Abstract
Background
Compared to direct laryngoscopy, videolaryngoscopy (VL) can provide improved laryngeal and glottic view, higher intubation success rates in patients with a known or predicted difficult airway and reduced incidence of laryngeal/airway trauma. However, the cost and availability of these devices handicap its use in resource-restricted facilities. The objective was to design and manufacture a novel VL using additive manufacturing (AM) and evaluate its usability on an intubation manikin by comparing it to one of the most common video laryngoscopes used in clinical practice, the CMAC®, by measuring the time to first pass of the endotracheal tube as the main outcome.
Methods
A randomised cross-over study was performed with 36 anaesthetists attempting tracheal intubation of a manikin. The novel 3D-printed hyperangulated VL blade was compared to a CMAC® VL (D-blade). Participants had no prior experience or training with the novel device. The participants included consultants, registrars/trainees and medical officers in the Department of Anaesthesiology at the University of the Free State (UFS) in South Africa.
Results
The CMAC® had a statistically shorter time to first pass (median 13.8 seconds) compared to the 3D-printed model (median 19.0 seconds) (95% confidence interval [CI] 1.0–6.2; P=0.0013). No failed attempts occurred with either device.
Conclusion
Intubation times were faster with the CMAC® than with the novel device. However, with a comparable intubation success rate, 3D printing technology potentially can improve access to video laryngoscopy. Further design improvements, validation of materials and manufacturing processes are required before 3D-printed laryngoscope blades can be used in human subjects.
Introduction
Since 2000, several different indirect video laryngoscopes (VLs) have been developed.Citation1 The VL can provide an improved laryngeal and glottic view, a higher intubation success rate in patients with a known or predicted difficult airway and a reduced incidence of laryngeal/airway trauma compared to direct laryngoscopy.Citation2–4 Videolaryngoscopy devices have become more affordable in recent years but are still relatively expensive and underutilised in modern airway management.Citation5
The basic design of a VL consists of an instrument to displace the tongue and soft tissue, namely the laryngoscope blade, a camera and a projection device. Due to the ubiquitous availability of smartphones and their increased use in clinical practice,Citation6 we attempted to manufacture a novel laryngoscope blade that can be connected to a smartphone to form a fully functional VL to intubate a manikin.
Additive Manufacturing (AM) is defined by the American Society for Testing and Materials (ASTM) F2792 as “a process of joining materials to make objects from 3D model data, usually layer upon layer, as opposed to subtractive manufacturing methodologies”.Citation7 AM includes a wide variety of processes that use different materials and binding mechanisms. For this study, entry-level Fused Deposition Modelling (FDM) was used. FDM printers selectively extrude plastic filament in adjacent lines to form a layer. Once the layer is completed, it extrudes the same plastic filament onto the previously extruded layer, thus producing a 3D object.Citation8 These entry level FDM printers are readily available and have opened numerous opportunities to produce on-demand spare parts or final-use products. An extensive variety of polymer filaments are available that can be processed by FDM printers.
FDM printing has been used extensively in recent years to manufacture “do-it-yourself” medical devices. A variety of medical device designs are freely available on websites such as ThingiverseCitation9 and Instructables.Citation10 In a previous preliminary study, Cohen and Nishioka investigated the usability of a 3D-printed laryngoscope.Citation11 Their aim was to compare the performance of a low-cost, 3D-printed video laryngo-borescope blade and a standard Macintosh blade for endotracheal intubation for success rate and time to intubate on a difficult airway simulator. When used by anaesthesia providers, the 3D-printed blade showed superiority with first pass success rate, Cormack-Lehane view and time to see the laryngeal inlet. The data also suggested the 3D-printed blade could be superior for time to intubate. In that preliminary study, the 3D-printed video laryngo-borescope blade was found to be a feasible, low-cost option for difficult airway situations. However, this study was limited by not addressing patient safety, strength and sterility of the device for patient use. Additionally, the VL was not compared to a device of the same technology.Citation11 De Villiers and co-workers recently found that their novel low-cost disposable hyperangulated VL was clinically equivalent to the industry standard CMAC® VL (Karl Storz SE & Co. KG.; Tuttlingen, Germany) with its D-blade when intubating a simulated difficult airway.Citation12
The study aimed to determine the usability of a 3D-printed hyperangulated VL blade for intubation of a manikin, compared to the CMAC® VL with its D-blade, by comparing the time to first pass of the endotracheal (ET) tube through the vocal cords as the primary outcome.
