?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Automatic 24-hour ambulatory blood pressure (BP) monitoring (ABPM) is a basic procedure performed in adults with arterial hypertension, but ABPM monitors have become widely used in pediatric practice only recently. The main problem is the lack of common normative data sets for ABPM in children and the small number of appropriate monitors that can be used for analysis of the 24-hour BP profile in this age group. The aim of this study was to validate the BPLab® ABPM monitor according to the 1993 British Hypertension Society (BHS-93) protocol, as well as to work out solutions regarding the feasibility of this device in pediatric practice.
Methods
Our study included 30 children of both sexes and aged 5–15 years, ie, “older” children according to the BHS-93 protocol. Before starting the study, we obtained ethical approval from the regional scientific ethics committee. All participants and their parents signed their written consent for participation in the study. The data were simultaneously obtained by three experts, who had completed a noninvasive BP measurement training course. BP values were measured using the Korotkoff auscultatory method (Phase I for systolic BP and Phase V for diastolic BP). Discrepancies in the systolic and diastolic BP measurements (n=180; 90 for each expert) were analyzed according to the criteria specified in the BHS-93 protocol.
Results
The device was graded “A” for both systolic BP and diastolic BP according to the criteria of the BHS-93 protocol.
Conclusion
The BPLab ABPM device may be recommended for extensive pediatric use.
Introduction
Automatic noninvasive blood pressure (BP) measurement is widely used in adults for self-monitoring and for clinical evaluation of the circadian BP profile. Ambulatory BP monitoring (ABPM) is added to the standard plan for the diagnosis and treatment of adults with arterial hypertension according to the European Society of Hypertension and European Society of Cardiology guidelines. In recent years, ABPM has been more widely used in the pediatric population.Citation1–Citation4 Use of ABPM in all children and adolescents with hypertension can be even more important than in adults according to the recommendations of the European Society of Hypertension.Citation4 However, the lack of common normative data for ABPM in children and the small number of appropriate monitors recommended for evaluation of the circadian BP profile prevent that method from being widely used in pediatric practice.
Information on 24-hour ABPM monitors that have successfully passed the independent tests and comply with national standards (Association for the Advancement of Medical Instrumentation and British Hypertension Society [BHS]) can be found on the Internet: however, only two among 25 monitors are recommended for use in pediatric practice.Citation5 The first BHS protocol was published in 1990, and its aim was to standardize the process of verification of monitors for noninvasive BP measurement.Citation6 The protocol was revised in 1993 and received international recognition.Citation7 In 2011, the results of validation of the BPLab® monitor (PetrTelegin, Nizhny Novgorod, Russia) for BP measurement in adult patients were published. According to the obtained results, the BPLab monitor was assigned to the “A/A” accuracy class for systolic and diastolic BP.Citation8 We consider these results as a first phase of the present study.
Materials and methods
Subject selection was based on the requirements of Part II of the 1993 BHS (BHS-93) protocol.Citation7 A group of 30 “older” children (aged 5–15 years according to the BHS-93 protocol) of both sexes (15 boys and 15 girls) of different ages (5 years, n=2; 6 years, n=2; 7 years, n=2; 8 years, n=3; 9 years, n=1; 10 years, n= 3; 11 years, n=2; 12 years, n=5; 13 years, n=2; 14 years, n=3; 15 years, n=5) who had received detailed instructions about the measurement procedure were recruited to participate in this study. Before the start of the study, we had received the approval of the regional scientific ethical committee. All participants and their parents signed their written consent for participation in the study.
Exclusion criteria were congenital heart or blood vessel disease, arrhythmia, pregnancy, and body mass index less than the 10 percentile or more than the 90 percentile according to sex and age. Subjects with Korotkoff sounds tending towards zero during examination were excluded from participation.
Test measurement conditions
According to the BHS-93 protocol, the results of BP measurement obtained by a qualified operator using the BPLab device were compared with BP values measured using the Korotkoff auscultatory method (Phase I for systolic BP and Phase V for diastolic BP) simultaneously by two experts, both of whom were pediatric cardiologists with over 25 years of experience, who had completed a noninvasive BP measurement standardization training course. Thus, there were three observers: the first observer measured BP using the test unit (BPLab device), and the second observer and third observer measured BP using the Korotkoff auscultatory method.
Measurements were taken in the morning in a comfortable setting (including ambient temperature 23°C–25°C and no irritating sounds) after 10 minutes of relaxation in a seated position. Stimulants (tea and coffee) were not allowed 8 hours before measurement; patients were only allowed a light breakfast not later than half an hour prior the test. The children were require not to have taken any medications within the 3 days before the test.
BP measurements were performed on the nondominant arm by two experts simultaneously using a high-quality stethoscope with one head and two headbands and two individual cali brated sphygmomanometers. The cuff pressure deflation rate was 2 mmHg per second. The cuff size depended on the patient’s arm circumference.
Measurement schedule
The BP of each patient was measured nine times, alternating between the experts and the test device according to the following schedule: measurement A, expert; measurement B, instrument; measurement 1, expert; measurement 2, instrument; measurement 3, expert; measurement 4, instrument; measurement 5, expert; measurement 6, instrument; and measurement 7, expert.
BP was measured on the nondominant arm. The interval between measurements was 30–60 seconds. After the experts had obtained their BP values, they made records in their own tables.
Three measurement error values were calculated for each patient (for systolic BP and diastolic BP, separately) according to the following formulae:
where BPn is a BP measurement result corresponding to the measurement number n. Measurement A was used to assign a patient to each group according to their BP level (); measurement B was treated as a calibration measurement. Discrepancies in systolic and diastolic BP measurements (n=180; 90 for each expert) were subsequently analyzed according to the criteria specified in the BHS-93 protocol.
