Abstract
Endoluminal occlusion has been performed since the early beginning of interventional radiology. Over recent decades, major technological advances have improved the techniques used and different devices have been developed for changing conditions. Most of these occlusion devices have been implemented in the vascular territory. Early embolization materials included glass particles, hot contrast, paraffin, fibrin, and tissue fragments such as muscle fibers and blood clots; today, occlusion materials include metallic devices, particles, and liquid materials, which can be indicated for proximal or distal occlusion, high-flow and low-flow situations, and in large-caliber and small-caliber vessels, based on need. Technological progress has led to a decreased size of delivery catheters, and an increase in safety due to release systems that permit the withdrawing and replacement of embolization material. Furthermore, bioactive embolization materials have been developed to increase the efficacy of embolization or the biological effect of medication. Finally, materials have been modified for changing indications. Intravascular stents were initially developed to keep an artery open; however, by adding a covering membrane, these stents can be used to occlude the wall of a vessel or other endoluminal structures. This article gives an overview of the devices most utilized for occlusion of endoluminal structures, as well as their major purpose in the endovascular territory.
Introduction
Endoluminal occlusion has been performed since the early beginning of interventional radiology. However, over the last few decades, major technological advances have improved the techniques used and different devices have been developed for changing conditions. Most of these devices have been implemented in the vascular territory, and embolization is now performed for countless procedures. Through technological progress, occlusion material has been exposed to the most important changes. Some materials, such as detachable balloons have become obsolete, because they might deflate and migrate into distal vascular territories. Nowadays, better alternatives are available and conventional balloon catheters are only used for temporary occlusions, such as blocking an artery during active bleeding or in combination with other embolization material to avoid migration, or to achieve blood stasis and improve the effect of the agent.
Early embolization materials included glass particles, hot contrast, paraffin, fibrin, and tissue fragments such as muscle fibers and blood clots; today, occlusion materials are designed for proximal or distal occlusion, high-flow and low-flow situations, and for large-caliber and small-caliber vessels, based on need. The size of the catheter delivery devices has been reduced due to technological progress, and further investigations have led to bioactive materials, such as hydrocoils and embolization particles. Hydrocoils improve packing density by increasing the cross-sectional diameter, whereas embolization particles, initially developed for small artery occlusions, can be loaded with medication like chemotherapeutic agents or radiation to potentiate the biological effect of ischemia in a tumoral lesion.
An additional aspect of the technological advances includes the increase in safety. Previously, coils had to be pushed through the delivery catheter by a guide wire or had to be flushed with saline solution; today, release systems permit the withdrawing and replacement of an unsatisfactorily placed coil. Newer liquid embolization agents allow precise occlusion by controlling the amount of embolization material delivered, without the risk of the catheter tip sticking in the glue. Finally, materials have been modified for changing indications. Intravascular stents were initially developed to keep an artery open; however, by adding a covering membrane, these stents can be used to occlude the wall of a vessel or other endoluminal structures.
This manuscript gives an overview of the devices most utilized for occlusion of endoluminal structures, consisting of metal occlusion devices, particles, and liquids, and their major purpose in the vascular territory.
Metal occlusion devices
Amplatzer vascular plug
Description
An Amplatzer® vascular plug (AVP, AGA Medical, Golden Valley, MN, USA) is a braided Nitinol device that can be used to achieve permanent vessel occlusion. The AVP family includes the AVPI, AVPII, AVPIII, and AVP4.Citation1,Citation2 The AVPI is a single-lobe, single-layer Nitinol mesh in a cylindrical shape. The densely woven Nitinol mesh reduces flow velocity and induces further coagulation. The AVPI is available in different diameters, ranging from 4 mm to 16 mm at 2 mm increments. In contrast with the AVPI, the AVPII was designed as a multilayer Nitinol mesh and has a three-lobe design to increase the occlusive surface and induce faster vessel occlusion. The central cylindrical lobe is surrounded by a proximal and distal disc, which assures a stable position for the plug inside the vessel. The AVPII is available in sizes from 3 mm to 22 mm. The AVPIII device has a multilayer Nitinol mesh with two lobes. The distal lobe has a body and an extended rim whereas the proximal lobe is a single ellipsoid disc. The asymmetric, ellipsoid cross-sectional shape allows perfect adaptation to a variety of different vessel configurations. The AVPIII is available in nine sizes ranging from 4 mm to 14 mm in length in 2 mm increments. Plugs with a diameter ≥10 mm are available with a width of 3 mm or 5 mm. Finally, the AVP4 is a short, two-lobed, low-profile vascular plug that can be delivered through a 0.038 inch diagnostic catheter. The AVP4 is available in diameters of 4–8 mm.
All AVPs present radiopaque markers at the proximal and distal end of the plug. The AVPs are connected to a Nitinol delivery wire, which is connected to the plug by a microscrew at the proximal end.
