Abstract
The treatment of symptomatic aortoiliac occlusive disease has shifted from open to endovascular repair. Both short- and long-term outcomes after percutaneous angioplasty and stenting rival those after open repair and justify an endovascular-first approach. In this article, we review the current endovascular treatment strategies in patients with aortoiliac occlusive disease, indications for primary and selective stenting in the iliac artery, and physical properties and future perspectives of self-expanding stents.
Introduction
The prevalence of peripheral arterial disease (PAD) in the US is more than 4% among adults aged 40 years and over. PAD increases dramatically with age and the prevalence exceeds 14% among those aged 70 years or over.Citation1 One subset of PAD is aortoiliac occlusive disease (AIOD), defined as any stenosis or occlusion from the distal aorta to the common femoral artery (CFA). Conventional surgical revascularization of AIOD is associated with excellent long-term patency rates.Citation2,Citation3 However, open repair is also associated with a significantly longer hospital stay and higher complication rates and inpatient costs, compared with endovascular treatment.Citation4 The TransAtlantic Intersociety Consensus (TASC) II, published in 2007, recommends endovascular therapy for straightforward AIOD (TASC A lesions) and surgery for complex AIOD (TASC D lesions).Citation5 However, due to the rapid development of endovascular techniques and improved competence, experienced centers advocate an “endovascular first” approach. In recent years, endovascular treatment has become widespread and is the preferred method of treatment nowadays for lower extremity arterial obstructions.Citation6
Endovascular treatment of AIOD consists of percutaneous transluminal angioplasty (PTA) with or without stenting. In a meta-analysis of six PTA studies (1,300 patients) and eight PTA and stent studies (816 patients), additional stenting was associated with an increased technical success rate and improved long-term patency.Citation7 The results of endovascular treatment of AIOD have been described in multiple publications. Technical success and both short- and long-term patency rates have been satisfactory, even in challenging lesions.Citation8,Citation9 These results justify an endovascular-first approach for symptomatic AIOD treatment.
Overview of current endovascular treatment for AIOD
Primary versus selective stenting
The Dutch Iliac Stent Trial enrolled 279 patients with intermittent claudication on the basis of iliac artery stenosis of >50%. The study randomly assigned 143 patients to direct stent placement (group I) and 136 to primary angioplasty, with selective stent placement in case of a residual mean pressure gradient >10 mmHg across the treated lesion (group II). The primary endpoint was clinical success, defined as improvement of at least one clinical category in the Fontaine classification.Citation5 In group II, stents were selectively placed in 43% of the patients. Less than 10% of patients were treated for iliac artery occlusions and the stenosis length was <2 cm in 56% patients. Most lesions corresponded to TASC A and B lesions. Clinical success, cumulative patency, and reintervention rates at 2 years were similar between the groups.Citation10 Long-term results (after 5–8 years) showed a better clinical outcome in patients with PTA and selective stenting in the iliac artery. Iliac patency, ankle–brachial index, and quality of life did not support a difference between groups.Citation11
More recent studies showed that primary stenting has significant benefits over angioplasty alone in TASC C and D aortoiliac lesions. In a nonrandomized series of 151 patients with iliac stenosis, a total of 110 consecutive patients (149 lesions) underwent primary stenting. The results were compared with 41 patients (41 lesions) who had PTA followed by selective stenting for suboptimal PTA. The overall early clinical success rate was superior for the primary stent group (). For TASC A and B lesions, the initial and late clinical success rates were comparable but were inferior in selective stenting for TASC C and D lesions.Citation12
Figure 1 Percutaneous transluminal angioplasty of the stenosis in the right common iliac artery and occluded external iliac artery (A) resulted in a significant residual stenosis and dissection, respectively (B). Additional stent placement resulted in technical success (C).