Methods
Ethics Approval
This randomised cross-over study was approved by the Health Sciences Research Ethics Committee (HSREC) of the University of the Free State (UFS) (ethics clearance reference number UFS-HSD2019/1980/2605) after registration with the Free State Province Department of Health. The study was conducted at Universitas Academic Hospital, a tertiary-level training hospital in Bloemfontein, South Africa, between October 15, 2020 and December 19, 2020. Written informed consent was obtained from all the participants.
Inclusion Criteria
Consultants, trainees/registrars and medical officers working in the Department of Anaesthesiology at the UFS at the time of commencing the study were able to participate in this study.
Exclusion Criteria
The consultant taking part in the data collection, members of the research project, members of the department on leave at the time of data collection, and any refusal of participation were applied as exclusion criteria.
The Novel Device
A 3D-printed hyperangulated VL blade with a handle was designed and manufactured by the Department of Anaesthesiology at the UFS in collaboration with the Product Development and Technology Station (PDTS) based at the Central University of Technology Free State (CUT). The best features from multiple designs were combined and evaluated to create the final design. This final design consisted of a rounded handle with four finger grips for an ergonomic grip and a hyperangulated blade profile with a cut-away on the right-hand side to form a channel for an ET tube to pass. The sleeve for fitting the borescope camera was an oval-shaped cut-away to prevent the sleeve and camera from rotating ().
Figure 1 Computer-assisted design model of video laryngoscope blade. (A) Oblique view of the handle. (B) Frontal view of the blade tip. (C) Endotracheal tube cut-away in early design.
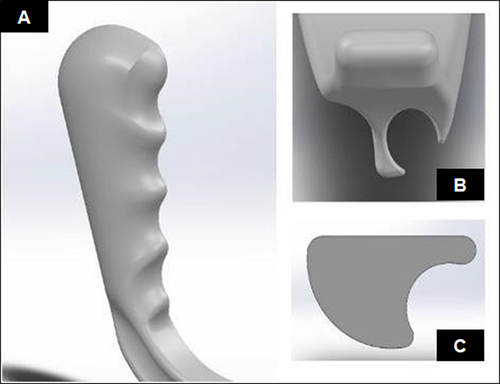
The camera was also offset to the left to create space for the ET tube and positioned so that the blade did not obstruct the view of the camera (except for the tip of the blade assisting the user in orientating the blade position). A commercially available 6.5 mm borescope camera was inserted into the blade and attached to a Samsung® Galaxy A11 smartphone (Samsung Group; Seoul, South Korea) (). The collaborative development process and testing of materials was published in the South African Journal of Industrial Engineering in 2020.Citation13
Study Process
Participants in this study were asked to intubate a Laerdal Airway Management Trainer (Laerdal Medical Corporation; Wappingers Falls, NY, USA) using the CMAC® VL with its D-blade, as well as a novel 3D-printed VL attached to a 6.5 mm borescope camera and a Samsung® Galaxy A11 smartphone.
Forty-seven eligible participants in the department were given a two-week period to volunteer for participation in this study. Thirty-six participants enrolled, including 11 consultants, 21 registrars/trainees and four medical officers. The participants were randomised by an independent biostatistician using a computer-generated randomisation list to determine which device would be used first.
