Abstract
Recently, the development of effective strategies for enhancing drug delivery to the brain has been a topic of great interest in both clinical and pharmaceutical fields. In this review, we summarize our studies evaluating nose-to-brain delivery of drugs and small interfering ribonucleic acids in combination with cell-penetrating peptide-modified polymer micelles. Our findings show that the use of polymer micelles with surface modification with Tat peptide in the intranasal administration enables the non-invasive delivery of therapeutic agents to the brain by increasing the transfer of the administered drug or small interfering ribonucleic acid to the central nervous system from the nasal cavity.
Introduction
The development of novel therapeutic systems that would be effective against intractable central nervous system (CNS) diseases such as Alzheimer’s disease, Parkinson’s disease, and brain tumors is expected to bring about breakthroughs in the future. In order to achieve therapeutic effectiveness, novel drug delivery systems capable of reaching target tissues are needed, in addition to new drugs.
In general, the blood–brain barrier (BBB) poses a major challenge to the drug development efforts targeting CNS disorders, since it limits the distribution of systemically administered therapeutics to the CNS.Citation1,Citation2 Therefore, the development of effective delivery systems capable of achieving therapeutic drug concentrations in the brain is of major interest not only in pharmaceutical research but also in the clinical field. Recently developed methodologies for brain drug delivery have been characterized as either invasive or non-invasive. The invasive methodologies consist of direct drug delivery by means of intraventricular or intracerebral administration and temporary disruption of the BBB, allowing the drug to enter the CNS.Citation3 Non-invasive methodologies involve systemic drug delivery that uses drug carriers to deliver agents through a receptor-mediated or adsorptive transcytosis,Citation4 or alternatively, by bypassing the BBB by using the nose-to-brain route.Citation5–Citation9
Intranasal delivery is a recognized non-invasive method of direct delivery of drugs to the CNS.Citation5–Citation9 In general, drugs administered through the nose-to-brain route are widely distributed within the cerebral regions, typically by migrating to the olfactory nerve, which is located in the epithelial tissue of the nasal olfactory mucosa inside the nasal cavity. Citation10–Citation12 Migration also occurs along the trigeminal nerve in the nasal cavity respiratory mucosa.Citation10–Citation12 Transport may also occur through the capillaries, lymphatics, and cerebrospinal fluid present in the nasal mucosa or by nasal mucociliary movement.Citation10–Citation14 Low-molecular-weight drugs,Citation15,Citation16 peptides,Citation10,Citation17 proteins,Citation18,Citation19 small interfering ribonucleic acid (siRNA),Citation20,Citation21 and cellsCitation22–Citation24 are reported to reach the CNS following intranasal delivery in animals.Citation13 In addition, insulin,Citation25–Citation29 melanocortin,Citation30–Citation33 angiotensin II,Citation34 vasopressin,Citation35 and oxytocinCitation36–Citation41 were shown to be directly delivered to the CNS following intranasal administration in humans.Citation13,Citation42 Intranasal administration also provides a painless and convenient route that could be used for self-administration of drugs by patients.Citation9–Citation12,Citation43 Therefore, nose-to-brain delivery is a highly versatile route, which, in combination with novel drugs being developed for treating intractable CNS diseases, is a promising approach for the treatment of disorders such as Alzheimer’s disease, Parkinson’s disease, and brain tumors. Furthermore, nano-sized drug carriers may improve nose-to-brain drug delivery by their capability to increase the stability of the encapsulated drug against chemical and biological degradation. The modification of the surface of nanocarriers with the incorporation of cell-penetrating peptides (CPPs) could further improve the specific drug delivery into the target cells. In this review, we will introduce our studies evaluating anti-cancer drugs and/or siRNAs’ delivery to the brain using a combination of the nose-to-brain route and CPP-modified polymer micelles.Citation44–Citation47
Polymeric micelles for nose-to brain delivery
