Abstract
We used a safety-valve (Trans-Urethral Catheterisation Safety Valve, Class Medical, Limerick, Ireland) to prevent urethral trauma due to inflation of the anchoring balloon in the urethra during catheterisation of male spinal cord injury patients in a spinal unit. The safety-valve is attached to the balloon channel of a Foley catheter. If the balloon is inflated when it is in the urethra, the pressure valve is activated. Any fluid pushed into the balloon channel leaks out and balloon inflation stops, indicating that the balloon is not inside the bladder. The safety-valve was used in 44 catheterisations. There was leakage of water during three catheterisations. In the first case, the health professional did not inflate and deflate the balloon prior to its use. This “pre-valve inflation” step overcomes the baseline resistance pressure of the balloon and prevents fluid leaking from the valve when the catheter is in the correct position. In the second instance, the valve was found to be defective. In the third case, the catheter had been misplaced; it was removed and repositioned; there was no leakage of water during inflation of the balloon. In one out of 44 catheterisations, the catheter had been misplaced; leakage of water from the safety-valve stopped inflation of the balloon and prevented iatrogenic urethral trauma. The safety-valve may be used during catheterisation of male patients in the spinal unit to prevent urethral trauma caused by inflation of the balloon of Foley catheter in the urethra. However, health professionals should remember the few shortcomings of the catheter safety-valve.
Introduction
Urethral trauma during catheterisation may occur in some patients with spinal cord injury. The predisposing factors are: (1) Patients may not have urethral sensation; (2) Some patients may get spasm of the pelvic floor muscles; (3) Spasm of urethral sphincter may occur during catheterisation. These patients are at increased risk for misplacement of the anchoring balloon of Foley catheter in the urethraCitation1 When the balloon of Foley catheter is inflated in the urethra, there is likely to be urethral mucosal tear or even rupture. Urethral trauma can result in potentially life-threatening complications including urosepsis. This may lead to litigation of the health care provider for clinical negligence.
To prevent such iatrogenic urethral trauma, a safety-valve can be used. Davis and associates evaluated the feasibility of the safety-valve device for preventing catheter related urethral trauma during urethral catheterization in 100 patients with a mean age of 76 years. The valve was activated in 7% (n = 7/100) patients during attempted urethral catheterisation, indicating that the catheter anchoring balloon was incorrectly positioned in the patient’s urethra. In these 7 cases, the catheter was successfully manipulated into the urinary bladder and inflated.Citation2
The transurethral catheter safety valve (Class Medical Ltd, Annacotty, County Limerick, Ireland) is a single-use device consisting of a flow restrictor and a pressure valve. () The red tab is pulled before using the safety valve. The safety-valve’s female luer lock is fitted to a 10 mL syringe containing water and the male luer slip is attached to the balloon port of the Foley catheter (). The catheter retaining balloon is then inflated as normal, allowing 10 to 15 seconds longer because the flow restrictor in the valve causes it to take longer to inflate. When the balloon is correctly placed in the lumen of the urinary bladder and then inflated, there will be no leakage of water from the safety port. After inflating the balloon, the safety-valve and the 10 mL syringe should be immediately disconnected. Failure to do so may result in the catheter balloon deflating and the catheter slipping from the urinary bladder.
Figure 2 Transurethral catheter safety valve is attached to the balloon inflation channel of a Foley catheter. A 10 mL syringe filled with sterile water is connected to the safety valve.
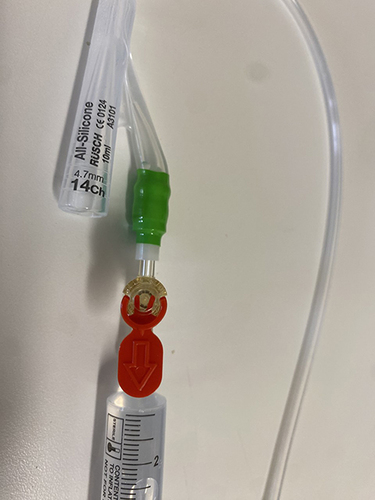
If the balloon is misplaced in the urethra and inflated, the pressure valve is activated. Any fluid pushed into the balloon channel of the catheter at that point leaks out of the safety-valve and balloon inflation ceases, indicating that the balloon is not inside the bladder. At this point the balloon should be deflated, fluid drawn back into the syringe, and the catheter removed. A senior health professional should be called to perform catheterisation.
Before inserting the Foley catheter into the urethra, the balloon of the Foley catheter should be inflated with 2–5mL of water before attaching the valve and then deflated fully by removing the water. This “pre-valve inflation” step overcomes the baseline resistance pressure of the anchoring balloon, and it prevents fluid leaking from the valve when the catheter is in the correct position.
The catheter safety-valve remains external to the human body. The catheter safety-valve is a single-use sterile item.
