Abstract
Objectives
The aim of this study was to evaluate two commonly used advanced bipolar devices (ENSEAL® G2 Tissue Sealers and LigaSure™ Blunt Tip) for compression uniformity, vessel sealing strength, and consistency in bench-top analyses.
Methods
Compression analysis was performed with a foam pad/sensor apparatus inserted between closed jaws of the instruments. Average pressures (psi) were recorded across the entire inside surface of the jaws, and over the distal one-third of jaws. To test vessel sealing strength, ex vivo pig carotid arteries were sealed and transected and left and right (sealed) halves of vessels were subjected to burst pressure testing. The maximum bursting pressures of each half of vessels were averaged to obtain single data points for analysis. The absence or presence of tissue sticking to device jaws was noted for each transected vessel.
Results
Statistically higher average compression values were found for ENSEAL® instruments (curved jaw and straight jaw) compared to LigaSure™, P<0.05. Moreover, the ENSEAL® devices retained full compression at the distal end of jaws. Significantly higher and more consistent median burst pressures were noted for ENSEAL® devices relative to LigaSure™ through 52 firings of each device (P<0.05). LigaSure™ showed a significant reduction in median burst pressure for the final three firings (cycles 50–52) versus the first three firings (cycles 1–3), P=0.027. Tissue sticking was noted for 1.39% and 13.3% of vessels transected with ENSEAL® and LigaSure™, respectively.
Conclusion
In bench-top testing, ENSEAL® G2 sealers produced more uniform compression, stronger and more consistent vessel sealing, and reduced tissue sticking relative to LigaSure™.
Introduction
Advanced bipolar and ultrasonic energy devices are now routinely utilized for sealing and transecting blood vessels in a number of surgical specialties such as gynecologic, urologic, cardio-thoracic, and colorectal.Citation1–Citation4 Advantages provided by modern energy technologies over conventional monopolar electrosurgery include limited risk of thermal injury, elimination of dispersive electrodes, and enhanced sealing capability, especially in blood vessels larger than 1–2 mm in diameter.Citation5 In addition, the need for laparoscopic suture ligation, which is technically demanding and time consuming, has been greatly reduced. Advanced bipolar devices are particularly advantageous for sealing larger vessels up to 5–7 mm in diameter through uniform compression and efficient energy delivery.Citation5,Citation6 Surgical hemostasis with advanced bipolar technology is accomplished with precise delivery of high-frequency/low-voltage electric current that is converted to thermal energy, which collapses vessel walls and denatures collagen and elastin, forming a hemostatic seal.Citation7 Traditional bipolar devices also employ heat to cause clotting; coaptation and crosslinking of vessel collagen create a seal, followed by thrombus formation that blocks the flow of blood (). However, these earlier devices may exhibit incomplete sealing as a result of inefficient energy delivery secondary to suboptimal compression and more stray current () relative to modern advanced bipolar instruments. Advanced bipolar devices, on the other hand, are able to create stronger and more uniform compression, and they employ unique ways of controlling the delivery of energy and heat. These features work in combination to provide stronger vessel seals.
Figure 1 Vessel sealing with traditional bipolar devices.