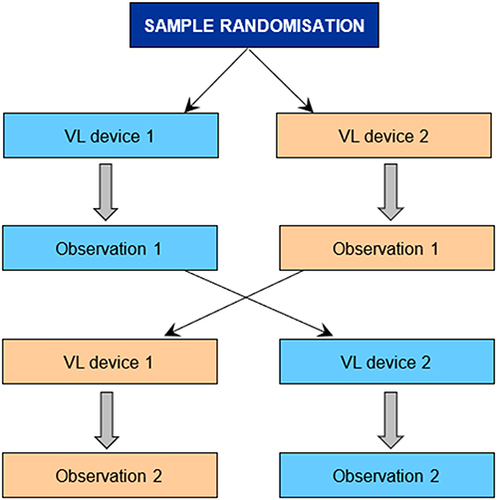
An intubation workstation was set up in a standardised configuration. Of note was that for both devices, the size 7.0 ET tube was preloaded with an intubation stylet that was preformed to fit the profile of the hyperangulated blade that was used. The ET tube was lubricated by the investigator to prevent damage to the airway trainer. A video recording of the attempts at intubation was made. Once the recording of the first device used was completed, the workstation was reset to the same standard configuration for the second device. After approximately one minute, the second observation was recorded (). The participants had no previous experience or training with the novel device.
Figure 3 Diagrammatic presentation of the study process showing sample randomisation. Participants were randomised to either of the two devices for their first attempt at intubation. A videorecording of this attempt was made. Thereafter, the alternative device was used in the same manner.
A non-participating consultant from the anaesthesiology department was asked to conduct the measurements of time to seeing the vocal cords, time to first pass of the ET tube through the vocal cords, determining the Cormack-Lehane score and stating whether it was a successful intubation attempt or not. This assessment was done by watching the recordings of the attempts.
The cross-over design of the study was chosen to evaluate the presence of memory bias towards the instrument used in the second attempt.Citation14
Statistical Analysis
Categorical variables were summarised by frequencies and percentages, and numerical variables by medians, interquartile ranges and ranges, due to skew distributions. Categorical variables were compared using chi-square, Fisher’s exact or McNemar tests. Numerical variables were compared using Mann–Whitney and Wilcoxon signed-rank tests. Analysis was performed by the Department of Biostatistics, UFS, using SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
The median age of the participants was 34 years (range 28–58 years) with a median of three years (range 1–15 years) of experience in anaesthesiology. The findings for the main outcomes are summarised in .
Table 1 Comparative Summary of the Main Outcomes (Time to Seeing Vocal Cords and Time to First Pass) Achieved with the CMAC® Video Laryngoscope and the Novel Device (N=36)
The median time to seeing the vocal cords with the CMAC® was 4.4 seconds less than with the novel device. The 95% confidence interval (CI) for the median difference was –5.7 to –3.5. With the CMAC®, the median time to first pass was 5.2 seconds less than with the novel device. The 95% CI for the median difference was –6.2 to –1.0, P=0.001.
The Cormack-Lehane score was used to evaluate the best laryngeal view. Only class 1 and 2 views were obtained with both devices. The CMAC® had 80.6% (n=29) class 1 views and the novel device 50.0% (n=18) (P=0.0045). No failed attempts occurred with either device, representing a 100% success rate for intubation.
The results when separating the observations into the group that used the CMAC® as their first modality and the group using the novel device as the first modality are shown in . For participants who used the CMAC® first, both the time to seeing the vocal cords and the time to first pass were significantly shorter with the CMAC® (P<0.0001 and P=0.009, respectively). When the novel device was used first, the time to seeing the vocal cords was still significantly shorter with the CMAC® (P<0.0001) but not the time to first pass (P=0.1084).
Table 2 Comparative Summary of the Main Outcomes (Time to Seeing Vocal Cords and Time to First Pass) According to Device Use First
Participants who used the CMAC® first obtained 72.2% class 1 views with the CMAC® and 50.0% class 1 views with the novel device (P=0.1573). In comparison, participants who used the novel device first obtained 88.9% class 1 views with the CMAC® and 50.0% with the novel device (P=0.0082). The only statistically significant difference between participants who used the novel device first and those who used the CMAC® first, was that the former group had a significantly shorter time to seeing the vocal cords with the novel device (medians 8.5 and 11.3 seconds, respectively; P=0.0162).