A key property of the amphiphilic block copolymer is its capability to form nano-sized micelles by self-assembly in a particular solvent.Citation48–Citation50 The nano-sized polymer micelles have been utilized as core-shell-type colloidal carriers for delivery of drugs and genes.Citation51–Citation53 The use of these carriers could increase the availability of drugs to the CNS, since their small diameter would potentially allow the nanoparticles to be transported transcellularly along the neurons to the brain through various endocytic pathways of sustentacular or neuronal cells present in the nasal membrane.Citation11
We selected methoxypolyethylene glycol (MPEG)-polycaprolactone (PCL) (MPEG-PCL) co-polymers, which can be used to form nano-sized micelles, as nanocarriers and prepared coumarin-loaded MPEG-PCL micelles at a range of diameters. At first, we examined the brain- and bio-distribution of fluorescein model drugs (coumarin) in rats following intranasal administration of coumarin-loaded MPEG-PCL micelles, ranging in diameter from 100 to 600 nm.Citation44 No significant difference was observed between micelles with diameters ranging from 200 to 600 nm. However, coumarin concentration in the brain following administration of coumarin-loaded PEG-PCL micelles with a diameter of 100 nm was significantly higher than that observed with micelles of a larger diameter, indicating that nano-sized polymer micelles with a diameter of around 100 nm can facilitate nasal absorption and increase the delivery of drugs into the CNS. In previous reports, smaller diameters may allow nanoparticles to be transported to the brain through various endocytic pathways of sustentacular or neuronal cells in the olfactory membrane.Citation6,Citation11,Citation12,Citation43,Citation54 Therefore, these findings indicate that small nanoparticles are the most promising for use in intranasal brain delivery. The coumarin concentrations in non-targeted tissues, such as the liver, heart, kidney, and spleen, were found to be lower than those measured in the brain, indicating that intranasal administration of drugs may decrease the risk of side effects in non-CNS tissues.
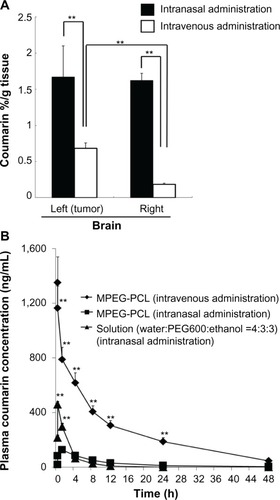
We compared the efficiency of brain delivery following intravenous and intranasal routes of administration of MPEG-PCL nanomicelles.Citation44 As shown in , the amount of coumarin measured in brain tissue after intranasal administration was significantly higher than that observed after intravenous administration, providing further evidence that the intranasal route is an effective method for brain-targeted drug delivery. Additionally, the duration of coumarin presence in blood circulation after intranasal administration was significantly shorter than that observed after intravenous injection (). In particular, we were only able to detect very low levels of coumarin in the blood of rats following intranasal administration of coumarin-loaded MPEG-PCL micelles, indicating that drugs loaded within the micelles are delivered intranasally directly to the brain without significant leakage into systemic circulation. However, coumarin was found to enter the systemic blood circulation soon after intranasal administration of coumarin solution alone. Therefore, administration of drug solution is associated with the risk of side effects resulting from toxic effects on non-CNS tissues. These findings suggest that intranasal delivery of drugs into brain tissue using nano-sized micelles is more likely to be effective and less likely to produce side effects in non-CNS tissues.
Figure 1 Distribution in brain tissue and blood concentration of coumarin after intravenous or intranasal administration of coumarin-loaded MPEG-PCL micelles.
Abbreviations: MPEG-PCL, methoxypolyethylene glycol-polycaprolactone; SE, standard error; h, hours; PEG, polyethylene glycol.
CPP-modified polymeric micelles for nose-to-brain delivery
The CPPs, which are cationic or amphiphilic molecules derived from sources such as the human immunodeficiency virus Tat protein and Drosophila antennapedia homeoprotein, can enhance the intracellular delivery of molecules.Citation55 A basic domain of Tat was previously reported to be the minimum sequence responsible for cellular uptake mediated by the 11-amino acid epitope YGRKKRRQRRR.Citation56
Conjugation of the CPP to the surface on the nanocarriers was expected to increase the penetration across the nasal mucosa and target cell membrane into the brain. Therefore, we developed MPEG-PCL copolymers conjugated with Tat peptide (MPEG-PCL-Tat) and evaluated the potential for intranasal brain delivery of coumarin-loaded MPEG-PCL-Tat micelles ().Citation44
Figure 2 Structure of Tat-modified MPEG-PCL micelle.
Abbreviations: MPEG, methoxypolyethylene glycol; PCL, polycaprolactone; CPP, cell-penetrating peptide; G, glycine; R, arginine; K, lysine; Q, glutamine.