Scientific Principle of the Safety-Valve
The safety-valve functions as a one-way pressure relief valve that allows pressurised fluid to flow from an auxiliary passage out of the system in a regulated manner. The purpose of the pressure valve is to prevent inflation of the Foley catheter’s anchoring balloon when inadvertently misplaced in the normal, healthy urethra and to allow inflation when correctly positioned in the urinary bladder. Once the valve has “popped”, it automatically deactivates so that the valve can be used repeatedly. The flow restrictor prevents rapid inflation of the anchoring balloon, which has the potential to allow a portion of the fluid to bypass the pressure valve and cause partial inflation of the balloon even when mispositioned in the normal, healthy urethra.
In experimental studies, urethral rupture in the thinnest urethras occurred when external urethral diametric stretch was 1.26 and greater, and balloon inflation pressures of 120 kPa or more.Citation3 A mean balloon inflation pressure across the 3 urethral ruptures was 165.16 kPa. The safety-valve eliminates user variability in inflation of the anchoring balloon and restricts balloon inflation pressure to below a safe level of under 150 kPa.Citation4,Citation5
Pilot Study in a Spinal Unit
We investigated the feasibility of using the safety-valve during urethral catheterisation of male persons with spinal cord injury. The study was conducted in a regional spinal injuries centre in the northwest of England.
If there is leakage of water from the safety-valve during inflation of the anchoring balloon, this will indicate that the catheter balloon is not located within the urinary bladder and inflation of the anchoring balloon may lead to urethral trauma. This study will indicate how frequently the catheter is misplaced during catheterisation of male spinal cord injury patients in the spinal unit and whether using the safety-valve can prevent such potential mishap. Further, we wished to know any shortcomings of the safety-valve.
Methodology
The use of the safety-valve for this pilot study was approved by the New Interventional Procedures, Techniques and Advancing Practice Committee of Southport and Ormskirk Hospital NHS Trust. The audit was approved by the Clinical Lead for Audit in the spinal unit and by the Clinical Audit and Effectiveness Officer of the hospital. The audit proposal approved by the relevant officers and the audit certificate are appended as Supplementary Files.
Both authors underwent online training by the Chief Medical Officer of Class Medical, Ireland and in person demonstration of the safety-valve by a staff member of MedTech Connect Ltd, England. Subsequently, the senior author (BMS) trained the nurses in the spinal unit how to use the safety-valve. The safety-valve was used during routine catheterisation of male persons with spinal cord injury during a four-month period (January to April 2022). An evaluation form was filled by the health professional online immediately after using the safety-valve during urethral catheterisation of male patients. Data was stored securely in the hospital intranet.
As per our routine practice, we discussed the use of safety valve with the patients, provided relevant information, discussed the risks and benefits, gave time for the patients to think, reflect and decide. We proceeded with the use of the safety valve if the patients gave informed consent. Our policy has always been to respect the patient’s decision and wishes and work in partnership with the patients.
Results
All patients gave verbal informed consent. No patient asked for additional information or declined.
The transurethral catheter safety-valve was used in 44 urethral catheterisations of male spinal cord injury patients in the spinal unit. The findings are summarised in .
Table 1 Summary Sheet of the Evaluation Form for the Use of the Safety Valve
All health professionals found it easy to use the safety-valve during urethral catheterization. There was no adverse event related to the use of the safety-valve. No patient developed bleeding from the urethra during or after catheterization.
In 21 of 44 catheter insertions, urine did not come out of the catheter when the catheter was inserted. These patients underwent routine change of catheter. Therefore, the urinary bladder was empty when catheterisation was done and safety valve was used.
Leakage of water from the safety port of the valve was observed during three urethral catheterizations. In the first instance (marked with one asterisk in the table), the senior nurse felt that the catheter was correctly placed; therefore, the nurse decided to leave the catheter in place. Subsequently, the catheter drained urine; there was no bleeding per urethra. The safety valve could not be checked because the used transurethral catheter safety valve had been discarded. It is possible that leakage of water occurred from the safety port in this patient because the user did not follow the instructions to inflate and deflate the balloon of the Foley catheter before inserting the catheter into the urethra. (Question 3 in the table). Some of the Foley catheters’ anchoring balloons have a baseline resistance pressure that is >150kPa before they are inflated for the first time. For this reason, the manufacturers advise to instil 2–5mL of water into the balloon port of the catheter before attaching the valve and then remove/ extract the 2–5mL of water. This “pre-valve inflation” step overcomes the baseline resistance pressure of the anchoring balloon, and it prevents fluid leaking from the valve when the catheter is in the correct position. This step is explained in step one of the instructions for the use of transurethral catheter safety valve.Citation6
In the second instance when leakage of water was observed (marked with two asterisks in the table), the catheter was removed and then reinserted. When the Foley balloon was inflated. Leakage of water occurred the safety port of the valve. The safety-valve was disconnected from the catheter. Water was injected as though the balloon of the catheter was being inflated. There was leakage of water through the safety port even when the valve was not connected to a catheter. This confirmed that the safety-valve was defective.