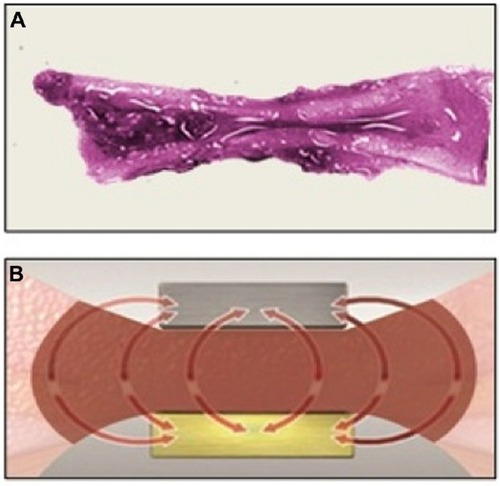
Essential parameters necessary for blood vessel electrocoagulation include homogeneously distributed compression, critical temperature, and time, allowing for optimal formation of the coagulum and strong vascular sealing.Citation1,Citation8–Citation10 Variations in compression force were shown to have a substantial effect on sealing of carotid arteries in ex vivo analyses.Citation10 A potential weakness in some advanced bipolar sealing devices is uneven (decreasing) compression from the proximal to the distal tip of the instrument’s end effector, which reduces sealing quality and consistency.Citation1 Another limitation, most prominent in nonarticulating devices, is the difficulty of approaching target tissue perpendicularly (especially during laparoscopic procedures). A perpendicular (90°) approach with respect to vessels minimizes the length of the seal and therefore has the potential to affect seal strength.Citation11
Advanced bipolar devices should ideally provide uniform tissue compression across the full jaw length to allow for consistent sealing. In complex procedures, a single sealing device may be fired many times, requiring stable performance for adequate hemostasis. These observations highlight the importance of evaluating bipolar devices in terms of their compression parameters and sealing reliability over multiple firings. Therefore, the aim of this report was to characterize the compression uniformity, seal strength, and sealing performance over time for two commonly utilized advanced bipolar devices: the ENSEAL® G2 family of tissue sealers and the LigaSure™ Blunt Tip laparoscopic instrument.
Materials and methods
Study design
Comparative, bench-top studies were performed to assess compression strength and vessel sealing quality of advanced bipolar instruments over multiple firings. Devices included the ENSEAL® G2 Tissue Sealers (ENSEAL), ENSEAL® G2 Articulating Tissue Sealers (ENSEAL Articulating; Ethicon Endo-Surgery, Cincinnati, OH, USA), and the LigaSure™ 5 mm Blunt Tip laparoscopic instrument (LigaSure; LF1537, Covidien, Mansfield, MA, USA).
Compression testing
Compression forces for the ENSEAL Articulating and LigaSure devices were assessed utilizing a foam pad and sensor (Tekscan 5027) placed between closed jaws of the instruments. Closed-jaw pressures (psi) were recorded with I-Scan® software (Tekscan® Inc., Boston, MA, USA). For each measurement, the average pressure over the tissue contact surface of the jaws was calculated as the mean of all data points above 24 psi. Values less than 24 psi were considered equivalent to background. For ENSEAL, full jaw pressure is exerted when the I-BLADE® is completely deployed, which closes and holds the jaws to a set gap. Because deployment of the I-BLADE® would destroy the foam pad/sensor, a close-jaw fixture was used to hold the jaws at this same set gap, thus replicating the applied pressure. The close-jaw fixture consisted of a c-clamp configured to engage the flat cam surfaces of both the upper and lower jaws normally engaged by the I-BLADE®. Closing the c-clamp brought these cam surfaces together, which is how the I-BLADE® closes the jaws. With the pad/sensor placed in the device jaws, a micrometer mounted to the c-clamp was used to measure the closure distance to ensure the fixture produced exactly the same gap as the (normally) deployed I-BLADE®. Specific devices tested in the analysis were five ENSEAL Articulating straight jaw, six ENSEAL Articulating curved jaw, and five LigaSure. Each individual instrument was tested six times, to provide a sample size of n≥30. For analysis of the total jaw, all data points along the entire surface in contact with the sensor were averaged; whereas for the distal tip, only data from the distal third of the jaw were counted. Testing for normality was performed using the Anderson–Darling method; and where normal data distributions were found, two-sample t-tests were employed to compare means for the determination of statistical significance. Most data sets exhibited normal distributions except for the distal tip data for ENSEAL straight jaw and LigaSure. Therefore, a comparison of medians was the most appropriate statistic for distal tip analyses and the Mann–Whitney test was utilized. For all comparisons, P-values less than 0.05 were considered significant.
Vessel sealing and burst pressure
Fresh Porcine carotid arteries were obtained from Animal Biotech Industries (Danboro, PA, USA). Vessels were examined for leak integrity, measured, and sorted according to outer diameter sizes of 5 mm (4.5–5.4 mm), 6 mm (5.5–6.4 mm), and 7 mm (6.5–7.5 mm). Carotid arteries were sealed and transected with ENSEAL and LigaSure devices. Each device was operated according to the accompanying instructions for use.Citation12,Citation13 In general, each tissue specimen was clamped in the device end effector, energy was applied until the generator signaled cycle completion, and the tissue was transected and released from the end effector. Sticking of vessel tissue to the instruments’ end effector was determined as follows: If noticeable residue remained on the device following transection, which was not easily removed (ie, was not released by simple manipulation of the device), the vessel was documented as sticking. The number of sticking events was expressed as a proportion (percentage) of the total transections for each device, and the difference in proportions test was employed to examine for statistical differences in the occurrence of sticking between device types.