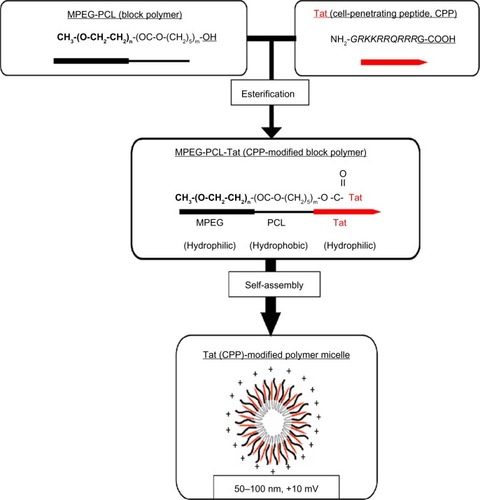
MPEG-PCL-Tat micelles showed high loading efficiency of coumarin. MPEG-PCL-Tat micelles had a smaller particle size (diameter of approximately 100 nm) than the MPEG-PCL micelles without Tat and exhibited a positive charge, whereas MPEG-PCL micelles were negatively charged. These findings suggest that synthesized MPEG-PCL-Tat forms nanoparticles, possibly as polymer micelles. Tat is almost completely presented on the surface of the polymer micelles, since MPEG-PCL-Tat nanoparticles exhibited the positive charge of the Tat peptide.
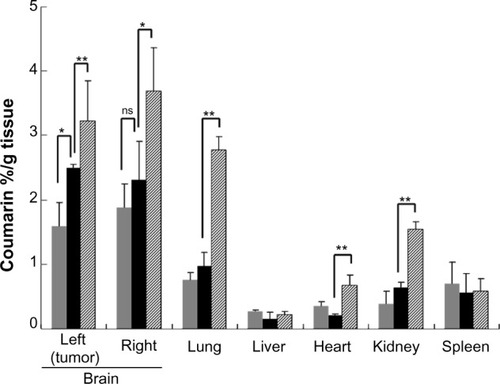
We first compared the efficacy of nose-to-brain delivery between MPEG-PCL and MPEG-PCL-Tat micelles. At 4 hours following intranasal administration, coumarin concentration in brain tissue of rats administered coumarin-loaded MPEG-PCL-Tat was significantly higher than in rats administered coumarin-loaded MPEG-PCL micelles. We subsequently determined the biodistribution of coumarin in rats after intranasal administration of coumarin-loaded MPEG-PCL-Tat micelles. As shown in , the concentrations of coumarin in non-targeted tissues, such as liver, lung, heart, kidney, and spleen, in rats administered coumarin-loaded MPEG-PCL-Tat were lower than those measured following administration of coumarin solution without the polymer micelles. These findings indicate that CPP-modified nano-sized micelles can improve the nose-to-brain drug delivery.
Figure 3 Biodistribution of coumarin in rats after intranasal administration of coumarin-loaded MPEG-PCL-Tat micelles.
Abbreviations: MPEG-PCL, methoxypolyethylene glycol-polycaprolactone; SE, standard error; ns, not significant.
Nose-to-brain delivery of drug-loaded polymer micelles for treatment of brain tumors
Although chemotherapy is widely applied in treatment of brain tumors, the outcomes continue to be unsatisfactory. In general, an increase in local anti-cancer drug concentration in the tumor tissue improves the outcome of the drug treatment. Therefore, we expected that the delivery of anti-cancer drugs using a combination of nose-to-brain route and MPEG-PCL-Tat micelles could increase the concentration of drugs at the intracranial tumors and suppress their growth.Citation45 Therefore, we prepared MPEG-PCL and MPEG-PCL-Tat micelles loaded with the anti-cancer drug camptothecin (CPT). The particle size of CPT-loaded MPEG-PCL and MPEG-PCL-Tat micelles was slightly larger than that before CPT loading. CPT encapsulation efficiencies of MPEG-PCL and MPEG-PCL-Tat were determined to be around 60%.
We evaluated the in vitro cytotoxicity in C6 rat glioma cells at a range of CPT concentrations. CPT-loaded Tat-modified MPEG-PCL exhibited stronger cytotoxicity than CPT-loaded MPEG-PCL. This observation may be due to the improved cellular uptake efficiency of CPT-loaded MPEG-PCL-Tat micelles by modification with CPP Tat on the surface of micelles. CPT-free MPEG-PCL-Tat did not exhibit any cytotoxicity at the higher concentrations tested. These results indicate that Tat-modified micelles strongly interact with the C6 rat glioma cells. Tat-modified polymer micelles would therefore be expected to result in greater delivery of CPT to tumor cells than polymer micelles without Tat.