In the third case where leakage of water was observed while inflating the balloon (marked with three asterisks in the table), the catheter was removed and reinserted. There was no leakage of water from the safety port of the valve following reinsertion of the catheter. This indicated that the anchoring balloon of the Foley catheter was not in the right place when the catheter was inserted initially. The safety-valve prevented a mishap. Leakage of water alerted the health professional to the misplacement of the catheter. Immediately, the doctor stopped inflating the balloon of the Foley catheter and prevented urethral trauma. Thus in one out of 44 catheter insertions, the catheter had been misplaced and the safety-valve protected the patient from developing urethral trauma. This is the real value of the transurethral catheter safety-valve.
Use of the Safety-Valve in Spinal Injury Persons with Dilated Prostatic Urethra or Wide-Open Bladder Neck
In a few spinal injury patients, the prostatic urethra may be dilated because of repeated misplacement of the Foley catheter and inflation of the Foley balloon in the prostatic urethra over several weeks or months. Or a patient may have undergone transurethral resection of bladder neck and prostate in the past. The urethral catheter safety-valve restricts balloon inflation pressure to below a safe level of under 150 kPa. The pressure required to inflate the anchoring balloon of a Foley catheter in a chronically dilated urethra may be well below 150 kPa. In patients with a chronically dilated prostatic urethra, the water may not leak through the safety port of the valve when inflating the balloon of a Foley catheter is misplaced in the dilated urethra. Therefore, in spinal injury patients with dilated prostatic urethra, the health professional should not rely upon the safety-valve to indicate correct positioning of the anchoring balloon in the urinary bladder.
Discussion
The risk factors for intra-urethral Foley catheter balloon inflation in spinal cord-injured patients include lack of sensation in urethra as a result of spinal cord injury, trauma to urethra during previous catheterisations resulting in urethral false passage, spasm of pelvic floor muscles and urethral sphincter which prevent the catheter from entering the bladder and leads to curling of Foley catheter in urethra, altered anatomy of lower urinary tract due to surgery in the past eg bladder neck resection, sphincterotomy. However, most cases where a catheter is misplaced, represent a failure of the correct catheterisation technique eg, where inadequate lubrication is used, or the balloon is inflated as soon as there is a return of urine and the tip of the catheter is in the bladder, but the balloon still resides in the prostatic or bulbous urethra. We believe that misplacement of the urethral catheter is often due to a failure of adequate education and training of nurses and doctors in the correct technique of urethral catheterisation. The question then arises as to whether the funds to pay for the devices such as the safety valve would be better spent on education and training of health professionals.
Intra-urethral Foley catheter balloon inflation in spinal cord-injured patients can be prevented by adequate lubrication, avoiding use of size 12CH in difficult cases due to risk of curling of the catheter, inserting the catheter to the hub, and inflating the balloon only when there is no resistance (an experienced person should be able to tell if the balloon is inflating properly), and flushing the catheter with 50 mL of 0.9% sodium chloride before inflating the balloon to help detect problems (ie if it bypasses right out the urethra, or will not inject at all). The safety valve may be used as an additional aid to validate correct positioning of the catheter. But the use of a safety valve should not be a substitute or replace the need for education, training, and good practice in urethral catheterisation.
We did not test the safety valve while 10% glycerine solution was used to inflate the balloon of the silicone Foley catheter. Some health professionals recommend 10% glycerine solution as filling medium to inflate the balloon of a silicone Foley catheter due to the higher rate of diffusion of sterile water compared with glycerine from the balloon of a Foley catheter. The balloon catheters made of silicone, which have been inflated with a glycerine solution, showed that the reduction in fill volume after an indwelling time of six weeks is negligible, whereas balloons filled with water suffer a reduction of over 50%.Citation7
The present audit on the use of catheter safety valve is unique due to the following reasons:
This is the first report of the use of the safety valve in patients with spinal cord injury and neuropathic bladder.
We discuss the limitations of using the safety valve in these patients. We highlighted potential shortcomings of the safety valve in patients with spinal cord injury and neuropathic bladder.
We discuss the role of catheter safety valve in the overall patient care; we reiterate that the use of a safety valve should not replace the need for education, training, and good practice in urethral catheterization.
Conclusion
In one out of 44 catheter insertions, the anchoring balloon of Foley catheter had been misplaced during routine catheterization. Leakage of water from the safety port of the transurethral catheter safety-valve alerted the health professional that the anchoring balloon was not located within the urinary bladder. The Foley catheter was removed and then repositioned in the right place. Thus, the transurethral catheter safety-valve protected this patient from sustaining iatrogenic urethral trauma.