To evaluate sealing strength over multiple firings, each individual instrument was fired through 52 activations (cycles). The protocol for each device consisted of three consecutive vessel transections followed by four sequential activations on porcine connective and fatty tissues (to simulate multi-use of the instrument). This pattern was performed seven times, followed by three final vessel transections for a total of 52 firings per instrument and 24 sealed/transected vessels. Three samples of each device type, (3) ENSEAL curved jaw, (3) ENSEAL straight jaw, and (3) LigaSure, were tested with this protocol.
The left and right halves of sealed vessels were subjected to testing with a burst pressure system consisting of a computer-controlled syringe pump (Harvard Apparatus, Holliston, MA, USA) with an inline pressure transducer. Physiologic saline was infused into the transected vessel halves at 47 mL/minute, and intraluminal pressure data were recorded until maximum burst pressure was reached at seal failure. The maximum bursting pressures of each half of vessels were averaged to obtain single data points for analyses. To determine statistical significance between burst pressure data sets, nonparametric Kruskal–Wallis comparisons of medians were performed.
Results
Compression strength and uniformity were evaluated for ENSEAL Articulating and LigaSure; pressure profiles across the entire jaw length are shown in . Whole-jaw and distal one-third (tip) compression values were calculated for ENSEAL Articulating devices compared to LigaSure (). The compression values for the distal tip as compared to the whole-jaw compression may be expressed as a ratio (percentage), which illustrates compression consistency from distal to proximal ends for each device jaws, as shown in . Mean whole-jaw and median distal tip compression for ENSEAL Articulating (both straight and curved jaw) were significantly higher than those for LigaSure, P<0.05.
Figure 2 Compression profiles for ENSEAL Articulating (A, B) and LigaSure (C) devices.
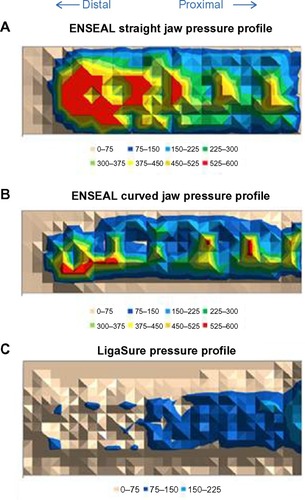
Table 1 Average and median compression values
The sealing consistency over multiple firings was examined in 5-mm-diameter porcine carotid arteries transected with ENSEAL and LigaSure devices, fired through 52 activations (cycles). shows burst pressures averaged at eight intervals, where each interval contains the average of three consecutive firings (ie, interval 1= cycles 1–3, interval 2= cycles 8–10, …, interval 8= cycles 50–52). The LigaSure device showed a reduction in burst pressures over multiple firings, including a 40% decrease in the mean value at the last interval relative to the first (). includes the median burst pressures calculated for all samples of each device at the first and last intervals (three of each device type were tested). The ENSEAL instruments maintained full sealing strength through 52 firings; no significant differences were noted between initial and last intervals. LigaSure exhibited a significant decrease of 727 mmHg at the last interval versus the first, P=0.027. depicts the overall burst pressure data (including all firings) for each device, represented as box plots. ENSEAL straight and curved jaw showed significantly higher overall median burst pressures relative to LigaSure.
Figure 3 Vessel burst pressures through multiple firing cycles of sealing devices.