We next determined the in vivo therapeutic effects in a rat model of intractable malignant glioma. CPT-loaded MPEG-PCL-Tat micelles exhibited high therapeutic efficiency after 7 days of continuous administration, unlike CPT-loaded MPEG-PCL. This observation suggests that Tat-modified MPEG-PCL micelles may be more effective because of high penetration of small molecule anti-cancer drugs across the nasal epithelium. The specificity achieved with intranasal delivery appears to be superior to the results obtained with the administration of simple CPT solution, which reportedly results in high drug levels in the systemic circulation.
siRNA delivery to the brain using nose-to-brain administration and CPP-modified polymer micelles
siRNA has great potential as a therapeutic agent against CNS diseases.Citation57–Citation61 Although the potential of using siRNA-based therapies against CNS disorders has been successfully demonstrated through in vitro studies, the function of BBB poses a major challenge to the drug development efforts aimed at treating CNS disorders. Therefore, the development of effective strategies that would enhance siRNA delivery to the brain is of great interest for both the clinical and pharmaceutical fields. In order to improve the efficiency of brain siRNA delivery systems, we developed an approach that combines siRNA nose-to-brain delivery system with CPP-modified nano-sized micelles.Citation46
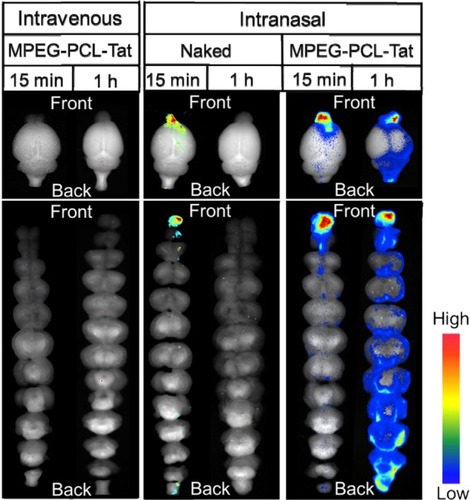
Significantly higher brain penetration was observed following intranasal administration of MPEG-PCL-Tat to rats than with intravenous injection, suggesting that superior delivery of nucleic acid to the brain is possible using peptide-modified micelles. Additionally, in our study of the mechanisms that promote nucleic acid transfer to the brain following intranasal administration using MPEG-PCL-Tat, we focused on the olfactory and trigeminal nerves, which are the two reported pathways of drug transport to the brain from the nasal cavity. First, nasal mucosal tissue was observed 15 minutes after intranasal administration of naked siRNA and complexes of siRNA with MPEG-PCL-Tat micelles. As shown in , animals administered siRNA complexed with MPEG-PCL-Tat exhibited more intense fluorescence in the mucosal epithelial surface and lamina propria mucosae than the animals administered naked siRNA, indicating that MPEG-PCL-Tat exhibits high mucosal penetration. Next, we assessed the distribution of the siRNA to the olfactory bulb after intranasal administration and found a significantly higher transfer of the siRNA to the olfactory bulb in the MPEG-PCL-Tat-treated animals, as compared to those administered the naked siRNA (). This finding suggests that the use of MPEG-PCL-Tat may increase the transfer of nucleic acid to the brain via the olfactory nerve pathway in the nasal mucosal tissue. Additionally, the observation of the trigeminal nerve after intranasal administration detected higher transfer of the fluorescein-labeled model siRNA (dextran, molecular weight: 10,000) to the trigeminal nerve in the MPEG-PCL-Tat group than in animals administered fluorescein-labeled model siRNA alone. This observation suggests that, as with the olfactory nerve, MPEG-PCL-Tat may also enable the nucleic acid to be delivered to the brain via the trigeminal nerve. demonstrates the distribution in the whole brain, from olfactory bulb to the brainstem, over time, measured following intravenous or intranasal administration of Anionic dextran labeled with Alexa Fluor® 678 (molecular weight: 10,000), (Alexa-dextran; Life Technologies, Carlsbad, MA, USA) model siRNA with and without MPEG-PCL-Tat. While the fluorescence of Alexa-labeled model siRNA did not distribute in the whole brain following intravenous administration, high accumulation was observed in the olfactory bulb 15 minutes after administration via the nose-to-brain pathway, both with or without MPEG-PCL-Tat. One hour after intranasal administration with MPEG-PCL-Tat, high accumulation was observed in the rostral brain tissue that appeared to follow the distribution trend into the olfactory bulb, while the caudal brain tissue and brain stem exhibited a high concentration that appeared to follow a distribution trend into the trigeminal nerve. Taken together, these observations suggest that, once transported to the olfactory bulb and brain stem via the olfactory nerve and trigeminal nerve pathways, nucleic acid is subsequently transported to other brain tissues. This shows that the proposed system of delivery of nucleic acid to the brain by intranasal administration using MPEG-PCL-Tat relies primarily on an increase in the transfer of nucleic acid to the brain via the olfactory nerve and trigeminal nerve pathways.