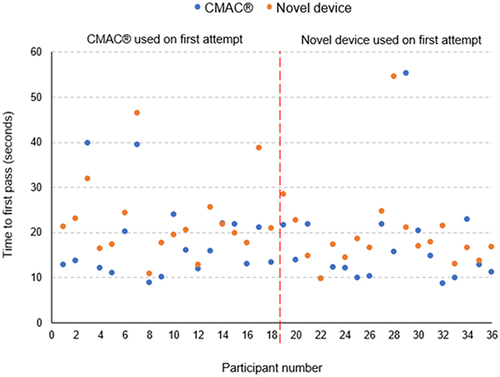
The dot graph () illustrates the time to first pass of each modality.
Figure 4 Time to first pass of CMAC® and novel device of all participants. The dot chart depicts participants time to first pass using the two different modalities. The vertical axis represents the time to first pass of the endotracheal tube. The blue and orange dots, respectively, represent the time to first pass with the CMAC® and the novel device. The horizontal axis corresponds with each of the participants and their matching attempts. The first 18 participants on the graph used the CMAC® for their first attempt and the second 18 participants used the novel device on their first attempt. Outlying measurements occurred with both modalities. Participant 22 had approximately the same numerical value for both devices, with participants 12 and 35 showing closely similar results.
Discussion
Key Results
A statistically significant difference in the median time to first pass of the CMAC® and the novel device was observed. The faster intubation times with the CMAC® could be explained by the participants’ previous experience with the CMAC®, as this is the VL they have access to as practitioners in our facility. No prior training with or exposure to the novel device was provided. The authors are of the opinion that with experience and training with the novel VL, first pass intubation times can improve substantially.
Despite no previous training, no failed attempts occurred when intubating a manikin with the novel device. Further research is needed to investigate the association between successful intubation with VL and previous VL experience across different devices. Determining this possible association was beyond the scope of the study and no data in this regard were collected.
Better laryngeal views were obtained using the CMAC®, which could be attributed to the participants’ previous experience and usage of the CMAC® in our facility. Our impression was that the image obtained by the novel device displayed enhanced clarity and contrast, although it had a slightly smaller angle of view. The wider angle of view in the CMAC® allows the observer to see more of the pharyngeal and glottic structures. The difference in laryngeal views can also be ascribed to a better hyperangulated blade angle and compact blade design of the CMAC®. Design alterations can easily be made to the novel device to overcome these challenges.
Participants who used the novel device first obtained more class 1 views with the CMAC than with the novel device. This finding might point to a memory bias in the performance of VL with two different VL devices in quick succession.
Interpretation
With reference to , the major trend observed was that the CMAC® had faster time to first pass in general. Both modalities have most of the intubation times within acceptable clinical limits. The minimum and maximum values for the time to first pass of the two devices were similar. Two sets of outlying measurements were from the same participant, which could be attributed to operator skill or experience. Three other single measurements were from different participants and with different devices and appeared to be random in nature.
The absence of any failed attempt suggests that intubation can be performed successfully with both modalities. It should be noted, however, that we do not know the performance of the novel device in humans. This observation would be expected from a well-known and commonly used instrument such as the CMAC®, which was supported by this study’s outcomes. Considering that the CMAC® is a very well established VL and in many centres regarded as the gold standard VL, it should be noted, however, that the difference in times to seeing the vocal cords and time to first pass was not as pronounced as might be expected.
Limitations
There was no time limit placed on the intubation attempts made by participants. A time limit would be prudent when conducting a similar study on human subjects. Introducing a time limit would likely increase the number of failed attempts during this study, as desaturation of patients during intubation in real-world conditions would necessitate aborting the attempt in favour of oxygenating the patient with manual bag mask ventilation in keeping with sound clinical practice guidelines.Citation15 The maximum time to first pass with the CMAC® was 55.1 seconds and 54.4 seconds with the novel device. These maximum times should not cause a noticeable desaturation in an adult pre-oxygenated American Society of Anesthesiologists (ASA) I or II patient.Citation16,Citation17 Benumof and co-workersCitation18 used physiologic modelling to calculate the time to desaturation of SpO2 < 90% after the administration of succinylcholine for various patient groups following apnoea. The times to this level of desaturation were 8 minutes for healthy adults, 5 minutes for moderately ill adults, and 2.7 minutes for obese adults.Citation18
Using the Cormack-Lehane classification to evaluate the quality of the laryngeal view during videolaryngoscopy has been found to be sub-optimal in previous studies. Alternative methods, such as the video classification of intubation (VCI) score or the percentage of glottic opening (POGO) scale, have been suggested as giving a better description and being clinically more relevant for the laryngeal view during videolaryngoscopy.Citation19,Citation20
The non-participating consultant who recorded the times and evaluated the laryngeal views noted that there was no option for a class 2b Cormack-Lehane score.Citation21,Citation22 However, this score was only needed for one (1.4%) of 72 measurements.