For transcatheter embolization procedures, the devices can be delivered to the target arteries using guiding catheters, long sheaths in the case of the AVPI-III, or through a 0.038 inch diagnostic catheter if the AVP4 is to be used. For an adequate vessel wall apposition and to avoid migration of the device, it is recommended to oversize by 30%–50%. The vascular plug can be released by turning the Nitinol delivery wire counterclockwise, using proximal torque. The device can be recaptured and repositioned before releasing the delivery wire.Citation1
Indication
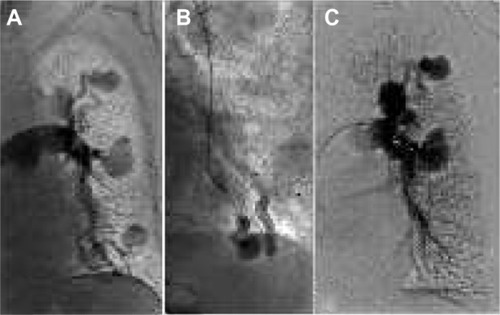
The AVP family is designed for permanent vessel occlusion.Citation1–Citation3 Depending on the blood flow and the device being used, complete occlusion can be achieved in 3–5 minutes, in the absence of coagulation disorders. Therefore, it has been indicated for use in bleeding situations.Citation4 In the case of a short landing zone, such as in a hypogastric artery occlusion before an endovascular aneurysm repair (EVAR), an AVPI is the ideal device to occlude the hypogastric artery at its origin, in order to avoid type II endoleaks and to preserve the distal gluteal vessels, further reducing the risk of buttock claudication.Citation5,Citation6 The AVPII and the AVPIII reduce the time to occlusion by creating multiple occlusive planes, and are therefore indicated for use in high-flow situations, such as occlusion of hemodialysis fistulas.Citation7 The AVP4 can be used without needing to exchange the catheter (). The plug can be applied in tortuous anatomy, and its unique design enables the vascular plug to be delivered in the distal arteries.Citation8,Citation9 The AVP has also been applied in portal vein occlusions to induce hypertrophy of the contralateral hepatic lobe and to occlude collateral pathways prior to selective internal radioembolization.Citation10,Citation11 Nonvascular applications of AVPs have been reported, such as the occlusion of bronchopleural, persistent biliary-cutaneous, and lower urinary tract fistulas.Citation12–Citation14
Figure 1 Pulmonary arteriovenous-fistula treatment.
Abbreviation: AVP, Amplatzer vascular plug.
Advantages and disadvantages
A strong advantage of the AVP is its easy and rapid delivery system. Once a target artery is catheterized, the plug can be deployed in a very short time, which reduces radiation exposure for both the patient and the operator.
Due to the wide range of plug types and sizes, almost all vascular characteristics can be covered, such as vessel size, blood flow and landing zones. The AVPII, which is available in diameters up to 22 mm, allows embolization of large-diameter arteries. In the event of insufficient occlusion, another plug can be placed to promote it. A major advantage is that the vascular plug can be recaptured and repositioned. This allows a precise placement, with low risk of mispositioning or migration. In case of incorrect sizing, the plug can be withdrawn and substituted. The AVP can also be used in combination with other embolization materials.
One major disadvantage of all the plugs is their dependence on the patient’s effective coagulation. In case of severe coagulation disorders, occlusion may not be achieved by an AVP. Occlusion time may also be unpredictable in the event of large vessel size and high-flow situations.Citation1 Distal embolization with the AVP is not possible; in these cases, use of other embolization agents might be necessary. Furthermore, depending on plug size, the AVPI-III devices need a large access sheath or guiding catheter. Finally, the delivery wires of these devices are rather rigid. Therefore, it may be difficult or impossible to navigate a larger-diameter device to its target artery.Citation4
Flow diverter
Description
Flow diverters (FDs) are new endovascular devices that are characterized by a tubular, narrow mesh. This design permits the redirection of blood flow and promotes intimal coverage of the mesh. The flow redirection decelerates the blood flow located outside the FD and therefore promotes occlusion. Redirection of blood flow facilitates a reorganization of the vessel wall by decreasing shearing forces. Furthermore, the intimal overgrowth caused by the inflammatory reaction of the vessel wall leads to complete exclusion of the dependent vascular area. Both effects are directly related to the amount of metal surface area coverage.Citation15 For optimal performance, the FD should have a low porosity (metal-free to metal-covered area) and a high pore density (number of pores per square millimeter). The narrow metal mesh can be made from different materials, such as platinum/cobalt chromium, Nitinol, or other materials.Citation16
Indication
FDs are mainly used in the cerebral arteries to treat non-ruptured, wide-neck aneurysms that are less or not amenable to endovascular coiling or open surgery. Complex or giant intracerebral aneurysms can be treated from different locations with less risk of recanalization than with conventional strategies.Citation16 FDs have also been used in peripheral arteries.Citation17
Advantages and disadvantages
FD devices are a good option to reconstruct vessel walls with endothelial overgrowth while keeping the side branches patent. This allows the placement of these devices in anatomically challenging situations. The placement of an FD can be combined with conventional coil placement, as an additional coil inside the aneurysm sac promotes occlusion. Nevertheless, the deployment of FD is sophisticated and requires a high level of experience. Because of their design, FDs must not be used in emergency situations, as complete closure of the dependent vascular area is not achieved instantly.
Covered stent/stent graft
Description
A covered stent or stent graft consists of a mesh in different shapes (the tubular one is the most common) that is covered by a membrane. A metallic stent body is commonly chosen for vascular placement, such as stainless steel or a titan nickel alloy (Nitinol). Nitinol stent bodies are manufactured from braided filaments or can be laser-cut from a Nitinol tube. In the past, autologous veins and arteries have been sutured over metallic stent bodies; however, synthetic materials are mainly used for this purpose nowadays.Citation18,Citation19 Most vascular stent grafts are covered with polyethylene terephthalate (Dacron) or polytetrafluoroethylene, as these materials are biocompatible, have high thromboresistance, and do not degrade over time.Citation18
Depending on the material of the stent graft body, covered stents can be balloon-expandable or self-expandable. Most of the available balloon-expandable stents are premounted on a balloon with a defined diameter. However, some balloon-expandable stents have to be mounted by the operator; this way, one stent can be used for different vessel diameters by preparing the stent on the most adequate balloon, after considering diameter and length. Self-expandable stents are usually loaded in a sheath. By withdrawing the sheath, the stent fully expands from the distal to the proximal end. Other techniques include liberation of the stent graft by pulling on a line so that the stent graft unfolds. In the gastrointestinal system, the use of plastic stent bodies has been reported.Citation20 Polyurethane as a covering membrane has little indication in the vascular system due to its moderate biodegradation, but has been placed in other endoluminal structures, such as the genitourinary and digestive systems.Citation21–Citation23 Silicone membranes have been used for stent covering to prevent food impaction.Citation24,Citation25 Membranes can also be loaded with different bioactive materials such as heparin to improve vascular patency.Citation26,Citation27
Indication
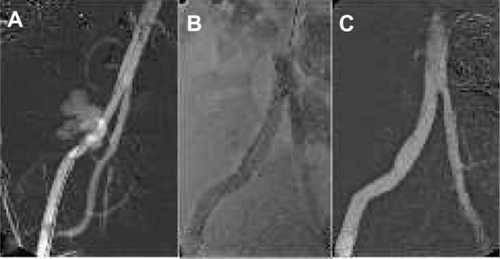
Covered stents or stent grafts have a wide variety of indications. As its main purpose is to separate an intraluminal from an extraluminal structure, the covered stents were first applied in the vascular system to control bleeding after pathologic or iatrogenic arterial lesions ().Citation28 Later on, stent grafts became an important tool in the treatment of aneurysms to prevent rupture or as a first-line treatment in already ruptured aneurysms. Modular devices have been developed to treat aneurysms involving aortoiliac bifurcation, and fenestrated and branched devices have become available for more complex aneurysm anatomies.Citation29–Citation31 Other vascular indications for stent grafts include arteriovenous fistulas and vascular graft lesions. Covered stents have also been used in the respiratory, gastrointestinal, and urinary system.Citation22–Citation25,Citation32–Citation34
Figure 2 External iliac artery rupture treatment.