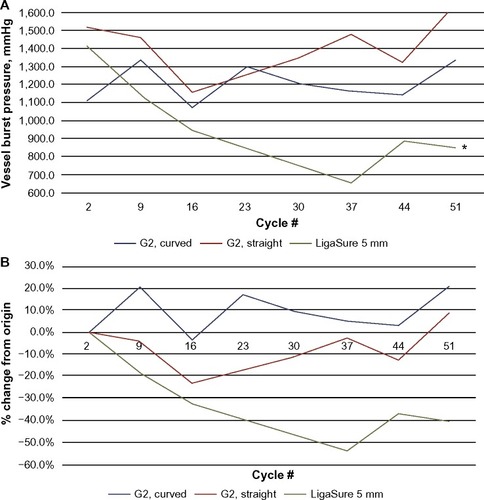
Figure 4 Ex vivo burst pressures for porcine carotid arteries transected with ENSEAL G2 and LigaSure.
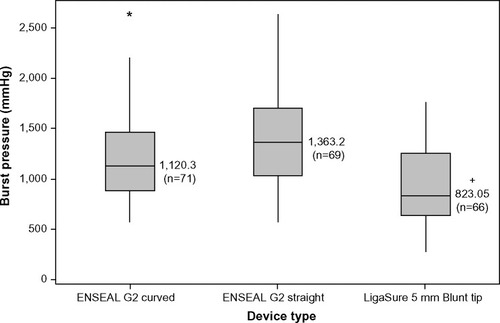
Table 2 Median burst pressure change over multiple firings: Interval 1 (cycles 1–3) and 8 (cycles 50–52); change = interval 8 minus interval 1
Tissue sticking was evaluated following transections of equal numbers of 5- to 7-mm-diameter porcine carotid arteries. Sticking events were noted for 1.39% of vessels transected with ENSEAL (straight and curved jaw combined), whereas LigaSure showed sticking in 13.3% of transections. The two proportions test yielded a statistical difference (P=0.007; 95% confidence interval 0.03259, 0.20660) comparing LigaSure with ENSEAL (straight and curved jaw combined). Sticking for ENSEAL straight jaw versus curved jaw (1.1% and 1.7%, respectively), did not differ significantly.
Discussion
A major objective of the current study was to determine if the advanced bipolar devices being tested would maintain uniform compression strength across the entire length of the instruments’ jaws. Statistically higher average whole-jaw and median distal (one-third) tip compression values were noted for ENSEAL Articulating (straight and curved jaw) devices compared to LigaSure. Moreover, the distal tip of the ENSEAL instruments maintained 100% or greater of their full jaw compression force, indicating consistent and fully distributed pressure. Reyes et al determined that variations in apposition force (compression) had a major effect on sealing strength of ex vivo sheep carotid arteries.Citation10 In that analysis, it was found that vessels were optimally sealed with pressures of 2.4–3.8 MPa (which equates to 348–551 psi), at 90°C and a clamp time of 10 seconds. Taken together, published results and data described here highlight the importance of uniform compression along the entire length of the jaw for producing strong vessel seals and adequate surgical hemostasis.
Complex procedures such as colorectal surgery may require more than one hundred activations of an energy device, including transection and/or sealing of multiple isolated blood vessels.Citation14 Therefore, consistency of the sealing and cutting mechanism in advanced bipolar instruments is critical for adequate hemostasis. For evaluating sealing performance over many firings, ex vivo burst pressure testing is a convenient method and this technique is widely utilized to test the integrity of blood vessel seals produced either in vivo or ex vivo.Citation15–Citation17 In the current burst pressure analyses, ENSEAL tissue sealers maintained consistent seal strength through 52 firings of the device, whereas seals performed with LigaSure showed a reduction in burst pressure over multiple firings.