Figure 4 Distribution of FAM-siRNA in slices of nasal mucosa after intranasal administration of naked FAM-siRNA and MPEG-PCL-Tat/FAM-siRNA.
Abbreviations: OE, olfactory mucosa epithelium; LP, lamina propria; NC, nasal cavity; MPEG-PCL, methoxypolyethylene glycol-polycaprolactone; N/P, ratio of amine to nucleic acid; FAM, 6-Carboxyfluorescein-aminohexyl; siRNA, small interfering ribonucleic acid.
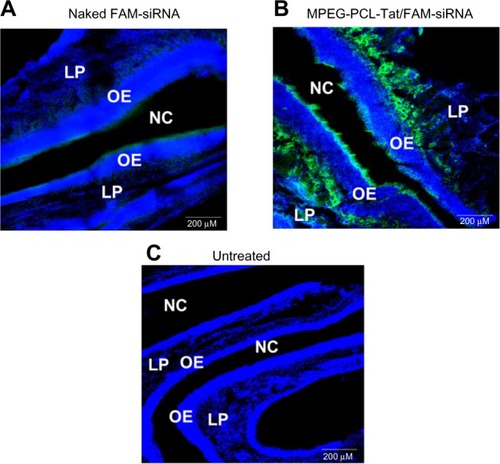
Figure 5 siRNA distribution in the olfactory bulb tissue after intranasal administration.
Abbreviations: GL, glomerular layer; EPL, external plexiform layer; MCL, mitral cell layer; GCL, granule cell layer; siRNA; small interfering ribonucleic acid; MPEG-PCL, methoxypolyethylene glycol-polycaprolactone; N/P, ratio of amine to nucleic acid; FAM, 6-Carboxyfluorescein-aminohexyl (FAM)-siRNA (Cosmo Bio Co., Ltd., Tokyo, Japan) as a fluorescent-labeled siRNA..
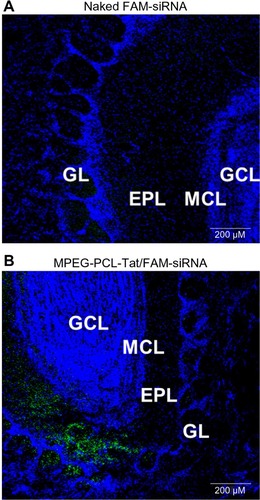
Figure 6 Dynamics of MPEG-PCL-Tat complex in brain tissue following intranasal or intravenous administration.
Abbreviations: MPEG-PCL, methoxypolyethylene glycol-polycaprolactone; h, hour; min, minutes.
Co-delivery of siRNA and drugs using nose-to-brain and CPP-modified polymer micelles for treatment of brain tumors
We investigated intranasal delivery of siRNA/drug co-loaded MPEG-PCL-Tat micelles using a rat model of malignant glioma.Citation47 The MPEG-PCL-Tat/siRNA complex and CPT-loaded MPEG-PCL-Tat/siRNA complex exhibited micelle diameters of approximately 60–200 nm, with a tendency of size to decrease as the ratio of amine to nucleic acid (N/P ratio) increased. The zeta-potential of these complexes was found to increase with increasing N/P ratio. Furthermore, on evaluation by the SYBR Green exclusion assay (Takara Bio Inc., Shiga, Japan), the fluorescence intensity from siRaf-1 of MPEG-PCL-Tat/siRaf-1 complexes strongly decreased at an N/P ratio of 1 (10%), compared with that of naked siRNA (100%). These results indicate that MPEG-PCL-Tat and CPT-loaded MPEG-PCL-Tat could form a stable complex with siRNA.