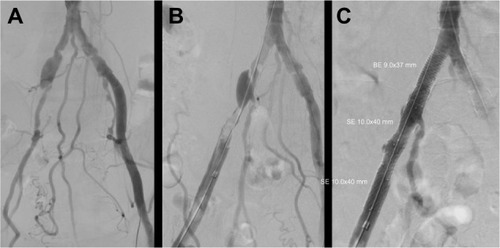
A recent meta-analysis of 16 reports including 958 patients with endovascular treatment of TASC C and D aortoiliac arterial lesions found better patency rates for primary stenting than for selective stenting.Citation8
The Stents Versus Angioplasty for the Treatment of Iliac Artery Occlusions (STAG) trial randomly assigned 112 patients with an iliac occlusion to PTA or primary stent placement. PTA was performed in 55 patients and primary stenting in 57. Technical success in the primary stenting group was higher (98% vs 84%) and major complications (predominantly distal embolization) occurred less frequently (5% vs 20%) compared with PTA. Patency rates did not differ after 1 and 2 years.Citation13
Predictors of success or failure
Independent predictors of iliac endovascular intervention success or failure have been described in multiple publications. The presence of two-vessel femoral runoff or at least two patent below-the-knee vessels, or both, is associated with improved iliac artery primary patency.Citation14 Poor outflow requiring a bypass is associated with decreased iliac artery primary patency rates.Citation3 In another study, iliac PTA and stenting, combined with an untreated superficial femoral artery stenosis >50% resulted in a decreased primary patency rate.Citation15
The presence of an iliac artery occlusion is considered an independent risk factor for patency loss.Citation16 However, similar results after treatment of iliac stenoses and occlusions have been published. In a series of 73 patients including 76 occluded iliac arteries (33 common, 34 external, and nine both) the primary patency was 79% at 1 year and 69% at 3 years.Citation17 In a prospective series of 223 patients with AIOD, endovascular treatment was performed for iliac occlusion in 109 patients and for iliac stenosis in 114 patients. No differences were observed in the complication rate or in short- and long-term patency rates.Citation18
Other predictors for decreased primary patency include diabetes mellitus,Citation3 age <50 years,Citation16 TASC C and D lesions,Citation19,Citation20 hypertension,Citation14 hypercholesterolemia,Citation14 chronic renal insufficiency,Citation14 external iliac artery (EIA) disease,Citation21 female sex,Citation21 and smoking history.Citation19
Differences between the common and external iliac artery
The iliac artery is subdivided as the common, external, and internal iliac or hypogastric artery. Most publications describe results after iliac artery stenting and do not differentiate between the common iliac artery (CIA) and EIA (). This may be the result of the TASC II classification.Citation5 The TASC II classification defines aortoiliac lesions, potentially involving the distal aorta, CIA, EIA, and CFA. The limitations of this rather generic aortoiliac TASC II classification have been described previously.Citation22 We believe that in trials investigating stents in the iliac arteries, a distinction must be made between the straight and relative immobile CIA and the tortuous and mobile EIA. Subgroups should be created according to the anatomic characteristics of the target lesion rather than by the TASC II classification.Citation23
Table 1 An overview of recent studies presenting primary patency rates of percutaneous angioplasty and bare-metal balloon-expandable (BE) or self-expandable (SE) stent placement in the iliac artery
One of the rare studies comparing stents in the CIA and EIA showed no differences in primary patency after 1, 2, and 3 years.Citation24 Two other studies, however, found EIA stenting was an independent predictor of decreased primary patency after iliac artery PTA and stenting.Citation21,Citation25 A more recent study evaluating a particular self-expandable stent showed no significant difference in the patency rates at 2 years among stents placed in the CIA, the EIA, and both the CIA and EIA.Citation26
Self-expanding stents and AIOD
Self-expanding stent
Most self-expanding stents are made of nitinol, an alloy of nickel and titanium. Elgiloy, a cobalt–chromium alloy, has also been used for self-expanding stents. An important feature of nitinol is its thermal shape memory and superelasticity, which means nitinol is able to return to its original shape after severe deformation.Citation41,Citation42 Besides being superelastic, nitinol is also biocompatible. The narrow temperature range within which nitinol’s superelasticity is exhibited includes body temperature.Citation43 Therefore, nitinol is an excellent material for a self-expanding stent design. The ability to recover their original shape without clinically relevant loss of lumen diameter is an important distinction between nitinol and stainless steel stents.