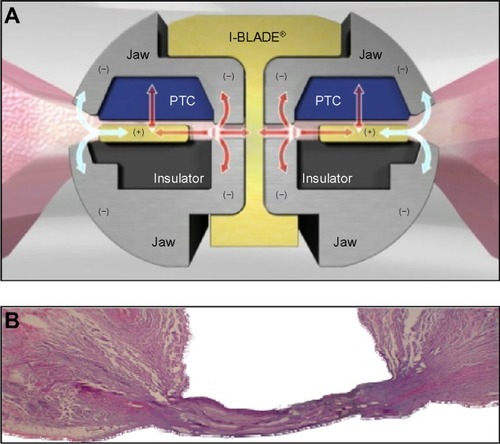
Tissue sticking is known to be a significant concern in electrosurgery.Citation18,Citation19 When heated tissue adheres to the sealing device’s hot surfaces, electrical resistance is increased, which inhibits energy delivery to targeted tissue making the process less effective and more time consuming. In addition, sticking tissue may lead to disruption or destruction of the seal as well as adjacent tissue, thereby causing bleeding and the need for additional sealing or touch ups. The model used in this study does not account for the effect of blood coagulum in vivo, which undoubtedly contributes to sticking in an operative setting. Therefore, it is uncertain as to whether the sticking results presented here could be applied to an in vivo operative context. Nonetheless, the ex vivo vessels in this experiment clearly showed less sticking to ENSEAL devices relative to LigaSure. This reduced sticking may contribute to the consistent sealing performance found over multiple firings with ENSEAL. The ENSEAL instruments contain an offset electrode configuration and positive temperature coefficient nanoparticles in the jaw (). This configuration minimizes thermal spread and sticking, while allowing complete hemostatic seals (). When the temperature in the jaw and tissue rises to approximately 100°C, the positive temperature coefficient polymer expands and the conductive chains break apart, thus interrupting the flow of electrical current and preventing further increases in temperature.
Figure 5 ENSEAL jaw structure and vessel sealing.
Although the scope of the current report was limited to key elements required for vascular sealing and hemostasis, published preclinical and clinical data have established the effectiveness of advanced bipolar sealing in vivo and are consistent with results presented here. Overhaus et al compared ENSEAL with conventional clamp and ligation for resection of bowel, colon, and kidney in a porcine model.Citation20 Blood loss and operation time were significantly lower for the ENSEAL surgeries, and lateral thermal spread was 1 mm or less. Bibi et al found that human mesenteric arteries sealed with ENSEAL during laparoscopic colorectal surgery exhibited mean (ex vivo) burst pressures of ≥ 853 mmHg and lateral thermal damage <1 mm.Citation3 Other clinical studies using ENSEAL during laparoscopic liver resections and hysterectomies each found equal or better outcomes in terms of effective sealing and estimated blood loss relative to comparative methods.Citation21,Citation22
Limitations of this analysis are mainly related to the ex vivo approach for assessing vessel seal quality (ie, burst pressures). The technique does not account for the effects of in vivo parameters such as tissue healing, chronic tensile strength and delayed fusion failures, compression ischemia, and pulsatile blood flow subjecting vessels to physiologic shear forces.Citation10 However, the reproducibility and supraphysiologic burst pressures achieved with this approach provide a reliable index of the safety and consistency that devices will deliver in vivo. Other limitations include relatively small sample size and restriction to 5-mm-diameter vessels in the burst pressure analysis. Finally, it must be noted that this work was funded and performed by the manufacturer of the ENSEAL tissue sealing technology and all authors are employees of Ethicon, Inc. However, risk of bias was minimized by using a standardized preclinical model for all devices in the respective studies.
Conclusion
These bench-top analyses have demonstrated superior sealing characteristics in porcine carotid arteries transected by the ENSEAL®-G2 devices. ENSEAL outperformed LigaSure with regard to uniformity of jaw compression, reduced tissue sticking, and increased burst pressures in sealed/transected vessels.
Acknowledgments
The authors wish to thank the personnel who participated in acquiring the data presented in this report: B Dickerson, K Huey, S Killinger, F Lewis, J Mallow, E Niehaus, A Pierce, R Rowe, B Schaefer, S Sell, D Shaw, and A Voegele.
Disclosure
This work was funded and performed by the manufacturer of the ENSEAL tissue sealing technology (Ethicon, Inc.). The authors report no other conflicts of interest in this work.