Advantages and disadvantages
The main advantage of using stent grafts is that occlusion can be achieved immediately, since covered stents are not dependent on the patient’s coagulation cascade. The device geometry does not impede the passage through the stent, thus preserving the target’s physiological function.
Regarding the vascular territory, balloon-expandable stent grafts have a higher radial force than self-expandable stents; however, they do not adapt to the artery very well. Shear forces promote intimal hyperplasia at the proximal and distal ends of the stent, especially in tortuous vessels or in locations where arteries are exposed to flexion. One advantage of the balloon-expandable stent over the self-expandable stent graft is its precise placement, which justifies its use in complex anatomic conditions. Balloon-expandable stents that can be mounted by the operator reduce hospital costs because a single-size stent can be placed in different artery diameters by choosing an adequate balloon size. Furthermore, balloon-expandable stents can be used to bridge large diameter differences by using only one stent.Citation28
One major disadvantage is that this stent can be lost during passage through a vessel as the stent preparation is operator-dependent; thus, protection using a long sheath is recommended. In the gastrointestinal tract, covered stents show a higher rate of migration than bare stents as a result of peristalsis.Citation35
Coils
Description
Coils are one of the most often applied embolization devices. They consist of spirals of different materials, such as stainless steel, platinum, alloys of these, or other materials, and may be covered by synthetic material to promote coagulation. Lately, new bioactive coils have been developed that consist of a layer of acrylic hydrogel polymer, which increases the effective coil thickness up to four times once it comes into contact with blood or liquid.Citation36,Citation37 Other bioactive coils may be covered with an absorbable copolymer that disappears after a few months.
Coils come in various designs; while the “regular” coil has a helical structure, many different shapes have been developed. Coils might have a predefined two-dimensional or three-dimensional shape for optimal filling or framing. Other coils are designed with deflection points that have the ability to seek out and fill open spaces. Other characteristics are important for increasing packing volume, such as coil softness, conformability, and mechanical stability. Coils may also vary in length and loop diameter, depending on the target vessel configuration. Coils are usually supplied in an introducer sheath. They have to be introduced into a catheter by pushing the coil with a connected pusher wire or using a loader for pushable or flushable coils. Some coils can be advanced through a diagnostic catheter, while other coils, such as those used for cerebral interventions, can be pushed through microcatheters. Once the coil is delivered to its target, it has to be released mechanically either by electricity or with a hydraulic release system. Pushable coils may be transferred to their target by flushing the catheter with physiological saline solution, or they may be pushed using a correctly sized guide wire.
Indication
For interventions in small-sized arteries, such as neurovascular procedures, or intestinal vessels, microcatheters must be used for navigation through tortuous vessels due to the small target vessel size. For that reason, microcoils have to be used, and are characterized by small cross-sectional diameters (). Coils of different shapes are indicated to treat aneurysms. Framing coils, mostly used in neurovascular procedures, are usually long three-dimensional-shaped coils used to build a structure inside the aneurysm space to accommodate following coils. Dense aneurysm packing is achieved after filling the “nest” with coils of different stiffness and length. A final finishing coil, which is commonly a soft or ultrasoft coil, is used to avoid protruding loops into the vessel. Low cross-sectional-shaped coils are applied to occlude cerebral arteriovenous malformations and arteriovenous fistulas, but can also be indicated in tortuous peripheral vasculature, as diagnostic catheters may not be navigated to the target vessels and microcatheters are the preferred delivering catheter.Citation38,Citation39
Figure 3 Lower intestinal hemorrhage treatment.
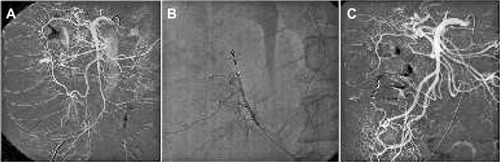
Large-diameter coils are suggested for peripheral aneurysms or peripheral vessel occlusion. High blood flow may impede adequate occlusion of the arterial system, so hydrocoils may be chosen to provide an increased filling density.Citation36 Another indication for coil placement is for the occlusion of endoleaks after EVAR.
Advantages and disadvantages
Once a catheter is navigated to the target vessel, coils can be placed safely and in a controlled way by using a release system. In the event of a failed coil placement, whether due to an unstable position or equivocal size election, the coil can be withdrawn. Most coils need an effective coagulation cascade because they may not occlude the vessel sufficiently, even if dense packing has been performed. In this regard, the hydrocoil may be a good alternative, as its increase in cross-sectional coil diameter is independent of coagulation.