Table 2 Grouping of patients according to different parameters
Results
The statistical distribution of the patients’ clinical parameters is given in , and their distribution according to BP level and arm circumference is shown in .
Table 1 Patients’ clinical profile parameters (15 boys, 15 girls)
Detailed results of the data analysis are given in in the way recommended by the BHS-93 protocol.Citation7 The number of measurement error values which are within the ranges envis aged by the protocol is indicated in the form of a percentage ratio in relation to the total number of measurements.
Table 3 Grading criteria, means, and mean differences in blood pressure for test device and for both observers
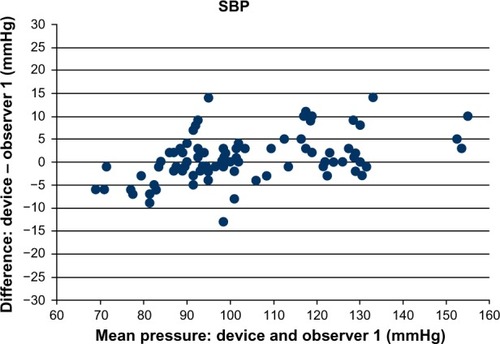
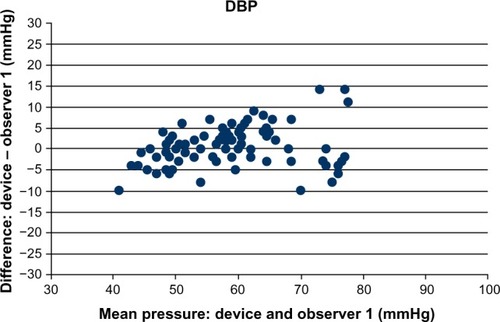
and are a graphical illustration of discrepancies in each measurement depending on the relevant BP value (separately for systolic BP and diastolic BP). As recommended in the BHS-93 protocol, the figures show only the results of comparison with the expert who obtained closer results (observer 1 for systolic BP, observer 1 for diastolic BP).
Discussion
ABPM is the method of choice for the diagnosis and control of arterial hypertension in both pediatric and adult patients.Citation1–Citation4 Although ABPM is widely used in children, there are still some open questions connected with normative data sets in children and appropriate validation of devices for use in the pediatric population.
The BPLab ABPM device is known to have accuracy class “A/A” for measuring peripheral arterial BP in adults.Citation8 Moreover, the device was clinically validated for measuring central aortic pressure and parameters of arterial stiffness,Citation9–Citation11 which provides normative data for arterial stiffness and central BP indices.Citation12 However, validation of the accuracy of the device in the pediatric population has not been performed as yet. Thus, the aim of this study was to validate the accuracy of the device for measuring peripheral arterial BP in “older” children (aged 5–15 years).
The results of this study suggest that the BPLab ABPM device has passed the test in a special group of patients, ie, “older” children (5–15 years) in accordance with the requirements of the BHS-93 protocol, and may be assigned to the “A/A” accuracy class as per the above-mentioned protocol. The tested device may be recommended for extensive pediatric use.
Disclosure
The authors report no conflicts of interest in this work.
References
- FlynnJTImpact of ambulatory blood pressure monitoring on the management of hypertension in childrenBlood Press Monit2000521121611035862
- KochVHColliASaitoMIComparison between casual blood pressure and ambulatory blood pressure monitoring parameters in healthy and hypertensive adolescentsBlood Press Monit2000528128911153052
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and AdolescentsThe fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescentsPediatrics200411455557615286277
- LurbeECifkovaRCruickshankKManagement of high blood pressure in children and adolescents; recommendations of the European Society of HypertensionJ Hypertens2009271719174219625970
- Dabl Educational TrustDevices for Ambulatory Blood Pressure Measurement Available from: http://dableducational.org/sphygmomanometers/devices_3_abpm.html#AbpmTableAccessed December 29, 2014
- O’BrienEPetrieJLittlerWAThe British Hypertension Society protocol for the evaluation of automated and semi-automated blood pressure measuring devices with special reference to ambulatory systemsJ Hypertens199086076192168451
- O’BrienEPetrieJLittleWAThe British Hypertension Society protocol for the evaluation of blood pressure measuring devicesJ Hypertens199311Suppl 2S43S62
- KoudryavtcevSALazarevVMValidation of the BPLab® 24-hour blood pressure monitoring system according to the European standard BS EN 1060-4:2004 and British Hypertension Society protocolMed Devices (Auckl)2011419319622915946
- RogozaANKuznetsovAACentral aortic blood pressure and augmentation index: comparison between Vasotens® and SphygmoCor® technologyResearch Reports in Clinical Cardiology201232733
- PosokhovINPulse wave velocity 24-hour monitoring with one-site measurements by oscillometryMed Devices (Auckl)20136111523549868
- KotovskayaYVKobalavaZDOrlovAVValidation of the integration of technology that measures additional “vascular” indices into an ambulatory blood pressure monitoring systemMed Devices (Auckl)20147919724833924
- KuznetsovaTYKornevaVABryantsevaENVasotens Registry CollaboratorsThe 24-hour pulse wave velocity, aortic augmentation index, and central blood pressure in normotensive volunteersVasc Health Risk Manag20141024725124812515
- De SwietMFayersPShinebourneEABlood pressure in first 10 years of life: the Brompton studyBMJ1992304P23P26