Stents have to survive pulsatility, external forces, and bending fatigue. Balloon expandable stents are sufficiently rigid to prevent the native artery from stretching and expanding due to the pulse pressure. The fatigue lifetime of nitinol far exceeds that of ordinary metals. However, extreme bending and crushing, which may be experienced under the inguinal ligament and in the popliteal or subclavian artery, may exceed the limitations of both balloon-expandable and self-expandable stents.
Nitinol stents have very low forces acting on the vessel wall (chronic outward force), but the force generated by a nitinol stent to resist compression (stiffness) increases rapidly with deflection; thus, a nitinol stent unloads its outward force when it reaches its intended diameter. Nitinol stents are able to adapt to the tortuous path of a vessel rather than forcing the vessel to straighten. Using a stiffer balloon-expandable stent may result in vessel straightening and concomitant vessel trauma.Citation44 The most important factor causing in-stent restenosis is the formation of neointimal tissue hyperplasia.Citation45 The underlying causes of intimal hyperplasia are migration and proliferation of vascular smooth muscle cells provoked by injury, inflammation, and stretch.Citation46
In a human cadaveric study, self-expanding stents in the CIA showed considerably lower radial expansion force than balloon-expandable stents. Moreover, precision and reproducibility of the achieved expansion was significantly lower in the self-expanding group.Citation47 As the self-expandable stent begins to emerge from the constraint, there is a natural tendency for it to spring forward that results from several stent properties, including bridge design, longitudinal stiffness, and friction. Although this tendency in a self-expandable stent can be reduced to a minimum nowadays, this potential source of inaccuracy does not exist in a balloon-expandable stent.Citation43
A stent is a compromise, and there is no single stent that is ideal for all indications.Citation44,Citation48 The physical properties of balloon-expandable and self-expandable stents both exhibit superior performance in different types of arteries. Comparative studies on the performance of self-expandable and balloon-expandable stents in a clearly defined arterial segment are scarce (). Based on results from mainly in vitro studies, most physicians will prefer a balloon-expandable stent in straight, focal, and calcified lesions or lesions adjacent to the aortic bifurcation; whereas, self-expanding stents are preferred in longer and tortuous lesions or for contralateral approaches. High-quality clinical data to support this practice are lacking, but this strategy has been advocated in many publications.Citation3,Citation15,Citation20,Citation27–Citation29,Citation31,Citation35,Citation36,Citation39
Limitations and future prospects
Stent fracture
The iliac artery, particularly the distal EIA prior to the inguinal ligament, is exposed to flexion by bending the hip joint. This may lead to stent fracture. In a series of 165 patients, a total 305 self-expandable stents were implanted in 216 iliac arteries.Citation33 Different stent types were used, according to the preferences of the physician. During follow-up, stent fracture was detected in eleven of 305 stents (3.6%). Stent fracture occurred in eleven of 222 nitinol stents (5.0%) but not in elgiloy stents. Multivariate analysis indicated stenting for chronic occlusion as a risk factor associated with stent fracture (hazard ratio: 6.09; P=0.008). No significant differences between stents in the CIA and EIA were observed. Reocclusion of the stented iliac artery was only detected in one of eleven iliac arteries with stent fracture.Citation49 The primary patency rates in iliac arteries with and without fractured stents at 8 years were 90% and 91%, respectively. These results are in contrast with the considerable risk of stent fractures in the femoropopliteal artery, which is associated with a higher in-stent stenosis and reocclusion rate.Citation50
Covered stents
