Am Coll Cardiol200647117518116386683
- VirmaniRGuagliumiGFarbALocalized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious?Circulation2004109670170514744976
- JonerMFinnAVFarbAPathology of drug-eluting stents in humans: delayed healing and late thrombotic riskJ Am Coll Cardiol200648119320216814667
- van der GiessenWJLincoffAMSchwartzRSMarked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteriesCirculation1996947169016978840862
- LewisALTolhurstLAStratfordPWAnalysis of a phosphorylcholine-based polymer coating on a coronary stent pre- and post-implantationBiomaterials20022371697170611922473
- FajadetJWijnsWLaarmanGJRandomized, double-blind, multicenter study of the Endeavor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: clinical and angiographic results of the ENDEAVOR II trialCirculation2006114879880616908773
- GuagliumiGIkejimaHSirbuVImpact of drug release kinetics on vascular response to different zotarolimus-eluting stents implanted in patients with long coronary stenoses: the LongOCT study (Optical Coherence Tomography in Long Lesions)JACC Cardiovasc Interv20114777878521777886
- MoriceMCSerruysPWSousaJEA randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularizationN Engl J Med2002346231773178012050336
- HeldmanAWChengLJenkinsGMPaclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosisCirculation2001103182289229511342479
- FinnAVNakazawaGJonerMVascular responses to drug eluting stents: importance of delayed healingArterioscler Thromb Vasc Biol20072771500151017510464
- BurkeSEKuntzRESchwartzLBZotarolimus (ABT-578) eluting stentsAdv Drug Deliv Rev200658343744616581153
- ClaessenBEHenriquesJPDangasGDClinical studies with sirolimus, zotarolimus, everolimus, and biolimus A9 drug-eluting stent systemsCurr Pharm Des201016364012402421208185
- WaksmanRPakalaRBaffourROptimal dosing and duration of oral everolimus to inhibit in-stent neointimal growth in rabbit iliac arteriesCardiovasc Revasc Med20067317918416945826
- FajadetJWijnsWLaarmanGJLong-term follow-up of the randomised controlled trial to evaluate the safety and efficacy of the zotarolimus-eluting driver coronary stent in de novo native coronary artery lesions: five year outcomes in the ENDEAVOR II studyEuroIntervention20106556256721044908
- LeonMBMauriLPopmaJJA randomized comparison of the Endeavor zotarolimus-eluting stent versus the TAXUS paclitaxel- eluting stent in de novo native coronary lesions 12-month outcomes from the ENDEAVOR IV trialJ Am Coll Cardiol201055654355420152559
- LeonMBNikolskyECutlipDEImproved late clinical safety with zotarolimus-eluting stents compared with paclitaxel-eluting stents in patients with de novo coronary lesions: 3-year follow-up from the ENDEAVOR IV (Randomized Comparison of Zotarolimus- and Paclitaxel-Eluting Stents in Patients With Coronary Artery Disease) trialJACC Cardiovasc Interv20103101043105020965463
- KandzariDELeonMBPopmaJJComparison of zotarolimus-eluting and sirolimus-eluting stents in patients with native coronary artery disease: a randomized controlled trialJ Am Coll Cardiol200648122440244717174180
- RasmussenKMaengMKaltoftAEfficacy and safety of zotarolimus-eluting and sirolimus-eluting coronary stents in routine clinical care (SORT OUT III): a randomised controlled superiority trialLancet201037597201090109920231034
- MaengMTilstedHHJensenLO3-Year clinical outcomes in the randomized SORT OUT III superiority trial comparing zotarolimus-and sirolimus-eluting coronary stentsJACC Cardiovasc Interv20125881281822917452
- KandzariDEMauriLPopmaJJLate-term clinical outcomes with zotarolimus- and sirolimus-eluting stents. 5-year follow-up of the ENDEAVOR III (A Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System Versus the Cypher Sirolimus-Eluting Coronary Stent System in De Novo Native Coronary Artery Lesions)JACC Cardiovasc Interv20114554355021596327
- BrugalettaSBurzottaFSabateMZotarolimus for the treatment of coronary artery disease: pathophysiology, DES design, clinical evaluation and future perspectiveExpert Opin Pharmacother20091061047105819364252
- BanerjeeSThe Resolute Integrity zotarolimus-eluting stent in coronary artery disease: a reviewCardiology and Therapy2013219
- BelardiJAAlbertalMIntegrity(R) coronary stent: a very limber bare-metal stentCatheter Cardiovasc Interv201178690922086779