The use of a manikin was necessitated by the lack of certification of the novel VL for use in human subjects because it is an electronic medical devices. However, due to this situation, the same intubating conditions for each attempt was ensured. Airway trainers and human subjects differ anatomically.Citation23,Citation24 General consensus is that it is not possible for the synthetic materials used to manufacture the manikin to simulate the same tissue compliance and the ability to manipulate the mouth and pharyngeal structures as it would be with human tissue. However, no research-based evidence for this point of view is available. Schalk and co-workersCitation25 noted that the dimensions of the Laerdal Airway Management Trainer were at least satisfactory. They suggested that the widespread use of airway management trainers as a replacement for in vivo trials in the field of airway management needs to be reconsidered.Citation25
Conclusion
After determining the usability of a 3D-printed VL by acceptable clinical first pass intubation times, comparable laryngeal views and a first pass success rate of 100%, this study supports the feasibility of developing a low-cost VL by means of 3D printing. 3D-printed medical devices can be used in resource-poor institutions and hospitals, especially in the low- to middle-income countries where academic and technical universities as well as private individuals have the expertise and equipment to produce these devices. Innovation with modern technologies can improve access to VL. Validation of materials, design processes and the safety of the use of electronic devices in human subjects are yet to be determined for this innovation.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest.
Acknowledgments
We would like to thank the following participating centres for their contributions to the success of this trial in the interest of advancing medical care through innovation: the Product Development and Testing Station, Central University of Technology Free State and Universitas Academic Hospital, Bloemfontein, South Africa. The authors acknowledge Dr. Daleen Struwig, medical writer/editor, Faculty of Health Sciences, UFS, for technical and editorial preparation of the article.
Additional information
Funding
References
- Pieters BM, Eindhoven GB, Acott C, van Zundert AAJ. Pioneers of laryngoscopy: indirect, direct and video laryngoscopy. Anaesthesia and Intensive Care. 2015;43(1_suppl):4–11. doi:10.1177/0310057X150430S103
- Lakticova V, Koenig SJ, Narasimhan M, Mayo PH. Video laryngoscopy is associated with increased first pass success and decreased rate of esophageal intubations during urgent endotracheal intubation in a medical intensive care unit when compared to direct laryngoscopy. J Intensive Care Med. 2015;30(1):44–48. doi:10.1177/0885066613492641
- Lewis SR, Butler AR, Parker J, Cook TM, Smith AF. Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation. Cochrane Database Syst Rev. 2016;11(11):CD011136. doi:10.1002/14651858.CD011136.pub2
- Michailidou M, O’Keeffe T, Mosier JM, et al. A comparison of video laryngoscopy to direct laryngoscopy for the emergency intubation of trauma patients. World J Surg. 2015;39(3):782–788. doi:10.1007/s00268-014-2845-z
- Zaouter C, Calderon J, Hemmerling TM. Videolaryngoscopy as a new standard of care. Br J Anaesth. 2015;114(2):181–183. doi:10.1093/bja/aeu266
- Boissin C, Fleming J, Wallis L, Hasselberg M, Laflamme L. Can we trust the use of smartphone cameras in clinical practice? Laypeople assessment of their image quality. Telemed J E Health. 2015;21(11):887–892. doi:10.1089/tmj.2014.0221
- Morrison RJ, Kashlan KN, Flanangan CL, et al. Regulatory considerations in the design and manufacturing of implantable 3D-printed medical devices. Clin Transl Sci. 2015;8(5):594–600. doi:10.1111/cts.12315
- Palermo E Fused deposition modeling: most common 3D printing method [homepage on the internet]; 2013. Available from: https://www.livescience.com/39810-fused-deposition-modeling.html. Accessed January 24, 2023.