As described previously, the additional primary or selective use of stents improves the clinical outcome. Unfortunately, stents also have limitations, such as subacute occlusion and restenosis. Neointimal hyperplasia may grow through the struts of the stent and cause in-stent restenosis. A covered stent or stent graft is a metal stent lined with polytetrafluoroethylene (PTFE) or Dacron. Covered stents may overcome this limitation by introducing a mechanical barrier between intimal hyperplasia and the arterial lumen. The covered stent potentially also prevents migration of macrophages in the vascular wall, which are attracted by proinflammatory mediators secreted by the damaged vessel wall. These macrophages release further cytokines, metalloproteinases, and growth factors that contribute to initiating the restenotic process.Citation51 This concept was tested using balloon-expandable stents that were covered with PTFE extending for one-half of the length of the stent. These grafts were used to treat 12 iliac artery occlusions in 12 high-risk patients. After 6 months of follow-up, the mean lumen diameter was significantly greater on the covered side than on the uncovered side.Citation52
A prospective evaluation of the Hemobahn PTFE-nitinol self-expanding stent (WL Gore & Associates, Flagstaff, AZ, USA), in 61 iliac arteries and 80 femoral arteries provided primary patency rates for the iliac arteries of 98% at 6 months and 91% at 12 months. During follow-up, one early occlusion (within 30 days) of a Hemobahn stent occurred in an iliac artery. Late occlusions (30 days to 12 months) were observed in an additional five iliac arteries.Citation53
The Cordis Covered Nitinol Stent (COVENT) study enrolled 98 patients, who received PTFE-covered nitinol stents in 60 iliac arteries and 47 superficial femoral arteries. The primary patency rates for the iliac arteries were 94.3% at 6 months and 90.7% at 12 months. Two iliac artery covered stents occluded, the first within 6 months and the second after 8 months. In-stent recurrent stenosis developed in two other iliac artery-covered stents.Citation54
Other authors have suggested that transgraft migration of endothelial cells may result in in-stent neointimal formation, which may lead to in-stent recurrent stenosis.Citation55,Citation56 Another study demonstrated significantly higher 5-year primary patency rates of 87% for predominantly self-expandable covered stents compared with 53% for bare metal stents (BMS) in patients undergoing simultaneous common femoral artery endarterectomy and iliac revascularization.Citation57 These results are promising, but long-term data are lacking. The explanation for the apparent benefit of covered stents in AIOD treatment is not completely clear. The decreased risk of iliac rupture may lead to improved dilatation with use of higher inflation pressure.Citation57
Three other studies showed similar results with covered balloon-expandable stents.Citation58–Citation60 The Covered Versus Balloon-Expandable Stent Trial (COBEST) randomly assigned 168 iliac arteries in 125 patients to receive a covered balloon-expandable stent or BMS. After 18 months of follow-up, covered stents and BMSs performed similarly in TASC B lesions; however, covered stents performed better in TASC C and D lesions than BMSs.Citation61 The Dutch Iliac Stent Trial: Covered Balloon-Expandable versus Uncovered Balloon-Expandable Stents in the Common Iliac Artery (DISCOVER) is currently enrolling patients with a CIA occlusion or stenosis >3 cm, who are randomized for a balloon-expandable covered stent or BMS.Citation62
Future perspectives
A new self-expanding interwoven nitinol stent has shown encouraging results in the popliteal artery.Citation63 This novel Supera stent (Abbott Vascular, Santa Clara, CA, USA), consists of woven nitinol wires braided in a tubular mesh configuration. This specific configuration results in a stent that is flexible, compliant, and self-expanding and that has a very high radial resistive force. This device may perform very well in complex iliac lesions; however, the largest currently available diameter is 8 mm.