- BennettJDuboisCA novel platinum chromium everolimus-eluting stent for the treatment of coronary artery diseaseBiologics2013714915923818756
- VerheyeSMartinetWKockxMMSelective clearance of macrophages in atherosclerotic plaques by autophagyJ Am Coll Cardiol200749670671517291937
- DingNPacettiSTangF-WGadaMRoordaWXience V stent design and rationaleJ Interv Cardiol200922s1s18s27
- SerruysPWOngATPiekJJA randomized comparison of a durable polymer Everolimus-eluting stent with a bare metal coronary stent: the SPIRIT first trialEuroIntervention200511586519758878
- SabateMCequierAIniguezAEverolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trialLancet201238098521482149022951305
- SerruysPWRuygrokPNeuznerJA randomised comparison of an everolimus-eluting coronary stent with a paclitaxel-eluting coronary stent: the SPIRIT II trialEuro Intervention20062328629419755303
- StoneGWMideiMNewmanWComparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trialJAMA2008299161903191318430909
- StoneGWMideiMNewmanWRandomized comparison of everolimus-eluting and paclitaxel-eluting stents: two-year clinical follow-up from the Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions (SPIRIT) III trialCirculation2009119568068619171853
- StoneGWRizviANewmanWEverolimus-eluting versus paclitaxel-eluting stents in coronary artery diseaseN Engl J Med2010362181663167420445180
- StoneGWRizviASudhirKRandomized comparison of everolimus- and paclitaxel-eluting stents. 2-year follow-up from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) IV trialJ Am Coll Cardiol2011581192521514084
- KedhiEJoesoefKSMcFaddenESecond-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trialLancet2010375971020120920060578
- SmitsPCKedhiERoyaardsKJ2-year follow-up of a randomized controlled trial of everolimus- and paclitaxel-eluting stents for coronary revascularization in daily practice. COMPARE (Comparison of the everolimus eluting XIENCE-V stent with the paclitaxel eluting TAXUS LIBERTE stent in all-comers: a randomized open label trial)J Am Coll Cardiol2011581111821514083
- PlanerDSmitsPCKereiakesDJComparison of everolimus-and paclitaxel-eluting stents in patients with acute and stable coronary syndromes: pooled results from the SPIRIT (A Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) and COMPARE (A Trial of Everolimus-Eluting Stents and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice) TrialsJACC Cardiovasc Interv20114101104111522017936
- ParkKWChaeIHLimDSEverolimus-eluting versus sirolimus-eluting stents in patients undergoing percutaneous coronary intervention: the EXCELLENT (Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting) randomized trialJ Am Coll Cardiol201158181844185422018294
- KaiserCGalatiusSErnePDrug-eluting versus bare-metal stents in large coronary arteriesN Engl J Med2010363242310231921080780
- JensenLOThayssenPHansenHSRandomized comparison of everolimus-eluting and sirolimus-eluting stents in patients treated with percutaneous coronary intervention: the Scandinavian Organization for Randomized Trials with Clinical Outcome IV (SORT OUT IV)Circulation2012125101246125522308301
- SerruysPWSilberSGargSComparison of zotarolimus-eluting and everolimus-eluting coronary stentsN Engl J Med2010363213614620554978
- SilberSWindeckerSVranckxPSerruysPWinvestigators RACUnrestricted randomised use of two new generation drug-eluting coronary stents: 2-year patient-related versus stent-related outcomes from the RESOLUTE All Comers trialLancet201137797731241124721459430
- TandjungKSenHLamMKClinical outcome following stringent discontinuation of dual antiplatelet therapy after 12 months in real-world patients treated with second-generation zotarolimus-eluting resolute and everolimus-eluting Xience V stents: 2-year follow-up of the randomized TWENTE trialJ Am Coll Cardiol201361242406241623602769
- von BirgelenCBasalusMWTandjungKA randomized controlled trial in second-generation zotarolimus-eluting Resolute stents versus everolimus-eluting Xience V stents in real-world patients: the TWENTE trialJ Am Coll Cardiol201259151350136122341737
- DammanPAbdel-WahabMMollmannHComparison of twelve-month outcomes after percutanous coronary intervention with everolimus-eluting versus zotarolimus-eluting or sirolimus-eluting stents from the PROENCY (PROmus ENdeavor CYpher) registryJ Invasive Cardiol2012241049550223043032
