Abstract
Catheter-related blood stream infections increase morbidity, mortality, and costs. This study investigated whether Certofix® protect antimicrobial catheters carry a surface charge and whether this inhibits biofilm formation. The capacitance of the catheter surfaces was measured and, to determine if the catheters released ions, distilled water was passed through and current measured as a function of voltage. With probes touching the inner and outer surfaces, capacitance was not voltage-dependent, indicating surfaces were uncharged or carried a similar charge. When one probe penetrated the catheter wall, capacitance was weakly voltage-dependent, indicating the presence of a surface charge. Standard and charged catheters were also exposed to phosphate buffered saline as controls or 2×106 colony forming units/mL (in phosphate buffered saline) of six different microorganisms for 60 or 120 minutes. When the growth of detached bacteria was measured, biofilm formation was significantly reduced, (P<0.05), for charged catheters for all organisms.
Introduction
Central venous catheters (CVCs) are essential in the treatment of critically ill patients. However, this increases the risk of catheter-related bloodstream infections (CRBSI), which increase morbidity, mortality, and costs. It has been estimated that 250,000–500,000 infections related to CVCs occur in the United States annually, adding $33,000–65,000 per case to health care costs.Citation1
There are many potential risk factors for CRBSI. These include, inter alia: longer duration of catheterization; the location of the CVC; the use of parenteral nutrition or multilumen CVCs; catheter care; and aseptic insertion technique.Citation2
Several recent studies have investigated preventative strategies; these include the routine replacement of intravenous catheters,Citation2,Citation3 the development of “CVC bundles” or guidelines designed to reduce catheter-related infection (CRI),Citation4 and antimicrobial, silver ion or antiseptic impregnated CVCs designed to prevent attachment of organisms. The latter has not conclusively demonstrated benefit, however.Citation2,Citation5 Implant-associated infections are caused by microorganisms that are adherent to the device surface and embedded in a complex, extracellular, three-dimensional, polymeric matrix called biofilm. Biofilm- associated infections are generally persistent, as the embedded organisms exchange mediators of resistanceCitation6 and evade eradication by the immune system, detergents and antimicrobials.Citation6–Citation8
Passerini et alCitation9 have shown that 81% of all vascular catheters which had been in situ for between 1–14 days were colonized by bacteria in biofilm. These confer 10–1,000-fold greater resistance to antibiotics and microbiocides, when compared to their planktonic counterparts.Citation6,Citation10,Citation11 Under unfavorable conditions such as overcrowding and nutrient deprivation, planktonic bacteria detach from the biofilm and enter the bloodstream causing bacteremia and colonization of new sites.Citation12 Single or multiple bacterial species can make up a biofilm, with a dental biofilm containing more than 500 different bacterial taxa.Citation13
In this regard, B Braun Melsungen AG (Melsungen, Germany) has recently developed a CVC (Certofix® protect antimicrobial catheter; catheter A) that is claimed to prevent biofilm formation by means of a surface charge generated by positively charged chemical structures on the internal and external surfaces (Certofix® protect 2013).Citation14
Aims
The aims of this study were to investigate whether the charge on the catheter actually exists and whether it inhibits microbial adherence or biofilm formation when using six different microorganisms on two CVCs, one charged and one uncharged, but similar in appearance.
Materials and methods
Investigation of catheter charge Catheters
The charged catheters were sourced from B Braun Melsungen AG and consisted of two components, a hydrophilic polymer, blended with polyurethane, and a polyhexanide functionalized with a methacrylate group (a biguanide salt). These two compounds have opposite charges and – as such – an ionic bond occurs between them with net polarization of the biguanide due to charge deficiency. This reportedly forms a polarized surface that releases ions extremely slowly. The other catheter was a standard central venous catheter, identical in appearance, manufactured from polyurethane and without charge or antiseptic, silver ion, or antibiotic impregnation. In the biofilm experiments, the investigators were blinded as to which catheter was which.
Investigation of polarization
To investigate the polarization of the exterior surface of the catheters, the capacitance between the inner and outer surfaces was measured as a function of voltage. Capacitors store electric charge, generally between two charged plates and dielectric materials, such as polyurethane, and can enhance capacitance. A fully discharged capacitor maintains zero volts across its terminals; whereas, one that is charged can maintain a potential across its terminals. Excess charge, in this case due to polarization of the catheter surface, can be detected through capacitance versus voltage measurements. The capacitive response of the catheters was measured at room temperature under atmospheric conditions as a function of applied voltage using a Janis micromanipulated probe station (Janis Research Company, LLC (Woburn, MA, USA) and an Agilent B1500A semiconductor device analyzer (Agilent Technologies, Santa Clara, CA, USA). The measurements were performed with one of the probes penetrating the surface of the catheter, while the other was in contact with the surface to determine if any surface charge was present.