Several promising stenting techniques are already available for noniliac arteries. In cardiology, the use of drug-eluting stents (DES) shows beneficial results. These coronary devices have been shown to be superior to BMS in tibial arteries too.Citation64,Citation65 A novel nitinol paclitaxel-eluting stent is available for the femoropopliteal artery. In a large randomized controlled trial, DESs showed superior 12-month event-free survival and primary patency rates compared with PTA. In the PTA group, 120 patients had acute PTA failure and underwent secondary random assignment to provisional DES or BMS. The provisional DES group exhibited superior 12-month primary patency and clinical benefit compared with the provisional BMS group.Citation66 This particular device is not yet available for the iliac arteries, but another self-expanding everolimus-eluting stent has been analyzed in an animal model. The iliac arteries of 24 Yucatan mini-swine were treated with the 8×28 mm nitinol everolimus-eluting stent. Bare nitinol stents were implanted in the contralateral iliac arteries to serve as controls. During the first 6 months, local arterial stent-mediated delivery of everolimus inhibited the formation of neointimal hyperplasia.Citation67
A drug-eluting bioresorbable vascular scaffold seems to be very promising in coronary arteries.Citation68,Citation69 This technology is currently being investigated in femoropopliteal lesions and may become available for the iliac artery in the future.
Conclusion
PTA and stenting is the preferred treatment modality in patients with AIOD and has been associated with satisfactory long-term results, even in challenging lesions. Primary stenting is indicated in iliac artery occlusions, while in iliac artery stenoses, selective stenting is preferred. Unfortunately, detailed information about the performance of different stent types in clearly defined iliac artery segments is limited. The unique properties of self-expanding stents make them particularly suitable for the treatment of long, tortuous, and mobile arteries, like the EIA. The most important limitation is in-stent restenosis resulting from neointimal hyperplasia. Use of covered or DESs seems promising, but more evidence is needed to finally prove these concepts ().
Table 2 Conclusions and level of evidence according to the Oxford Centre for Evidence Based Medicine
Disclosure
The authors report no conflicts of interest in this work.
References
- SelvinEErlingerTPPrevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000Circulation2004110673874315262830
- TimaranCHPraultTLStevensSLFreemanMBGoldmanMHIliac artery stenting versus surgical reconstruction for TASC (TransAtlantic Inter-Society Consensus) type B and type C iliac lesionsJ Vasc Surg200338227227812891108
- KashyapVSPavkovMLBenaJFThe management of severe aortoiliac occlusive disease: endovascular therapy rivals open reconstructionJ Vasc Surg20084861451145718804943
- IndesJEMandawatATuggleCTMuhsBSosaJAEndovascular procedures for aorto-iliac occlusive disease are associated with superior short-term clinical and economic outcomes compared with open surgery in the inpatient populationJ Vasc Surg20105251173117920691560
- NorgrenLHiattWRDormandyJANehlerMRHarrisKAFowkesFGTASC II Working GroupInter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II)J Vasc Surg200745Suppl SS5S6717223489
- GoodneyPPBeckAWNagleJWelchHGZwolakRMNational trends in lower extremity bypass surgery, endovascular interventions, and major amputationsJ Vasc Surg2009501546019481407
- BoschJLHuninkMGMeta-analysis of the results of percutaneous transluminal angioplasty and stent placement for aortoiliac occlusive diseaseRadiology1997204187969205227