Detachable coils are expensive in comparison with pushable coils; however, the lack of safety when using pushable coils limits their indications. Coil sizing is essential because coils that are too small can migrate, and oversized coils elongate and may therefore not occlude the vessel sufficiently. For coil placement, only end-hole catheters should be used, as coils may get stuck in the lateral holes of diagnostic catheters. One of the major concerns of coils is their proximal occlusion. If a coil is used in a bleeding condition, proximal occlusion may impede access to the bleeding spot in the event of an existing distal vascular network.
Others
Special indications require unique designs for vascular and nonvascular systems. For the occlusion of small vessel aneurysms, intrasaccular devices have been developed, consisting of a metallic mesh that functions as an intrasaccular FD.Citation40 For EVAR, aorto-uni-iliac endoprostheses are offered that require occlusion of the contralateral iliac axis in order to avoid type II endoleaks. For this purpose, contralateral iliac artery occlusion devices have been designed that consist of a metallic basket covered by a membrane. Other endoluminal occlusion systems are being investigated for occlusion of the venous and arterial vasculature.Citation41
Particles
Polyvinyl alcohol
Description
Polyvinyl alcohol (PVA) is a synthetic, nonreabsorbable embolic agent for permanent vessel occlusion. For many years, irregularly shaped PVA particles have been used; however, calibrated spherical microparticles have recently been introduced.Citation42 The embolization effect of earlier PVA particles was not only gained by mechanical vessel obstruction, as the irregularly shaped particles leave space for the blood to circulate, but was mainly caused by an important perivascular inflammatory reaction.Citation43 Newer microparticles do not show this inflammatory reaction.Citation44 An important physical property of PVA particles is that they tend to aggregate, so may obstruct the delivering catheter or cause undesired proximal vessel occlusion.Citation45 Calibrated spherical PVA microparticles are available in different sizes ranging from 45 μm to 1,200 μm, depending on the manufacturer. Most of the embolic particles are presented in syringes that are color-coded by particle size for safety.
For catheter injection, PVA particles have to be prepared in a solution of physiological saline and a contrast agent for visibility because PVA particles are nonradiopaque on fluoroscopy. To achieve an optimal suspension and therefore avoid aggregation of the particles, it is of the upmost importance to find the right balance between the saline solution and the contrast agent. The particles may be delivered through a microcatheter or by a conventional diagnostic catheter, depending on their size.
Indication
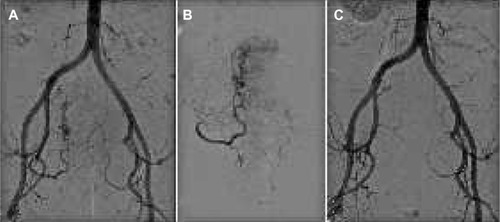
PVA particles can be used for embolization of arteriovenous malformations and small vessel bleeding, such as gastrointestinal bleeding and bronchial artery hemorrhage, and in several trauma scenarios.Citation46,Citation47 Indications for embolization also include benign tumor embolization, most commonly uterine fibroid treatment (), carotid artery glomus embolization, renal angiomyolipoma, and meningioma.Citation48 Solid organ embolization using PVA particles has been proposed for renal ablation and portal embolization in order to achieve hypertrophy of the contralateral hepatic lobe. More recently, benign prostatic hypertrophy has also become an indication for embolization using this agent.Citation49 Embolization of primary and secondary malignant hepatic tumors has been proposed because loading PVA with chemotherapeutics is now effective and gaining importance in this field.Citation50
Figure 4 Uterine fibroid treatment.
Advantages and disadvantages
PVA particles for embolization are a cheap alternative to other embolization materials. Furthermore, they are easy to use and the embolization of upstream territories can be performed without the need to navigate a catheter to the target arteries because embolization with PVA is flow-directed. However, the risk of nontarget embolization is elevated, as the particles are compressible and can travel to distal vascular territories. When blood flow is almost completely blocked, the particles might reflux, which can be the cause of severe complications, especially when drug-loaded spheres are used.Citation36,Citation51 Furthermore, particle aggregation property may lead to catheter occlusion or proximal vessel obstruction.
Acrylic microspheres
Description
Trisacryl gelatin microspheres are nonreabsorbable microspheres with a precisely calibrated particle size and uniform shape. Their smooth surface structure prevents the microparticles from agglomerating, which permits better penetration of the particles to the distal arteries.Citation52 The microspheres are also compressible, which allows for easy passage through the catheter and the vascular network. Trisacryl gelatin microspheres are available in different sizes ranging from 40 μm to 1,200 μm. As for PVA, trisacryl gelatin microspheres are usually delivered in color-coded syringes by particle size. The microspheres are also nonradiopaque to fluoroscopy; however, efforts are being made to improve their visibility.Citation53
Indication
Trisacryl gelatin microspheres have indications similar to those of PVA.
Advantages and disadvantages
In contrast with PVA particles, trisacryl gelatin microspheres have a smooth surface structure and are more uniform in size and shape; therefore, they do not aggregate as much as PVA particles. For this reason, catheter occlusion is less frequent.Citation45 Furthermore, trisacryl gelatin microspheres cause less inflammatory tissue reaction than PVA particles. The improved penetration characteristics of trisacryl microspheres may result in a greater tendency to find particles in small-caliber vessels, which could cause more infarction.
Gelatin sponge
Description
Gelatin sponge or gelfoam is a hemostatic agent that is absorbable; therefore, the embolization effect may not be permanent and vascular recanalization might occur. The gelfoam is made out of purified pork skin, has a porous structure, and is not antigenic. Its embolization action is due to physical effects, because gelfoam absorbs 45 times its weight when contacting liquids such as blood.Citation54 This drying effect and entrapment of blood platelets promote coagulation. For this reason, only a minimal inflammatory tissue reaction is observed after embolization. It is presented as sterile sheets, particles, or powder. The particles can have different predefined sizes and shapes, such as spherical or cubic. The sheets can be cut into small pieces that can be mixed with saline solution and contrast to form a gel-like liquid, or can be cut into thin lines to be used as “torpedoes”. Depending on the size and form that are being used, gelfoam can be applied through diagnostic catheters, microcatheters, and even through large French-sized sheaths.