Finally, to determine if the catheter released ions, distilled water was passed through the catheters and multiple measurements of current, as a function of voltage, were made and compared with that generated through distilled water alone.
Estimation of effect on biofilm
Microorganisms utilized
Staphylococcus aureus, S. epidermidis, Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella pneumoniae, and Candida albicans were grown overnight to the mid-log phase, at 37°C in an atmosphere of 5% CO2 in tryptic soy broth ([TSB]; Merck Millipore, Billerica, MA, USA), followed by turbidimetric numerical standardization of the culture, representing 2×106 colony forming units (cfu)/mL, in phosphate buffered saline ([PBS] 0.15 M; BD Diagnostics, Sparks Glencoe, MD, USA).
Chemicals and reagents
All chemicals and reagents, unless otherwise indicated, were obtained from Sigma-Aldrich Co (St Louis, MO, USA).
Biofilm formation on intravenous catheters
The catheters were cut into 15 mm sections and exposed to either PBS (untreated control) or 2×106 cfu/mL bacteria (in PBS) for 60 or 120 minutes at 37°C, 5% CO2. Following incubation, the nonadherent bacteria were removed by washing the catheter sections with PBS. The washed sections were placed in TSB and incubated for 24 hours at 37°C, 5% CO2. Following incubation, the growth of the detached bacteria was determined through both turbidity measurements at 540 nm using the PowerWaveX™ plate reader (Bio-Tek Instruments Inc., Winooski, VT, USA) and standard cfu enumeration procedures. Before the amount of biofilm could be determined, the nonadherent bacteria and medium were removed from the catheter sections by washing the sections three times with PBS. Following the removal of all the unbound bacteria, the catheter sections were stained with 0.1% crystal violet, and the excess dye was removed by washing with PBS. The crystal violetCitation15 was released from the adherent bacteria by the addition of 96% ethanol and the amount of biofilm formed correlated directly with the color intensity of the crystal violet, which was determined spectrophotometrically at a wavelength of 570 nm, using the PowerWaveX™ plate reader.
Statistical analysis
Two to three experiments comprising 18–36 replicates in each system for each microorganism were performed. The results are expressed as mean ± standard error of the mean. Statistical analysis was performed using the Mann–Whitney U test (unpaired, nonparametric, two-tailed). *P values of ≤0.05 were considered significant.
Results
Investigation of catheter charge
Assessment of polarization
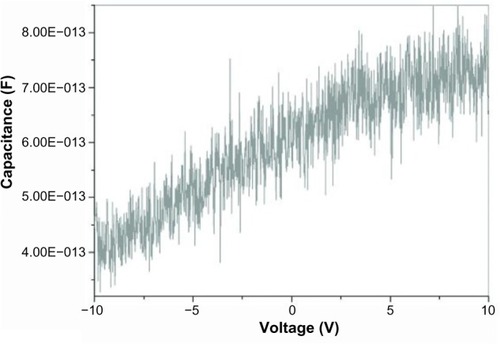
When the probes touched the inner and outer surfaces lightly, the capacitance was found to be voltage-independent (). This indicates that the inner and outer surfaces of the capacitor are either uncharged or have the same charge. In addition, the capacitance was enhanced by the fact that the catheters are dielectric; ie, each surface is insulated from the other.
Figure 1 Capacitance versus voltage when both probes are touching the outer surface of the capacitor.
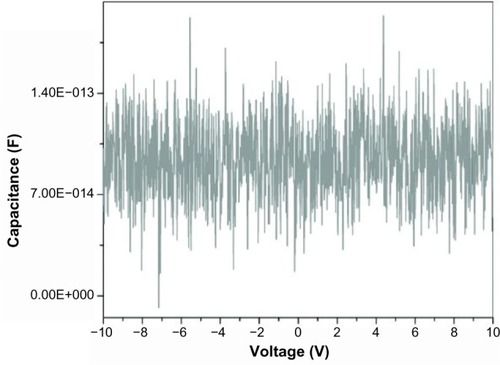
When one of the probes was inserted into the catheter wall, penetrating the outer surface, capacitance was found to be weakly voltage-dependent (). As the potential was varied from negative to positive, the charge on the probes changed. This could only occur if one of the probes was in contact with a charge, indicating that in this case, the outer surface is polarized. Also, since – as has been described previously – the capacitance is independent of voltage when both probes are in contact with the surface of the capacitor (), the inner and outer surfaces of the capacitor must have the same charge. This was confirmed by the fact that when the potential across the two probes was reversed, the capacitance decreased slightly with voltage as would be expected since the excess charge is in contact with the opposite probe in this case (not shown).