- YeWLiuCWRiccoJBManiKZengRJiangJEarly and late outcomes of percutaneous treatment of TransAtlantic Inter-Society Consensus class C and D aorto-iliac lesionsJ Vasc Surg20115361728173721609804
- JongkindVAkkersdijkGJYeungKKWisselinkWA systematic review of endovascular treatment of extensive aortoiliac occlusive diseaseJ Vasc Surg20105251376138320598474
- TetterooEvan der GraafYBoschJLRandomised comparison of primary stent placement versus primary angioplasty followed by selective stent placement in patients with iliac-artery occlusive disease. Dutch Iliac Stent Trial Study GroupLancet19983519110115311599643685
- KleinWMvan der GraafYSeegersJDutch iliac stent trial: long-term results in patients randomized for primary or selective stent placementRadiology2006238273474416371580
- AbuRahmaAFHayesJDFlahertySKPeeryWPrimary iliac stenting versus transluminal angioplasty with selective stentingJ Vasc Surg200746596597017905559
- GoodeSDClevelandTJGainesPASTAG trial collaborators. Randomized clinical trial of stents versus angioplasty for the treatment of iliac artery occlusions (STAG trial)Br J Surg201310091148115323842828
- GalariaIIDaviesMGPercutaneous transluminal revascularization for iliac occlusive disease: long-term outcomes in TransAtlantic Inter- Society Consensus A and B lesionsAnn Vasc Surg200519335236015818461
- KudoTRigbergDAReilTDChandraFAAhnSSThe influence of the ipsilateral superficial femoral artery on iliac angioplastyAnn Vasc Surg200620450251116732446
- YilmazSSindelTGolbasiITurkayCMeteALüleciEAortoiliac kissing stents: long-term results and analysis of risk factors affecting patencyJ Endovasc Ther200613329130116784315
- UherPNymanULindhMLindbladBIvancevKLong-term results of stenting for chronic iliac artery occlusionJ Endovasc Ther200291677511958328
- PulliRDorigoWFargionAEarly and long-term comparison of endovascular treatment of iliac artery occlusions and stenosisJ Vasc Surg2011531929820934841
- KudoTChandraFAAhnSSLong-term outcomes and predictors of iliac angioplasty with selective stentingJ Vasc Surg200542346647516171589
- OzkanUOguzkurtLTercanFTechnique, complication, and long-term outcome for endovascular treatment of iliac artery occlusionCardiovasc Intervent Radiol2010331182419768500
- TimaranCHStevensSLFreemanMBGoldmanMHExternal iliac and common iliac artery angioplasty and stenting in men and womenJ Vasc Surg200134344044611533595
- BekkenJAFiooleBRegarding “A comparison of covered vs bare expandable stents for the treatment of aortoiliac occlusive disease”J Vasc Surg20125551545154622542352
- DiehmNPattynamaPMJaffMRClinical endpoints in peripheral endovascular revascularization trials: a case for standardized definitionsEur J Vasc Endovasc Surg200836440941918692415
- LeeESSteensonCCTrimbleKECaldwellMPKuskowskiMASantilliSMComparing patency rates between external iliac and common iliac artery stentsJ Vasc Surg200031588989410805878
- PowellRJFillingerMBettmannMThe durability of endovascular treatment of multisegment iliac occlusive diseaseJ Vasc Surg20003161178118410842155
- JaffMRKatzenBTTwo-year clinical evaluation of the Zilver vascular stent for symptomatic iliac artery diseaseJ Vasc Interv Radiol201021101489149420801673
- BalzerJOGastingerVRitterRPercutaneous interventional reconstruction of the iliac arteries: primary and long-term success rate in selected TASC C and D lesionsEur Radiol200616112413115809828
- LevilleCDKashyapVSClairDGEndovascular management of iliac artery occlusions: extending treatment to TransAtlantic Inter-Society Consensus class C and D patientsJ Vasc Surg2006431323916414384
- De RoeckAHendriksJMDelrueFLong-term results of primary stenting for long and complex iliac artery occlusionsActa Chir Belg2006106218719216761475
- CarreiraJMReyesRGudeFLong-term follow-up of Symphony nitinol stents in iliac arteriosclerosis obliteransMinim Invasive Ther Allied Technol2008171344218270875