Indication
Gelfoam is a very flexible embolization material and can be used in different clinical situations. It is mainly used in hemorrhages, involving trauma, post-partum, and other bleeding conditions. Because it can be applied in pieces, puncture tract embolization after percutaneous hepatobiliary interventions can be performed. Gelfoam has also been indicated for use in tumor embolization prior to surgery.
Advantages and disadvantages
Gelfoam is an inexpensive embolization material, is accessible in most interventional departments, and does not require special training. It is available in different sizes and shapes, and can be prepared by the operator. Gelfoam is an absorbable agent and can be degraded biologically; therefore, it is applied in conditions when only a temporary occlusion of the artery is required such as in post-partum hemorrhage.
Gelfoam has been associated with infectious complications, which might be related to air retention with posterior bacterial colonization.Citation54 In coagulation disorders, where it is required to assure complete occlusion, gelfoam should not be used as a definitive coagulation agent.
Liquids
Onyx
Description
Onyx® (ev3 Neurovascular Inc., Irvine, CA, USA) is a nonadhesive liquid embolization material that consists of three primary elements: a copolymer of ethylene and vinyl alcohol (EVOH) that is responsible for occlusion, dimethyl sulfoxide (DMSO) as a dissolvent, and tantalum powder for radiopaque visualization. The relative viscosity of Onyx is defined by the concentration of the EVOH/DMSO solution. The most commercially available viscosities of Onyx are Onyx 18, Onyx 34, and Onyx HD-500, which correspond to EVOH concentrations of 6%, 8%, and 20%, respectively. Once the Onyx is injected into the vessel and comes in contact with aqueous solutions such as blood, the copolymer precipitates. The Onyx starts to solidify from the outside to the inside, which is how the dissolvent diffuses. Before the embolization material can be used, the Onyx vials have to be stirred for at least 20 minutes until injection because the tantalum powder must be in an optimal suspension in the EVOH/DMSO solution. Once the catheter is navigated to the target vessels, the microcatheter has to be flushed with physiological saline solution. The dead space lumen of the catheter is then filled with DMSO to separate the EVOH from the blood; otherwise, precipitation could occur inside the catheter and an irreversible blockage will require a catheter exchange. Care should be taken, as not all microcatheters are compatible with DMSO. Finally, the Onyx solution is injected at a low speed to avoid vascular endothelial damage of the DMSO.
Indication
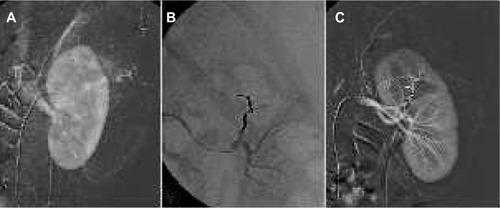
Because injection of the embolization material can be controlled and performed very slowly, it is indicated in neurointerventional procedures. Onyx has been described for use in the embolization of arteriovenous malformations and arteriovenous fistulas. Onyx HD-500 was developed for the embolization of complex cerebral aneurysms because its high density decreases the likelihood of reflux in the parent artery.Citation55 In the peripheral vasculature, Onyx is indicated for use in small-vessel and medium-vessel hemorrhages and can be combined with other embolization materials. It has been described for use in both malignant and benign tumors, such as angiomyolipoma () and aggressive vertebral hemangioma as a primary treatment or prior to surgical resection for preoperative devascularization.Citation56,Citation57 It can also be used for portal vein embolization to achieve hypertrophy of the liver parenchyma and for embolization of the gastroduodenal artery before selective internal radioembolization to avoid complications caused by migration of the radioactive particles.Citation58 In the arterial system, Onyx is a safe embolization material to occlude persistent endoleaks after EVAR.Citation59,Citation60
Figure 5 Renal angiomyolipoma treatment.
Advantages and disadvantages
The major advantage of Onyx is its safety due to perfect control. Onyx can be injected slowly, and injection can be stopped and continued as desired by the operator. Because of the tantalum, Onyx has excellent visibility using fluoroscopy. Also, the catheter does not get stuck in the Onyx and can be withdrawn by applying continuous tension onto the catheter. If the microcatheter is wedged into the Onyx cast around the tip of the catheter or using proximal balloon occlusion, embolization can be achieved, even in more distal vascular territories, by pushing the Onyx sponge, thanks to its lava-like behavior.
One of the major concerns of using Onyx is its painful injection. DMSO can also damage the vascular endothelium, which may result in severe spasm and even vascular necrosis. If injected close to superficial anatomic structures, the dark color may be visible through the skin.
N-butyl cyanoacrylate
Description
Cyanoacrylates (ethyl-2-cyanoacrylate, methyl-2-cyanoacrylate) have been used for many years in industry and are also known as “super glue”. N-butyl cyanoacrylate (NBCA) is an adhesive and liquid embolization material that is used for medical purposes and has been developed to reduce toxicity. NBCA is a monomer that polymerizes once it comes into contact with ionic fluids. NBCA is nonradiopaque, so ethiodized oil (Lipiodol®) or tantalum powder should be added to the mixture for better visualization. Its mixture with Lipiodol not only defines its radiopacity but also influences viscosity and polymerization time.Citation61 The more Lipiodol that is added to the mixture, the more peripherally embolization will occur from the catheter tip. In addition, a linear relationship has been described between the ratio of the NBCA/Lipiodol mixture and polymerization speed.Citation62
Once the NBCA is ready to be used, the embolization catheter should be flushed with 5% dextrose water solution to prevent premature polymerization during NBCA injection. Occlusion has to be executed rapidly in order to avoid the catheter sticking to the glue. After injection, aspiration should be performed during catheter retrieval.