References
- EickSLoudermilkBWalbergEWenteMNRationale, bench testing and in vivo evaluation of a novel 5 mm laparoscopic vessel sealing device with homogeneous pressure distribution in long instrument jawsAnn Surg Innov Res20137123289664
- SeehoferDMoglMBoas-KnoopSSafety and efficacy of new integrated bipolar and ultrasonic scissors compared to conventional laparoscopic 5-mm sealing and cutting instrumentsSurg Endosc20122692541254922447285
- BibiSCoralicJVelchuruVA prospective study of in vivo and ex vivo sealing of the human inferior mesenteric artery using an electrothermal bipolar vessel-sealing deviceJ Laparoendosc Adv Surg Tech A201424747147424987843
- O’KeeffeKFuchsKLaparoscopic-assisted vaginal hysterectomy with bipolar coagulation cutting forceps (ENSEAL(r) trio device) versus suture technique vaginally: A comparative analysisJ Gynecol Surg2013293131134
- LyonsSDLawKSKLaparoscopic vessel sealing technologiesJ Minim Invasive Gynecol201320330130723659750
- KondrupJDAndersonFQuickBUse of the ENSEAL(R) G2 tissue sealer in gynecologic surgerySurg Technol Int20132319119524081850
- KennedyJSStranahanPLTaylorKDChandlerJGHigh-burst-strength, feedback-controlled bipolar vessel sealingSurg Endosc19981268768789602010
- SigelBDunnMRThe mechanism of blood vessel closure by high frequency electrocoagulationSurg Gynecol Obstet196512148238315320121
- WallwienerCWRajabTKZubkeWThermal conduction, compression, and electrical current-an evaluation of major parameters of electrosurgical vessel sealing in a porcine in vitro modelJ Minim Invasive Gynecol200815560561018640881
- ReyesDAGBrownSICochraneLMottaLSCuschieriAThermal fusion: effects and interactions of temperature, compression, and duration variablesSurg Endosc201226123626363322773229
- VoegeleACKorvickDLGutierrezMClymerJWAmaralJFPerpendicular blood vessel seals are stronger than those made at an angleJ Laparoendosc Adv Surg Tech A201323866967223755852
- Package insert for ENSEAL® G2 Tissue SealerCincinnati OH, USAEthicon Endo-Surgery, Inc2011
- Package insert for LigaSure™ Blunt Tip Laparoscopic Sealer/DividerMansfield, MA, USACovidien, LLC2011
- ParkerGTsaiBDipeNClinical utility of a new articulating tissue sealer in laparoscopic colorectal surgeryWorld J Colorectal Surg2014414Abstract presented at the XXVI Biennial Congress of the International Society of University Colon and Rectal SurgeonsCape Town, South AfricaSeptember 4–7 2014 Available from: http://services.bepress.com/cgi/viewcontent.cgi?article=1167&context=wjcs
- KraneCPinnellMGardnerCThompsonMColemanJWilkensRMechanical test methods for assessing porcine carotid and uterine artery burst pressure following ex vivo ultrasonic ligature seal and transectionJ Test Eval2011394
- CarbonellAMJoelsCSKercherKWMatthewsBDSingRFHenifordBTA comparison of laparoscopic bipolar vessel sealing devices in the hemostasis of small-, medium-, and large-sized arteriesJ Laparoendosc Adv Surg Tech A200313637738014733701
- NovitskyYWRosenMJHarrellAGSingRFKercherKWHenifordBTEvaluation of the efficacy of the electrosurgical bipolar vessel sealer (LigaSure) devices in sealing lymphatic vesselsSurg Innov200512215516016034506
- OuKLChuJSHosseinkhaniHChiouJFYuCHBiomedical nanostructured coating for minimally invasive surgery devices applications: characterization, cell cytotoxicity evaluation and an animal study in ratSurg Endosc20142872174218824619328
- BrillAIBipolar electrosurgery: convention and innovationClin Obstet Gynecol200851115315818303509
- OverhausMSchaeferNWalgenbachKHirnerASzyrachMNTolbaRHEfficiency and safety of bipolar vessel and tissue sealing in visceral surgeryMinim Invasive Ther Allied Technol201221639640122292919
- RothmundRKraemerBBruckerSLaparoscopic supracervical hysterectomy using ENSEAL vs standard bipolar coagulation technique: randomized controlled trialJ Minim Invasive Gynecol201320566166623791399
- MbahNABrownREBowerMRScogginsCRMcMastersKMMartinRCGDifferences between bipolar compression and ultrasonic devices for parenchymal transection during laparoscopic liver resectionHPB (Oxford)201214212613122221574