- GandiniRFabianoSChiocchiMChiappaRSimonettiGPercutaneous treatment in iliac artery occlusion: long-term resultsCardiovasc Intervent Radiol20083161069107618663521
- SixtSAlawiedAKRastanAAcute and long-term outcome of endovascular therapy for aortoiliac occlusive lesions stratified according to the TASC classification: a single-center experienceJ Endovasc Ther200815440841618729553
- HigashiuraWKubotaYSakaguchiSPrevalence, factors, and clinical impact of self-expanding stent fractures following iliac artery stentingJ Vasc Surg200949364565219268770
- KoizumiAKumakuraHKanaiHTen-year patency and factors causing restenosis after endovascular treatment of iliac artery lesionsCirc J200973586086619282607
- MaurelBLanceleveeJJacobiDBleuetFMartinezRLermusiauxPEndovascular treatment of external iliac artery stenoses for claudication with systematic stentingAnn Vasc Surg200923672272819748218
- IchihashiSHigashiuraWItohHSakaguchiSNishimineKKichikawaKLong-term outcomes for systematic primary stent placement in complex iliac artery occlusive disease classified according to Trans-Atlantic Inter-Society Consensus (TASC)-IIJ Vasc Surg201153499299921215582
- SogaYIidaOKawasakiDREAL-AI investigatorsContemporary outcomes after endovascular treatment for aorto-iliac artery diseaseCirc J201276112697270422864278
- KordeckiKLukasiewiczANowickiMAssessment of effectiveness of endovascular treatment of common and external iliac artery stenosis/occlusion using self-expanding Jaguar SM stentsPol J Radiol2012774222923269933
- BosiersMDelooseKCallaertJBRAVISSIMO: 12-month results from a large scale prospective trialJ Cardiovasc Surg (Torino)2013542235253
- ArakiMHiranoKNakanoMTwo-year outcome of the self-expandable stent for chronic total occlusion of the iliac arteryCardiovasc Interv Ther2013291404624068528
- DuerigTWPeltonARStöckelDThe utility of superelasticity in medicineBiomed Mater Eng1996642552668980834
- ChenJTDuerigTWPeltonARStöckelDAn apparatus to measure the shape memory of properties of nitinol tubes for medical applicationsJ Phys IV1995512471252
- DuerigTWTolomeoDEWholeyMAn overview of superelastic stent designMinim Invasive Ther Allied Technol200093–423524620156021
- DudaSHWiskirchenJTepeGPhysical properties of endovascular stents: an experimental comparisonJ Vasc Interv Radiol200011564565410834499
- HoffmannRMintzGSDussaillantGRPatterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound studyCirculation1996946124712548822976
- NewbyACZaltsmanABMolecular mechanisms in intimal hyperplasiaJ Pathol2000190330030910685064
- GrenacherLRohdeSGängerEDeutschJKauffmannGWRichterGMIn vitro comparison of self-expanding versus balloon-expandable stents in a human ex vivo modelCardiovasc Intervent Radiol200629224925416328696
- DyetJFWattsWGEttlesDFNicholsonAAMechanical properties of metallic stents: how do these properties influence the choice of stent for specific lesions?Cardiovasc Intervent Radiol2000231475410656906
- HigashiuraWSakaguchiSMorimotoKKichikawaKStent fracture and reocclusion after placement of a single self-expanding stent in the common iliac artery and endovascular treatmentCardiovasc Intervent Radiol20083151013101718266031
- ScheinertDScheinertSSaxJPrevalence and clinical impact of stent fractures after femoropopliteal stentingJ Am Coll Cardiol200545231231515653033
- ElsnerMAuch-SchwelkWBrittenMWalterDHSchächingerVZeiherAMCoronary stent grafts covered by a polytetrafluoroethylene membraneAm J Cardiol199984333533810496448
- MarinMLVeithFJCynamonJEffect of polytetrafluoroethylene covering of Palmaz stents on the development of intimal hyperplasia in human iliac arteriesJ Vasc Interv Radiol1996756516568897327