Indication
NBCA is primarily used in hemorrhagic conditions when fast occlusion of the bleeding vessel is required. It has also been described for this use in high-flow situations, such as cerebral arteriovenous malformations and arteriovenous fistulas, because NBCA polymerizes very fast into a solid cast, especially when using high ratios of NBCA/Lipiodol. Other indications in the vascular system include varicocele embolization, primary and secondary hepatic tumor embolization, treatment of endoleaks after EVAR, and embolization of peripheral pseudoaneurysms.Citation62,Citation63 Because NBCA polymerizes when it comes into contact with fluids, occlusion of any endoluminal structure outside the vascular territory such as bronchopleural fistulas, anorectal fistulas, or aneurysmal bone cysts can be achieved.Citation64–Citation66
Advantages and disadvantages
NBCA is a cheap, fast embolization agent; therefore, radiation exposure might be reduced during intervention. One of the major inconveniences is its adhesive nature; if the catheter tip is not removed fast enough, then the catheter sticking to the glue might require breaking of the catheter. Catheters with detachable tips are now available to overcome this problem. Furthermore, reflux from too hasty injection or inappropriate injection volumes may cause nontarget embolization.Citation67 Migration of the solidified material can also occur when retracting the catheter without aspiration due to stripping the material attached to the catheter tip. Other causes of migration include premature polymerization and an inadequate relationship between polymerization speed and blood flow volume in high-flow vascular malformations.
Alcohol
Description
Ethanol is an alcohol that is considered to be a permanent embolization agent. The occlusion effect is achieved by denaturing proteins, as well as by damaging the endothelium and subsequent vasospasm. Furthermore, it activates the coagulation system and promotes occlusion. Absolute alcohol also causes perivascular necrosis. Recanalization is unlikely to happen, and formation of collateral vessels may be avoided, as alcohol causes microvascular occlusion. Because alcohol is a liquid agent, it can be applied through microcatheters, diagnostic catheters, and even needles in the case of percutaneous alcohol instillation. For better visualization, ethanol can be mixed with contrast material.Citation68 A balloon occlusion could be performed to increase the contact of alcohol with the vessel wall in order to accelerate thrombosis.
Indication
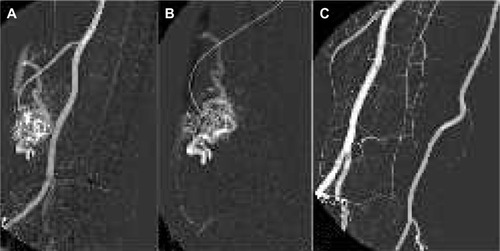
Ethanol can be used for organ ablation, such as kidney embolization. It is also a good alternative for embolizing peripheral vascular malformations ().Citation69 Furthermore, absolute alcohol has been used for occlusion of varicose veins. It can also be an alternative in hemorrhagic conditions, if no other embolization material is available.
Figure 6 Peripheral arteriovenous malformation treatment.
Advantages and disadvantages
Absolute ethanol is a sclerosing agent that is widely available, well known, and inexpensive. Alcohol can penetrate into the distal arteries, but if the alcohol enters the main circulation, the toxic effect of the agent disappears due to dilution. However, alcohol has some limitations. Absolute ethanol can be toxic at higher dosages and cause transitory hypertension and compartment syndrome. Its injection is also painful and, if used in peripheral malformations, can cause skin necrosis, muscle contractions, and transitory or permanent paralysis, and rarely, amputation.
Others
Some liquid agents have been developed for sclerotherapy, such as polidocanol and sodium tetradecyl sulfate. These agents can be applied in their liquid forms or by producing a foam with air using the double-syringe system technique.Citation70
Conclusion
Embolization procedures are increasingly performed not only as a primary treatment but also to manage complications after open surgery and minimal invasive interventions, such as hemorrhagic conditions. In recent years, new occlusion devices has been developed for challenging conditions to increase treatment efficacy and decrease complications arising from embolization procedures. However, it is mandatory for optimization of treatment that the operator is familiar with new devices and techniques.
Disclosure
The authors report no conflicts of interest in this work.