- ParkKWLeeJMKangSHSafety and efficacy of second-generation everolimus-eluting Xience V stents versus zotarolimus- eluting resolute stents in real-world practice: patient-related and stent-related outcomes from the multicenter prospective EXCELLENT and RESOLUTE-Korea registriesJ Am Coll Cardiol201361553654423273394
- PalmeriniTKirtaneAJSerruysPWStent thrombosis with everolimus-eluting stents: meta-analysis of comparative randomized controlled trialsCirc Cardiovasc Interv20125335736422668554
- Boston ScientificLandmark analysis of year two data from PLATINUM Workhorse Trial demonstrates superior efficacy of PROMUS Element platinum chromium stent compared with Promus (Xience V) Stent2012 Available from: http://bostonscientific.mediaroom.com/index.php?s=24889&item=125347Accessed November 12, 2013
- von BirgelenCSenHLamMKThird-generation zotarolimus-eluting and everolimus-eluting stents in all-comer patients requiring a percutaneous coronary intervention (DUTCH PEERS): a randomised, single-blind, multicentre, non-inferiority trialLancet2014383991541342324183564
- ByrneRAMehilliJIijimaRA polymer-free dual drug-eluting stent in patients with coronary artery disease: a randomized trial versus polymer-based drug-eluting stentsEur Heart J200930892393119240066
- CarrieDNEXT A prospective, randomized trial comparing Cre8, a polymer-free stent eluting sirolimus, to a paclitaxel-eluting stentPaper presented at the Transcatheter Cardiovascular Therapeutics 23rd Annual Scientific SymposiumNovember 7–11, 2011San Francisco, CA, USA
- GrubeEBIOFREEDOM: a prospective randomized trial of polymer-free bioliums a9-eluting stents and paclitaxel-eluting stents in patients with coronary artery diseasePaper presented at at the Transcatheter Cardiovascular Therapeutics 22rd Annual Scientific SymposiumSeptember 23–25, 2010Washington, DC, USA
- StellaPRDemonstr8 randomized trial results: Cre8: reduced anti-platelet therapy with effective DESPaper presented at the EuroPCR meetingMay 21–24, 2013Paris, France
- HanYLZhangLYangLXA new generation of biodegradable polymer-coated sirolimus-eluting stents for the treatment of coronary artery disease: final 5-year clinical outcomes from the CREATE studyEuroIntervention20128781582223171802
- WindeckerSSerruysPWWandelSBiolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trialLancet200837296441163117318765162
- StefaniniGGKalesanBSerruysPWLong-term clinical outcomes of biodegradable polymer biolimus-eluting stents versus durable polymer sirolimus-eluting stents in patients with coronary artery disease (LEADERS): 4 year follow-up of a randomised non-inferiority trialLancet201137898071940194822075451
- RaberLKelbaekHOstojicMEffect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trialJAMA2012308877778722910755
- ChristiansenEHJensenLOThayssenPBiolimus-eluting biodegradable polymer-coated stent versus durable polymer-coated sirolimus-eluting stent in unselected patients receiving percutaneous coronary intervention (SORT OUT V): a randomised non-inferiority trialLancet2013381986766166923374649
- SmitsPCHofmaSTogniMAbluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trialLancet2013381986765166023374650
- MeredithITVerheyeSDuboisCLPrimary endpoint results of the EVOLVE trial: a randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stentJ Am Coll Cardiol201259151362137022341736
- MeredithITVerheyeSWeissmanNJSix-month IVUS and two-year clinical outcomes in the EVOLVE FHU trial: a randomised evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting stentEuroIntervention20139330831523872647
- OrmistonJASerruysPWRegarEA bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trialLancet2008371961689990718342684
- OnumaYDudekDThuesenLFive-year clinical and functional multislice computed tomography angiographic results after coronary implantation of the fully resorbable polymeric everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB Cohort A trialJACC Cardiovasc Interv2013610999100924156961
- DilettiRSerruysPWFarooqVABSORB II randomized controlled trial: a clinical evaluation to compare the safety, efficacy, and performance of the Absorb everolimus-eluting bioresorbable vascular scaffold system against the XIENCE everolimus-eluting coronary stent system in the treatment of subjects with ischemic heart disease caused by de novo native coronary artery lesions: rationale and study designAm Heart J2012164565466323137495