- LammerJDakeMDBleynJPeripheral arterial obstruction: prospective study of treatment with a transluminally placed self-expanding stent-graft. International Trial Study GroupRadiology200021719510411012429
- WiesingerBBeregiJPOlivaVLPTFE-covered self-expanding nitinol stents for the treatment of severe iliac and femoral artery stenoses and occlusions: final results from a prospective studyJ Endovasc Ther200512224024615823072
- VirmaniRKolodgieFDDakeMDHistopathologic evaluation of an expanded polytetrafluoroethylene-nitinol stent endoprosthesis in canine iliofemoral arteriesJ Vasc Interv Radiol199910444545610229474
- DolmatchBDongYHHeeterZEvaluation of three polytetrafluoroethylene stent-grafts in a model of neointimal hyperplasiaJ Vasc Interv Radiol200718452753417446544
- ChangRWGoodneyPPBaekJHNolanBWRzucidloEMPowellRJLong-term results of combined common femoral endarterectomy and iliac stenting/stent grafting for occlusive diseaseJ Vasc Surg200848236236718572359
- BosiersMIyerVDelooseKVerbistJPeetersPFlemish experience using the Advanta V12 stent-graft for the treatment of iliac artery occlusive diseaseJ Cardiovasc Surg (Torino)2007481712
- GilesHLesarCErdoesLSprouseRMyersSBalloon-expandable covered stent therapy of complex endovascular pathologyAnn Vasc Surg200822676276818922676
- GrimmeFASpithovenJHZeebregtsCJScharnDMReijnenMMMidterm outcome of balloon-expandable polytetrafluoroethylene-covered stents in the treatment of iliac artery chronic occlusive diseaseJ Endovasc Ther201219679780423210879
- MwipatayiBPThomasSWongJCovered Versus Balloon Expandable Stent Trial (COBEST) Co-investigatorsA comparison of covered vs bare expandable stents for the treatment of aortoiliac occlusive diseaseJ Vasc Surg20115461561157021906903
- BekkenJAVosJAAartsRAde VriesJPFiooleBDISCOVER: Dutch Iliac Stent trial: COVERed balloon-expandable versus uncovered balloon-expandable stents in the common iliac artery: study protocol for a randomized controlled trialTrials20121321523164097
- ScheinertDWernerMScheinertSTreatment of complex atherosclerotic popliteal artery disease with a new self-expanding interwoven nitinol stent: 12-month results of the Leipzig SUPERA popliteal artery stent registryJACC Cardiovasc Interv201361657123347863
- RastanABrechtelKKrankenbergHSirolimus-eluting stents for treatment of infrapopliteal arteries reduce clinical event rate compared to bare-metal stents: long-term results from a randomized trialJ Am Coll Cardiol201260758759122878166
- BosiersMScheinertDPeetersPRandomized comparison of everolimus-eluting versus bare-metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive diseaseJ Vasc Surg201255239039822169682
- DakeMDAnselGMJaffMRZilver PTX InvestigatorsPaclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study resultsCirc Cardiovasc Interv20114549550421953370
- ZhaoHQNikanorovAVirmaniRSchwartzLBInhibition of experimental neointimal hyperplasia and neoatherosclerosis by local, stent-mediated delivery of everolimusJ Vasc Surg20125661680168822841285
- DudekDOnumaYOrmistonJAThuesenLMiquel-HebertKSerruysPWFour-year clinical follow-up of the ABSORB everolimus-eluting bioresorbable vascular scaffold in patients with de novo coronary artery disease: the ABSORB trialEuroIntervention2012791060106121959320
- DilettiRFarooqVGirasisCClinical and intravascular imaging outcomes at 1 and 2 years after implantation of absorb everolimus eluting bioresorbable vascular scaffolds in small vessels. Late lumen enlargement: does bioresorption matter with small vessel size? Insight from the ABSORB cohort B trialHeart20139929810523118346
- PhillipsBBallCSackettDLevels of evidence and grades of recommendations Available at: http://www.cebm.net/index.aspx?o=1025Accessed March 11, 2014Oxford, UKOxford Centre for Evidence-Based Medicine