References
- WangWLiHTamMDZhouDWangDXSpainJThe amplatzer vascular plug: a review of the device and its clinical applicationsCardiovasc Intervent Radiol201235472574022526108
- GuneyliSCinarCBozkayaHParildarMOranIApplications of the amplatzer vascular plug to various vascular lesionsDiagn Interv Radiol201420215515924047719
- ZanderTBaldiSRabellinoMSuccessful occlusion of a ruptured aortic aneurysm using the amplatzer vascular plug: a technical noteCardiovasc Intervent Radiol201134Suppl 2S136S14120431884
- ManginiMLaganaDFontanaFUse of amplatzer vascular plug (AVP) in emergency embolisation: preliminary experience and review of literatureEmerg Radiol200815315316018193463
- LibicherMPavlidisDBangardCGawendaMOcclusion of the internal iliac artery prior EVAR: comparison of coils and plugsVasc Endovascular Surg2012461343922156161
- PellerinOCarubaTKandounakisYEmbolization of the internal iliac artery: cost-effectiveness of two different techniquesCardiovasc Intervent Radiol20083161088109318612682
- PowellSNarlawarROdetoyinboTEarly experience with the amplatzer vascular plug II for occlusive purposes in arteriovenous hemodialysis accessCardiovasc Intervent Radiol201033115015619937024
- YildizAEPeynirciogluBCilBEApplications of the amplatzer vascular plug 4Diagn Interv Radiol201218222523021922460
- BaldiSRostagnoRDZanderTRabellinoMMaynarMOcclusion of a pulmonary arteriovenous fistula with an amplatzer vascular plugArch Bronconeumol2007434239241 Spanish17397589
- LibicherMHerbrikMStippelDPoggenborgJBovenschulteHSchwabeHPortal vein embolization using the amplatzer vascular plug II: preliminary resultsRofo2010182650150620084595
- PechMKraetschAWienersGEmbolization of the gastroduodenal artery before selective internal radiotherapy: a prospectively randomized trial comparing platinum-fibered microcoils with the amplatzer vascular plug IICardiovasc Intervent Radiol200932345546119137369
- PieperCCMeyerCHauserSWilhelmKESchildHHTransrenal ureteral occlusion using the Amplatzer vascular plug II: a new interventional treatment option for lower urinary tract fistulasCardiovasc Intervent Radiol201437245145723748732
- FruchterOBruckheimerERavivYRosengartenDSauteMKramerMREndobronchial closure of bronchopleural fistulas with Amplatzer vascular plugEur J Cardiothorac Surg2012411464921600781
- IerardiAMFontanaFManginiMUse of amplatzer vascular plug to treat a biliary cutaneous fistulaKorean J Radiol201314580180424043976
- PierotLFlow diverter stents in the treatment of intracranial aneurysms: Where are we?J Neuroradiol2011381404621257202
- ZanatyMChalouhiNTjoumakarisSIRosenwasserRHGonzalezLFJabbourPFlow-diversion panacea or poison?Front Neurol201452124592254
- BusoRRattazziMLeoniMPuatoMPaolaFDPaulettoPAn unusual case of fibromuscular dysplasia with bilateral renal macroaneurysms: three-year outcome after endovascular treatmentOpen Cardiovasc Med J20137750753
- JamshidiPMahmoodyKErnePCovered stents: a reviewInt J Cardiol2008130331031818672301
- StefanadisCTsiamisEVlachopoulosCArterial autologous graft-stent for treatment of coronary artery disease: a new techniqueCathet Cardiovasc Diagn19974033023079062730
- HirdesMMVleggaarFPSiersemaPDStent placement for esophageal strictures: an updateExpert Rev Med Devices20118673375522029470
- KimJHSongHYShinJHJungHYKimSBParkSIMembrane degradation of covered stents in the upper gastrointestinal tract: frequency and clinical significanceJ Vasc Interv Radiol2008192 Pt 122022418341953
- ShinJHSongHYParkHRemoval of retrievable self- expandable urethral stents: experience in 58 stentsEur Radiol20061692037204316528559
- KanasakiSFurukawaAKaneTMurataKPolyurethane-covered Nitinol Strecker stents as primary palliative treatment of malignant biliary obstructionCardiovasc Intervent Radiol200023211412010795835
- ChaputUHeresbachDAudureauEComparison of a standard fully covered stent with a super-thick silicone-covered stent for the treatment of refractory esophageal benign strictures: a prospective multicenter studyUnited European Gastroenterol J20131293102
- MartinRCWoodallCDuvallRScogginsCRThe use of self-expanding silicone stents in esophagectomy strictures: less cost and more efficiencyAnn Thorac Surg200886243644018640310
- LensveltMMFritschyWMvan OostayenJAHolewijnSZeebregtsCJReijnenMMResults of heparin-bonded ePTFE-covered stents for chronic occlusive superficial femoral artery diseaseJ Vasc Surg201256111812522342154
- ZhuHDGuoJHMaoAWConventional stents versus stents loaded with 125iodine seeds for the treatment of unresectable oesophageal cancer: a multicentre, randomised phase 3 trialLancet Oncol201415661261924742740
- MaynarMBaroMQianZEndovascular repair of brachial artery transection associated with traumaJ Trauma20045661336134115211146
- ZanderTBaldiSRabellinoMBifurcated endograft (Excluder) in the treatment of isolated iliac artery aneurysm: preliminary reportCardiovasc Intervent Radiol200932592893619449072
- RabellinoMGarcANLZanderTEndovascular treatment for a thoracic-abdominal aortic aneurysm without fenestrations or branchesMinim Invasive Ther Allied Technol201120635235521247249
- ZieglerPPerdikidesTPAvgerinosEDUmscheidTStelterWJFenestrated and branched grafts for para-anastomotic aortic aneurysm repairJ Endovasc Ther200714451351917696626
- OtaKTakeuchiTHiguchiKTemporary insertion of a covered self-expandable metal stent for spontaneous esophageal ruptureDig Endosc201426460760824861336
- LeeKMShinSJHwangJCComparison of uncovered stent with covered stent for treatment of malignant colorectal obstructionGastrointest Endosc200766593193617767930
- KimJHShinJHSongHYLeeSCKimKRParkJHUse of a retrievable metallic stent internally coated with silicone to treat airway obstructionJ Vasc Interv Radiol20081981208121418656015
- WaidmannOTrojanJFriedrich-RustMSEMS vs cSEMS in duodenal and small bowel obstruction: high risk of migration in the covered stent groupWorld J Gastroenterol201319376199620624115817
- Lopez-BenitezRHallscheidtPKratochwilCProtective embolization of the gastroduodenal artery with a one-HydroCoil technique in radioembolization proceduresCardiovasc Intervent Radiol201336110511022414984