We subsequently determined the cellular uptake of siRNA and evaluated its in vitro cytotoxicity in rat glioma C6 cells. The intracellular siRNA uptake of siRNA/MPEG-PCL-Tat complexes increased with increasing N/P ratio, with the highest intracellular uptake efficacy observed with the complexes at an N/P ratio of 30. To evaluate the in vitro transfection efficiency of the MPEG-PCL-Tat/siRNA complexes at an N/P ratio of 30, we assessed the cytotoxicity induced by Raf-1 gene silencing in C6 cells using the WST-8 assay (Dojindo Laboratories, Kumamoto, Japan). These results showed that the cell viability significantly decreased with an increase in the concentration of Raf-1 siRNA (siRaf-1), indicating that MPEG-PCL-Tat/siRaf-1 induces cell death in rat glioma cells due to the high cellular uptake of siRaf-1 by MPEG-PCL-Tat carrier.
We finally evaluated the in vivo therapeutic effects in glioma model rats of intranasally administered naked siRaf-1 without any micelles, MPEG-PCL-Tat/control siRNA, MPEG-PCL-Tat/siRaf-1 complex, CPT-loaded-MPEG-PCL-Tat micelles/control siRNA complex, and CPT-loaded MPEG-PCL-Tat micelles/siRaf-1 complexes. MPEG-PCL-Tat/siRaf-1 complex, CPT-loaded-MPEG-PCL-Tat micelles/control siRNA complex, and CPT-loaded MPEG-PCL-Tat micelles/siRaf-1 complexes showed high therapeutic efficacy after 7 days of continuous delivery. These results indicate that MPEG-PCL-Tat improved the delivery of siRNAs and CPT to the brain, and showed a marked prolongation of the mean survival period. No cytotoxicity was detected in neuronal cells and no signs of macroscopic damage in rat nasal mucosa, olfactory and trigeminal nerves, and brain tissue were observed.
Conclusion and future perspectives
Through the review, we have shown that the use of polymer micelles with surface-loaded Tat peptide in the intranasal administration of drugs and siRNAs enables the non-invasive delivery of therapeutic agents to the brain by increasing the transfer of drug or siRNA to the CNS from the nasal cavity. This improvement in delivery is not as pronounced with naked micelles. We feel that this review offers important insight that can be applied to improve drug delivery to the brain and shows the potential of using drug-loaded surface-loaded polymer micelles against intractable neuropsychiatric disorders, brain tumors, and cerebral infarction.
Acknowledgments
This work was supported in part by Grant-in-Aid for Young Scientists (B) (25870767) from the Japan Society for the Promotion of Science (JSPS) and in part by a Grant for Private Universities provided by the Promotion and Mutual Aid Corporation for Private Schools of Japan.
Disclosure
The author declares no conflicts of interest in this work.
References
- PathanSAIqbalZZaidiSMCNS drug delivery systems: novel approachesRecent Pat Drug Deliv Formul200931718919149731
- XiaHGaoXGuGLow molecular weight protamine-functionalized nanoparticles for drug delivery to the brain after intranasal administrationBiomaterials201132369888989821937105
- TosiGCostantinoLRivasiFTargeting the central nervous system: in vivo experiments with peptide-derivatized nanoparticles loaded with loperamide and rhodamine-123J Control Release200712211917651855
- KreuterJNanoparticulate system for brain delivery of drugsAdv Drug Deliv Rev2001471658111251246
- DhuriaSVHansonLRFreyWH2ndIntranasal delivery to the central nervous system: mechanisms and experimental considerationsJ Pharm Sci20109941654167319877171
- ThorneRGFreyWH2ndDelivery of neurotrophic factors to the central nervous system: pharmacokinetic considerationsClin Pharmacokinet2001401290794611735609
- IllumLTransport of drugs from the nasal cavity to the central nervous systemEur J Pharm Sci200011111810913748
- IllumLIs nose-to-brain transport of drugs in man a reality?J Pharm Pharmacol200456131714979996
- MistryAStolnikSIllumLNanoparticles for direct nose-to-brain delivery of drugsInt J Pharm2009379114615719555750
- ThorneRGPronkGJPadmanabhanVFreyWH2ndDelivery of insulin-like growth factor-1 to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administrationNeuroscience2004127248149615262337