Assessment of release of ions
In control samples consisting of distilled water, the current was measured as a function of voltage for 55 μL droplets. Current is directly related to conductivity, so the presence of ions can be detected by measuring current as a function of voltage. The same measurements were then performed when distilled water was passed through the catheters, and a voltage applied. At any given voltage, the current (representative of conductivity) was smaller in the droplets of water that had been passed through the CVCs, indicating that ions had been adsorbed onto the catheter surface with a consequent decrease in conductivity. No direct evidence of release of ions was found; however, after the passage of greater volumes of distilled water through the catheter, the results were closer to those of the controls, indicating that the catheter surface had been saturated by ions acquired from the distilled water.
Biofilm formation
The amount of biofilm, after the untreated control has been subtracted, for each organism still adherent to the catheters is shown in the panel A series of –. The results for S. aureus and S. epidermidis were similar, and only those for S. aureus are shown. It is evident that there is a statistically significant difference (P<0.05) between both the 60- and 120-minute exposure times for all six organisms, with the charged catheter forming statistically less biofilm than the uncharged catheter at both time points. The quantity of bacteria that detached from the biofilm was determined by turbidity and cfu/mL, and as both of these growth measurements showed similar results, only the cfu/mL measurements were shown as depicted in the panel B series of –, each of which is representative of a different microorganism. Significantly fewer bacteria were present in the supernatant of the charged catheters at both time points, indicating fewer detached cells. It is noteworthy that the growth of P. aeruginosa in the uncharged catheter sections was greatly underestimated as detached bacteria proliferated to the extent that they showed visible clumping, which could not be broken up for fear of disrupting biofilm formation. As a consequence, fewer bacteria were considered for the growth determinations. Irrespective of this limitation, the difference between the two catheters still reached significance.
Figure 3 Charged and uncharged catheter sections were exposed to Staphylococcus aureus for 60 or 120 minutes. The amount of biofilm formed (A), and the amount of bacteria that detached from the biofilm in 24 hours measured as cfu/mL (B), are shown as the mean ± SEM. Two to three experiments with between 18 and 36 replicates for each system were analyzed.
Abbreviations: cfu, colony forming units; SEM, standard error of the mean.
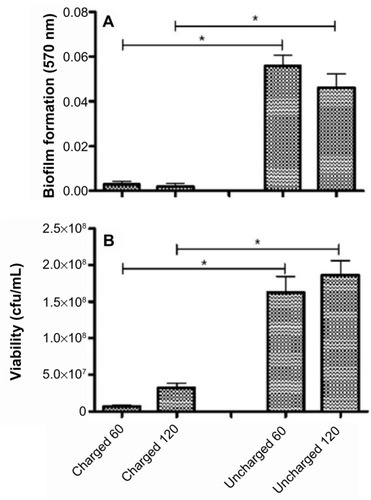
Figure 4 Charged and uncharged catheter sections were exposed to Pseudomonas aeruginosa for 60 or 120 minutes. The amount of biofilm formed (A), and the amount of bacteria that detached from the biofilm in 24 hours measured as cfu/mL (B), are shown as the mean ± SEM. Two to three experiments with between 18 and 36 replicates for each system were analyzed.
Abbreviations: cfu, colony forming units; SEM, standard error of the mean.
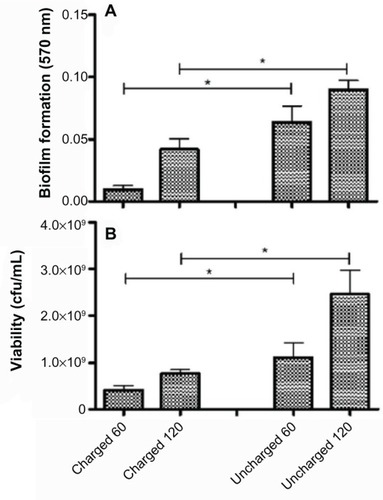
Figure 5 Charged and uncharged catheter sections were exposed to Klebsiella pneumoniae for 60 or 120 minutes. The amount of biofilm formed (A), and the amount of bacteria that detached from the biofilm in 24 hours measured as cfu/mL (B), are shown as the mean ± SEM. Two to three experiments with between 18 and 36 replicates for each system were analyzed.
Abbreviations: cfu, colony forming units; SEM, standard error of the mean.