- Lopez-BenitezRRuschOHeverhagenJThe “embowire” technique: concept and description of a novel embolization technique for narrow vesselsCardiovasc Intervent Radiol20133651393139823580113
- YoshidaWBSequeraJDe Abreu MaffeiFHLong-term histopathologic evaluation of inferior vena cava after modified Greenfield filter implantation. Experimental study in sheepInt Angiol200423217017615507896
- RabellinoMGarcia-NielsenLZanderTBaldiSLlorensRMaynarMStent-assisted coil embolization of a mycotic renal artery aneurysm by use of a self-expanding neurointerventional stentCardiovasc Intervent Radiol201134Suppl 2S109S11220811890
- DingYHLewisDAKadirvelRDaiDKallmesDFThe Woven EndoBridge: a new aneurysm occlusion deviceAJNR Am J Neuroradiol201132360761121330397
- VenbruxACRudakovLPlassAEmmertMYEbnerAA new occlusion device: application of the ArtVentive endoluminal occlusion system (EOS) – first in human clinical trialCardiovasc Intervent Radiol2014371859323703667
- LewisALAdamsCBusbyWComparative in vitro evaluation of microspherical embolisation agentsJ Mater Sci Mater Med200617121193120417143749
- GermanoIMDavisRLWilsonCBHieshimaGBHistopathological follow-up study of 66 cerebral arteriovenous malformations after therapeutic embolization with polyvinyl alcoholJ Neurosurg19927646076141545253
- StampflSBellemannNStampflUInflammation and recanalization of four different spherical embolization agents in the porcine kidney modelJ Vasc Interv Radiol200819457758618375304
- LaurentAWassefMNamurJMartalJLabarreDPelageJPRecanalization and particle exclusion after embolization of uterine arteries in sheep: a long-term studyFertil Steril200991388489218321492
- KatoAKudoSMatsumotoKBronchial artery embolization for hemoptysis due to benign diseases: immediate and long-term resultsCardiovasc Intervent Radiol200023535135711060364
- BandiRShettyPCSharmaRPBurkeTHBurkeMWKastanDSuperselective arterial embolization for the treatment of lower gastrointestinal hemorrhageJ Vasc Interv Radiol200112121399140511742013
- PelageJPPolyvinyl alcohol particles versus tris-acryl gelatin microspheres for uterine artery embolization for leiomyomasJ Vasc Interv Radiol200415878979115297581
- PiscoJMPinheiroLCBilhimTDuarteMMendesJROliveiraAGProstatic arterial embolization to treat benign prostatic hyperplasiaJ Vasc Interv Radiol2011221111921195898
- KosSWasanEWeirGElution characteristics of doxorubicin-loaded microspheres differ by drug-loading method and microsphere sizeJ Vasc Interv Radiol201122336136821353988
- Lopez-BenitezRRichterGMKauczorHUAnalysis of nontarget embolization mechanisms during embolization and chemoembolization proceduresCardiovasc Intervent Radiol200932461562219387732
- DerdeynCPGravesVBSalamatMSRappeACollagen-coated acrylic microspheres for embolotherapy: in vivo and in vitro characteristicsAJNR Am J Neuroradiol19971846476539127025
- SaralidzeKvan Hooy-CorstjensCSKooleLHKnetschMLNew acrylic microspheres for arterial embolization: combining radiopacity for precise localization with immobilized thrombin to trigger local blood coagulationBiomaterials200728152457246417257667
- AbadaHTGolzarianJGelatine sponge particles: handling characteristics for endovascular useTech Vasc Interv Radiol200710425726018572138
- DalyaiRTRandazzoCGhobrialGRedefining Onyx HD 500 in the flow diversion eraInt J Vasc Med2012201243549022121488
- HurleyMCGrossBASurdellDPreoperative Onyx embolization of aggressive vertebral hemangiomasAJNR Am J Neuroradiol20082961095109718372419
- GemmeteJJPatelSPandeyASPreliminary experience with the percutaneous embolization of juvenile angiofibromas using only ethylene-vinyl alcohol copolymer (Onyx) for preoperative devascularization prior to surgical resectionAJNR Am J Neuroradiol20123391669167522499846
- TappingCRDixonSLittleMWBoardmanPSharmaRAAnthonySLiquid embolization of the gastroduodenal artery before selective internal radiotherapy (SIRT)Clin Radiol201267878979222749385
- LarzonTHorerTPlugging and sealing technique by Onyx to prevent type II endoleak in ruptured abdominal aortic aneurysmVascular2013212879123508383
- Muller-WilleRWohlgemuthWAHeissPTransarterial embolization of type II endoleaks after EVAR: the role of ethylene vinyl alcohol copolymer (Onyx)Cardiovasc Intervent Radiol20133651288129523397186
- TakasawaCSeijiKMatsunagaKProperties of N-butyl cyanoacrylate-iodized oil mixtures for arterial embolization: in vitro and in vivo experimentsJ Vasc Interv Radiol201223912151221. e121122920981
- PollakJSWhiteRIJrThe use of cyanoacrylate adhesives in peripheral embolizationJ Vasc Interv Radiol200112890791311487670
- MittalRStephenEKeshavaSNMosesVAgarwalSPercutaneous cyanoacrylate glue embolization for peripheral pseudoaneurysms: an alternative treatmentIndian J Surg201274648348524293904
- RossiGRimondiEBartalenaTSelective arterial embolization of 36 aneurysmal bone cysts of the skeleton with N-2-butyl cyanoacrylateSkeletal Radiol201039216116719669138
- ChawlaRKMadanABhardwajPKChawlaKBronchoscopic management of bronchopleural fistula with intrabronchial instillation of glue (N-butyl cyanoacrylate)Lung India2012291111422345907
- BarillariPBassoLLarcineseAGozzoPIndinnimeoMCyanoacrylate glue in the treatment of ano-rectal fistulasInt J Colorectal Dis200621879179416625375
- PeynirciogluBErkusFCilBCiftciTDurhanGBalkanciFMesenteric angiography of patients with gastrointestinal tract hemorrhages: a single center studyDiagn Interv Radiol201117436837321204043
- LinPHBushRLErasoAZhouWIntraoperative renal artery embolization with concomitant nephrectomyEndovascular Today112005 Available from: http://evtoday.com/2005/11/EVT1105_F1_Linrenal.html/Accessed September 18, 2014
- GorrizECarreiraJMReyesRIntramuscular low flow vascular malformations: treatment by means of direct percutaneous embolizationEur J Radiol19982721611659639143
- RaoJGoldmanMPStability of foam in sclerotherapy: differences between sodium tetradecyl sulfate and polidocanol and the type of connector used in the double-syringe system techniqueDermatol Surg2005311192215720090