- MakerBot. Thingiverse – digital designs for physical objects [homepage on the internet]; 2022. Available from: https://www.thingiverse.com/. Accessed January 24, 2023.
- Instructables – Yours for the making [homepage on the internet]; 2022. Available from: https://www.instructables.com/. Accessed January 24, 2023.
- Cohen T, Nishioka H. Abstract #10: comparison of a low-cost 3D printed video laryngo-borescope blade versus direct laryngoscope for simulated endotracheal intubations. Proceedings of the Society for Technology in Anesthesia (STA) 2017 Annual Meeting, January 11–14, 2017, San Diego, California. Anesth Analg. 2017;124(5S):32–34.
- De Villiers CT, Alphonsus C, Eave D, Hofmeyr R. Innovation in low-cost video laryngoscopy: intubator V1-Indirect compared with Storz C-MAC in a simulated difficult airway. Trends Anaesth Crit Care. 2021;40:41–45. doi:10.1016/j.tacc.2021.07.002
- Huysamen HW, Kinnear WA, Fonternel TE, Turton EW, Yadroitsava I, Yadroitsev I. 3D printed laryngoscope for endotracheal intubation. South African J Ind Eng. 2020;31(3):209–217.
- Cleophas TJ, De Vogel EM. Crossover studies are a better format for comparing equivalent treatments than parallel-group studies. Pharm World Sci. 1998;20(3):113–117.
- Higgs A, McGrath BA, Goddard C, et al. Guidelines for the management of tracheal intubation in critically ill adults. Br J Anaesth. 2018;120(2):323–352. doi:10.1016/j.bja.2017.10.021
- Weingart SD, Levitan RM. Preoxygenation and prevention of desaturation during emergency airway management. Ann Emerg Med. 2012;59(3):165–175.e1. doi:10.1016/j.annemergmed.2011.10.002
- Roppolo LP, Wigginton JG. Preventing severe hypoxia during emergent intubation: is nasopharyngeal oxygenation the answer? Crit Care. 2010;14(6):1005. doi:10.1186/cc9197
- Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg intravenous succinylcholine. Anesthesiology. 1997;87(4):979–982. doi:10.1097/00000542-199710000-00034
- Chaggar RS, Shah SV, Berry M, Saini R, Soni S, Vaughan D. The Video Classification of Intubation (VCI) score: a new description tool for tracheal intubation using videolaryngoscopy: a pilot study. Eur J Anaesthesiol. 2021;38(3):324–326. doi:10.1097/EJA.0000000000001374
- Levitan RM, Ochroch EA, Kush S, Shofer FS, Hollander JE. Assessment of airway visualization: validation of the percentage of glottic opening (POGO) scale. Acad Emerg Med. 1998;5(9):919–923. doi:10.1111/j.1553-2712.1998.tb02823.x
- Yentis SM, Lee DJH. Evaluation of an improved scoring system for the grading of direct laryngoscopy. Anaesthesia. 1998;53(11):1041–1044. doi:10.1046/j.1365-2044.1998.00605.x
- Krage R, Van Rijn C, Van Groeningen D, Loer SA, Schwarte LA, Schober P. Cormack-Lehane classification revisited. Br J Anaesth. 2010;105(2):220–227. doi:10.1093/bja/aeq136
- Schebesta K, Hüpfl M, Rössler B, Ringl H, Müller MP, Kimberger O. Degrees of reality: airway anatomy of high-fidelity human patient simulators and airway trainers. Anesthesiology. 2012;116(6):1204–1209. doi:10.1097/ALN.0b013e318254cf41
- Blackburn MB, Wang SC, Ross BE, et al. Anatomic accuracy of airway training manikins compared with humans. Anaesthesia. 2021;76(3):366–372. doi:10.1111/anae.15238
- Schalk R, Eichler K, Bergold MN, et al. A radiographic comparison of human airway anatomy and airway manikins – implications for manikin-based testing of artificial airways. Resuscitation. 2015;92:129–136. doi:10.1016/j.resuscitation.2015.05.001