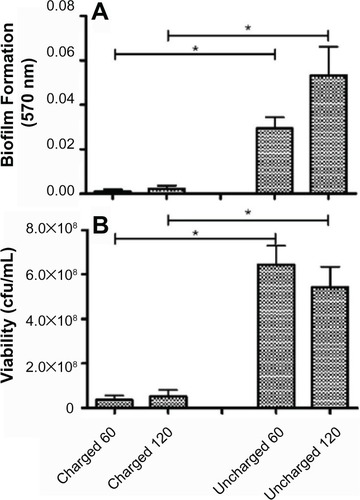
Figure 6 Charged and uncharged catheter sections were exposed to Acinetobacter baumanii for 60 or 120 minutes. The amount of biofilm formed (A), and the amount of bacteria that detached from the biofilm in 24 hours measured as cfu/mL (B), are shown as the mean ± SEM. Two to three experiments with between 18 and 36 replicates for each system were analyzed.
Abbreviations: cfu, colony forming units; SEM, standard error of the mean.
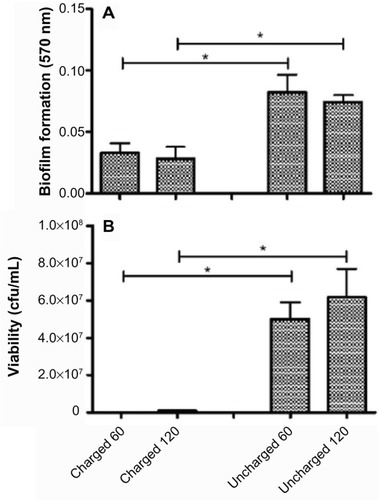
Figure 7 Charged and uncharged catheter sections were exposed to Candida albicans for 60 or 120 minutes. The amount of biofilm formed (A), and the amount of bacteria that detached from the biofilm in 24 hours measured as cfu/mL (B), are shown as the mean ± SEM. Two to three experiments with between 18 and 36 replicates for each system were analyzed.
Abbreviations: cfu, colony forming units; SEM, standard error of the mean.
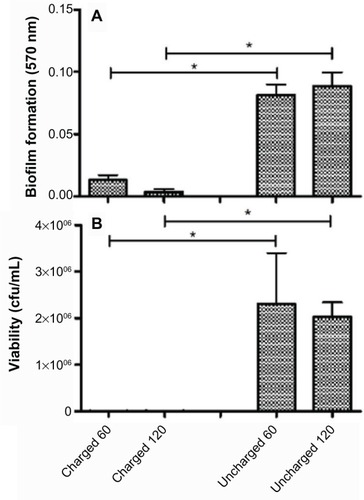
Discussion
Intravascular catheters are a major source of bloodstream infections in hospitalized patients. The prevalence of catheter-related infections is approximately five episodes per 1,000 catheter days in EuropeCitation16 and one to six episodes per 1,000 catheter days in the United States.Citation1 These infections often result in increased morbidity and mortality and increase costs considerably.Citation17 Microbial adherence is essential to the formation of biofilm;Citation18 however, this adherence is dependent on the ionic interaction between the microorganism and the surface.Citation19 It has been shown that a negative charge attracts bacteria,Citation20 suggesting that a positively charged surface will repel bacteria and, thereby, inhibit adhesion. This principle has recently been explored with the use of the copper coating of commonly touched surfaces in the ICU, which significantly reduces hospital-acquired infection.Citation21
This study investigated whether the Certofix® protect (charged) catheter had a polarized inner and outer surface as claimed, and whether adherence of microorganisms would be inhibited in comparison to an otherwise identical uncharged catheter.
We showed that both the external as well as the internal surfaces of the Certofix® protect catheter retained a small positive charge, which was maintained by ionic bonding and that no ions were released, indicating a stable product, which will retain its charge over an extended time period. This charge appeared to have a significant inhibitory effect on biofilm formation as well as decreasing the number of detached bacteria as determined by growth (optical density and cfu measurements), when compared to the uncharged catheters.
These differences were evident following 60- and 120-minute exposure times for all microorganisms tested. Even though this time period is short when compared to the time an intravenous catheter will be used, the catheters were exposed to a far higher concentration of microorganisms (2×106 cells/mL) than is likely to occur in the clinical setting.
An additional limitation of the experimental design was the cutting of catheter sections. Even though great care was taken, the surface of the catheter could have been damaged, which could have provided a nidus for attachment, growth, and biofilm formation. Unfortunately, there was no feasible alternative method by which these experiments could have been performed and, in addition, both catheters were sectioned in an identical manner, and – despite this – significant differences between the catheters persisted. The experimental design also catered only for total (surface and intraluminal) adherence and biofilm formation, and we could not distinguish between the two.