- LochheadJJThorneRGIntranasal delivery of biologics to the central nervous systemAdv Drug Deliv Rev201164761462822119441
- DjupeslandPGMessinaJCMahmoudRAThe nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overviewTher Deliv20145670973325090283
- ChapmanCDFreyWH2ndCraftSIntranasal treatment of central nervous system dysfunction in humansPharm Res201230102475248423135822
- MerkusFWVan Den BergMPCan nasal drug delivery bypass the blood-brain barrier?: questioning the direct transport theoryDrugs R D20078313314417472409
- ShingakiTInoueDFurubayashiTTransnasal delivery of methotrexate to brain tumors in rats: a new strategy for brain tumor chemotherapyMol Pharm2010751561156820695463
- SakaneTYamashitaSYataNSezakiHTransnasal delivery of 5-fluorouracil to the brain in the ratJ Drug Target19997323324010680979
- ThorneRGHansonLRRossTMTungDFreyWH2ndDelivery of interferon-beta to the monkey nervous system following intranasal administrationNeuroscience2008153278579718304744
- Alcalá-BarrazaSRLeeMSHansonLRMcDonaldAAFreyWH2ndMcLoonLKIntranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNSJ Drug Target201018317919019807216
- YangJPLiuHJChengSMDirect transport of VEGF from the nasal cavity to brainNeurosci Lett2009449210811118996442
- RennerDBFreyWH2ndHansonLRIntranasal delivery of siRNA to the olfactory bulbs of mice via the olfactory nerve pathwayNeurosci Lett2012513219319722387067
- NishinaKMizusawaHYokotaTShort interfering RNA and the central nervous system: development of nonviral delivery systemsExpert Opin Drug Deliv201310328929223289737
- DanielyanLSchäferRvon Ameln-MayerhoferAIntranasal delivery of cells to the brainEur J Cell Biol200988631532419324456
- DanielyanLSchäferRvon Ameln-MayerhoferATherapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson diseaseRejuvenation Res201114131621291297
- van VelthovenCTSheldonRAKavelaarsAMesenchymal stem cell transplantation attenuates brain injury after neonatal strokeStroke20134451426143223539530
- KernWBornJSchreiberHFehmHLCentral nervous system effects of intranasally administered insulin during euglycemia in menDiabetes199948355756310078556
- BenedictCHallschmidMHatkeAIntranasal insulin improves memory in humansPsychoneuroendocrinology200429101326133415288712
- BenedictCKernWSchultesBBornJHallschmidMDifferential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulinJ Clin Endocrinol Metab20089341339134418230654
- BenedictCBredeSSchiothHBIntranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy menDiabetes201160111411820876713
- RegerMAWatsonGSGreenPSIntranasal insulin improves cognition and modulates beta-amyloid in early ADNeurology200870644044817942819
- BornJLangeTKernWMcGregorGPBickelUFehmHLSniffing neuropeptides:a transnasal approach to the human brainNat Neurosci20025651451611992114
- FehmHLSmolnikRKernWMcGregorGPBickelUBornJThe melanocortin melanocyte-stimulating hormone/adrenocorticotropin(4–10) decreases body fat in humansJ Clin Endocrinol Metabol200186311441148
- SmolnikRPerrasBMolleMFehmHLBornJEvent-related brain potentials and working memory function in healthy humans after single-dose and prolonged intranasal administration of adrenocorticotropin 4–10 and desacetyl-alpha-melanocyte stimulating hormoneJ Clin Psychopharmacol200020444545410917406
- HallschmidMSmolnikRMcGregorGBornJFehmHLOverweight humans are resistant to the weight-reducing effects of melanocortin 4–10J Clin Endocrinol Metabol2006912522525
- DeradIWillekeKPietrowskyRBornJFehmHLIntranasal angiotensin II directly influences central nervous regulation of blood pressureAm J Hypertens1998118 Pt 19719779715790
- PietrowskyRStrubenCMolleMFehmHLBornJBrain potential changes after intranasal vs. intravenous administration of vasopressin: evidence for a direct nose–brain pathway for peptide effects in humansBiol Psychiatry19963953323408704064
- KosfeldMHeinrichsMZakPJFischbacherUFehrEOxytocin increases trust in humansNature2005435704267367615931222