Therefore, it is advisable to use the safety-valve routinely during urethral catheterisation of male patients in the spinal unit to prevent urethral trauma caused by inflation of the balloon of Foley catheter in the urethra. However, the health professionals should remember the few shortcomings of the safety-valve as listed below:
Very rarely, the transurethral catheter safety-valve may be defective. Therefore, health professionals should check the valve prior to attaching the valve to the Foley catheter. If the valve is defective, there will be leakage of water through the safety port when water is injected. If the valve is found to be defective, the valve, which has not been used yet in the patient, should be stored safely, and sent to the manufacturers for investigation.
The balloon of the Foley catheter should be inflated and deflated before urethral catheterization. If this step is not followed, there could be leakage of water through the safety port of the valve may occur even when the anchoring balloon of Foley catheter is placed within the lumen of the urinary bladder. Therefore, it is mandatory to follow the above step in all cases.
The safety-valve restricts balloon inflation pressure to below a safe level of under 150 kPa. The pressure required to inflate the anchoring balloon of a Foley catheter in a chronically dilated urethra may be well below 150 kPa. Thus, water may not leak through the safety port of the valve when inflating the balloon of a Foley catheter misplaced in a chronically dilated prostatic urethra. Therefore, in spinal injury patients with dilated prostatic urethra, the health professional should not rely upon the safety-valve to indicate correct positioning of the anchoring balloon in the urinary bladder.
Data Sharing Statement
All relevant data are provided in the manuscript. Details of individual patients such as names, date of birth, NHS number were removed to maintain confidentiality of the patients.
Ethical Statement
This study was approved by the Clinical Audit and Effectiveness Office of the Southport and Ormskirk Hospital NHS Trust vide audit number 22–154 “Audit on the use of transurethral catheter safety valve”. The use of the catheter safety valve was approved by the New Interventional Procedures, Techniques and Advancing Practice Committee of the Southport and Ormskirk Hospital NHS Trust. The safety valve was shown to the patients, the authors discussed how the safety valve worked and the potential benefit of using a safety valve and the shortcomings including the cost of the safety valve. Following the discussion, the patients gave verbal informed consent to use the safety valve during urethral catheterisation. No patient declined. This audit is a shared venture between the health professionals and the patients. This study complies with the Declaration of Helsinki.
Disclosure
All authors declare no conflicts of interest in this work.
Acknowledgment
The authors are grateful to Mr Niall Davis, Consultant Urological Surgeon, Royal College of Surgeons in Ireland, Tissue Engineering Research Group, Dublin, Ireland for valuable comments; Dr Hugh Flood, Chief Medical Officer, Class Medical, Unit 1 D, Annacotty Business Park, County Limerick, Ireland for online discussion; Mr James Wright, Managing Director, MedTech Connect Ltd, Saint Helens, England for providing the safety valves for this study. The authors are most grateful to Mr James Wright for payment of the article processing fee for this manuscript.
References
- Subramanian V, Soni BM, Hughes PL, Singh G, Oo T. The risk of intra-urethral Foley catheter balloon inflation in spinal cord-injured patients: lessons learned from a retrospective case series. Patient Saf Surg. 2016;10(1):14. doi:10.1186/s13037-016-0101-1
- Davis NF, Cunnane EM, Mooney RO, Forde JC, Walsh MT. Clinical evaluation of a safety-device to prevent urinary catheter inflation related injuries. Urology. 2018;115:179–183. doi:10.1016/j.urology.2018.02.026
- Davis NF, Cunnane EM, Mooney RO, Hess J, Walsh MT. Characterisation of human urethral rupture thresholds for urinary catheter inflation related injuries. J Mech Behav Biomed Mater. 2018;83:102–107. doi:10.1016/j.jmbbm.2018.04.015
- nice.org.uk. TUC Safety Valve to prevent balloon inflation in the urethra during transurethral catheterisation Medtech innovation briefing; 2020. Available from. www.nice.org.uk/guidance/mib210. Accessed September 8, 2022.
- Davis NF, Mooney RO, Cunnane CV, Cunnane EM, Thornhill JA, Walsh MT. Preventing urethral trauma from inadvertent inflation of catheter balloon in the urethra during catheterization: evaluation of a novel safety syringe after correlating trauma with urethral distension and catheter balloon pressure. J Urol. 2015;194(4):1138–1145. doi:10.1016/j.juro.2015.02.083
- Instructions for use for the TUC safety valve. Available from: www.https//classmedical.ie/pages/ifu. Accessed January 12, 2023.
- aresmedikal.com.tr. Rüsch PROFILCATH AQUAFLATE GLYCERINE The exceptional solution. Available from: 940557-000001_ProfilCath_1007.pdf. Accessed February 6, 2023.