It is possible that the absorbance of the ions from water, as well as the cutting of the catheters, may have had an impact on the charged catheter’s ability to prevent adherence of bacteria over longer periods of time; however, a well-designed, controlled clinical study should be able to answer this question. Several care facilities have introduced the utilization of bundles of care to reduce catheter-associated infections. Nonetheless, any additional intervention that could further impact on the morbidity and mortality associated with catheter use is to be welcomed.
Acknowledgments
B Braun Melsungen AG (Melsungen, Germany) supplied the catheters and sufficient funds to perform the biofilm studies in the form of an unrestricted grant. RC is supported by the National Research Foundation of South Africa. RM is supported by the National Research Foundation (SA) under the Nanotechnology Flagship Project grant and the University Research Council (Witwatersrand).
Disclosure
The authors report no conflicts of interest in this work.
References
- BrooksJLJeffersonKKStaphylococcal biofilms: quest for the magic bulletAdv Appl Microbiol201281638722958527
- MerMDuseAGGalpinJSRichardsGACentral venous catheterization: a prospective, randomized double-blind studyClin Appl Thromb Hemost2009151192618593746
- RickardCMWebsterJWallisMCRoutine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trialLancet201238098471066107422998716
- O’GradyNPAlexanderMDellingerEPGuidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and PreventionMMWR Recomm Rep200251RR-1012912233868
- McConnellSAGubbinsPOAnaissieEJAre antimicrobial-impregnated catheters effective? Replace the water and grab your washcloth, because we have a baby to washClin Infect Dis200439121829183315578407
- AslamSEffect of antibacterials on biofilmsAm J Infect Control20083610S175.e9S175.e1119084156
- FuxCACostertonJWStewartPSStoodleyPSurvival strategies of infectious biofilmsTrends Microbiol2005131344015639630
- Hall-StoodleyLCostertonJWStoodleyPBacterial biofilms: from the natural environment to infectious diseasesNat Rev Microbiol2004229510815040259
- PasseriniLLamKCostertonJWKingEGBiofilms on indwelling vascular cathetersCrit Care Med19922056656731572192
- MoscosoMGarcíaELópezRPneumococcal biofilmsInt Microbiol2009122778519784927
- GilbertPMaira-LitranTMcBainAJRickardAHWhyteFWThe physiology and collective recalcitrance of microbial biofilm communitiesAdv Microb Physiol20024620225612073654
- TejedorSCTongDSteinJTemporary central venous catheter utilization patterns in a large tertiary care centre: tracking the “idle central venous catheter”Infect Control Hosp Epidemiol2012331505722173522
- SureshGKEdwardsWHCentral line-associated bloodstream infections in neonatal intensive care: changing the mental model from inevitability to preventabilityAm J Perinatol2012291576421879458
- Certofix®protect – Antipathogenic central venous catheter (CPAC) for long-term protection against catheter infection [webpage on the Internet]German Healthcare Export Group Available from: http://www.gheg.de/en/products/product/4575_Certofix_protect_Antipatho-genic_central_venous_catheter_for_long-term_protection_against_catheter_infection/index.htmlAccessed January 6, 2014
- VesterlundSPalttaJKarpMOuwehandACMeasurement of bacterial adhesion–in vitro evaluation of different methodsJ Microbiol Methods200560222523315590097
- van Eck van der SluijsAOosterheertJJEkkelenkampMBHoepelmanIMPetersEJBacteremic complications of intravascular catheter tip colonization with Gram-negative micro-organisms in patients without preceding bacteremiaEur J Clin Microbiol Infect Dis20123161027103321909649
- DimickJBPelzRKConsunjiRSwobodaSMHendrixCWLipsettPAIncreased resource use associated with catheter-related bloodstream infection in the surgical intensive care unitArch Surg2001136222923411177147
- ZhangLGowardmanJRickardCMImpact of microbial attachment on intravascular catheter-related infectionsInt J Antimicrob Agents201138191521435841
- TobaFAVisaiLTrivediSLowyFDThe role of ionic interactions in the adherence of the Staphylococcus epidermidis adhesion SdrF to prosthetic materialFEMS Microbiol Lett20133881243023039791
- YamashitaYKunimoriATakeharaTEffect of calcium ions on cell surface electrostatics of Bacteroides gingivalis and other oral bacteriaZentralbl Bakteriol1991275146531930564
- SalgadoCDSepkowitzKAJohnJFCopper surfaces reduce the rate of healthcare-acquired infections in the intensive care unitInfect Control Hosp Epidemiol201334547948623571364