- KirschPEsslingerCChenQOxytocin modulates neural circuitry for social cognition and fear in humansJ Neurosci20052549114891149316339042
- DomesGHeinrichsMMichelABergerCHerpertzSCOxytocin improves “mind-reading” in humansBiol Psychiatry200761673173317137561
- HeinrichsMBaumgartnerTKirschbaumCEhlertUSocial support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stressBiol Psychiatry200354121389139814675803
- LabuschagneIPhanKLWoodAOxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorderNeuropsychopharmacology201035122403241320720535
- GuastellaAJEinfeldSLGrayKMIntranasal oxytocin improves emotion recognition for youth with autism spectrum disordersBiol Psychiatry201067769269419897177
- LalatsaASchatzleinAGUchegbuIFStrategies to deliver peptide drugs to the brainMol Pharm20141141081109324601686
- DhandaDSFreyWH2ndLeopoldDKompellaUBApproaches for drug deposition in the human olfactory epitheliumDrug Deliv Technol200556472
- KanazawaTTakiHTanakaKTakashimaYOkadaHCell-penetrating peptide-modified block copolymer micelles promote direct brain delivery via intranasal administrationPharm Res20112892130213921499835
- TakiHKanazawaTAkiyamaFTakashimaYOkadaHIntranasal delivery of camptothecin-loaded Tat-modified nanomicells for treatment of intracranial brain tumorsPharmaceuticals20125101092110224281259
- KanazawaTAkiyamaFKakizakiSTakashimaYSetaYDelivery of siRNA to the brain using a combination of nose-to-brain delivery and cell-penetrating peptide-modified nano-micellesBiomaterials201334369220922623992922
- KanazawaTMorisakiKSuzukiSTakashimaYProlongation of life in rats with malignant glioma by intranasal siRNA/drug codelivery to the brain with cell-penetrating peptide-modified micellesMol Pharm20141151471147824708261
- AllenCYuYMaysingerDEisenbergAPoly caprolactone– b-poly(ethylene oxide) block copolymer micelles as a novel drug delivery vehicle for neurotrophic agents FK506 and L-685,818Bioconjug Chem2008955645729736490
- OtsukaHNagasakiYKataokaKPEGylated nanoparticles for biological and pharmaceutical applicationsAdv Drug Deliv Rev200355340341912628324
- JangJSKimSYLeeSBKimKOHanJSLeeYMPoly(ethylene glycol)/poly (ε-caprolactone) diblock copolymeric nanoparticles for non-viral gene delivery: the role of charge group and molecular weight in particle formation, cytotoxicity and transfectionJ Control Release2006113217318216750279
- ShinIGKimSYLeeYMChoCSSungYKMethoxy poly(ethylene gricol)/ε-caprolactone amphiphilic block copolymeric micelle containing indomethacin. I. Preparation and characterizationJ Control Release19985111119685899
- KakizawaYKataokaKBlock copolymer micelles for delivery of gene and related compoundsAdv Drug Deliv Rev200254220322211897146
- SharmaRLeeJSBettencourtRCXiaoCKoniecznySFWonYYEffects of the incorporation of hydrophobic middle block into a PEG–polycation diblock copolymer on the physicochemical and cell interaction properties of the polymer–DNA complexesBiomacromolecules20089113294330718942877
- BrookingJDavisSSIllumLTransport of nanoparticles across the rat nasal mucosaJ Drug Target20019426727911697030
- YangSColesDJEspositoAMitchellDJTothIMinchinRFCellular uptake of self-assembled cationic peptide-DNA complex: multifunctional role of the enhancer chloroquineJ Control Release2009135215916519171168
- RajagopalanRXavierJRangarajNRaoNMGopalVRecombinant fusion proteins TAT-Mu. Mu and Mu-Mu mediate efficient non viral gene deliveryJ Gene Med20079427528617397090
- ChenSGeXChenYLvNLiuYuanWAdvances with RNA interference in Alzheimer’s disease researchDrug Des Devel Ther20137117125
- PiweckaMRolleKWyszkoENucleic acid-based technologies in therapy of malignant gliomasCurr Pharm Biotechnol20111211180521902632
- OrlacchioABernardiGOrlacchioAMartinoSRNA interference as a tool for Alzheimer’s disease therapyMini Rev Med Chem20077111166117618045220
- PappasTCBaderAGAndrussBFBrownDFordLPApplying small RNA molecules to the directed treatment of human diseases: realizing the potentialExpert Opin Ther Targets200812111512718076375
- DykxhoornDMRNA interference as an anticancer therapy:patent perspectiveExpert Opin Ther Pat200919447549119441927
