Abstract
The artificial urinary sphincter (AUS), which has evolved over many years, has become a safe and reliable treatment for stress urinary incontinence and is currently the gold standard. After 4 decades of existence, there is substantial experience with the AUS. Today AUS is most commonly placed for postprostatectomy stress urinary incontinence. Only a small proportion of urologists routinely place AUS. In a survey in 2005, only 4% of urologists were considered high-volume AUS implanters, performing >20 per year. Globally, ~11,500 AUSs are placed annually. Over 400 articles have been published regarding the outcomes of AUS, with a wide variance in success rates ranging from 61% to 100%. Generally speaking, the AUS has good long-term outcomes, with social continence rates of ~79% and high patient satisfaction usually between 80% and 90%. Despite good outcomes, a substantial proportion of patients, generally ~25%, will require revision surgery, with the rate of revision increasing with time. Complications requiring revision include infection, urethral atrophy, erosion, and mechanical failure. Most infections are gram-positive skin flora. Urethral atrophy and erosion lie on a spectrum resulting from the same problem, constant urethral compression. However, these two complications are managed differently. Mechanical failure is usually a late complication occurring on average later than infection, atrophy, or erosions. Various techniques may be used during revisions, including cuff relocation, downsizing, transcorporal cuff placement, or tandem cuff placement. Patient satisfaction does not appear to be affected by the need for revision as long as continence is restored. Additionally, AUS following prior sling surgery has comparable outcomes to primary AUS placement. Several new inventions are on the horizon, although none have been approved for use in the US at this point.
Introduction
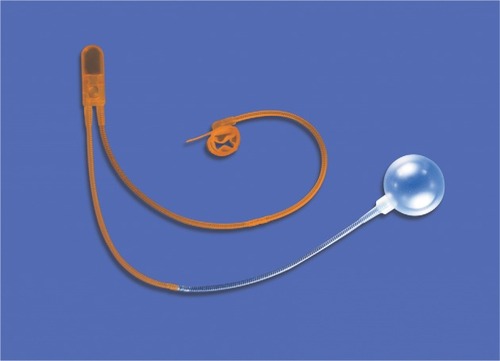
Urinary incontinence is a significant quality of life issue affecting a large proportion of the population.Citation1 It is estimated that ~22.6 million men are affected by urinary incontinence and 8.2 million have moderate to severe urinary incontinence.Citation2 Pure stress urinary incontinence (SUI) accounts for only a subset (12.5%) of these patients, yet it remains a substantial and growing problem, especially in the postprostatectomy setting. The artificial urinary sphincter (AUS), which has evolved over many years, has become a safe and reliable treatment for SUI and is currently the gold standard. The first description of an AUS dates back to 1947Citation3; however, the modern era of sphincters began in 1983 with the release of the AMS 800 (American Medical Systems, Minnetonka, MN, USA; ).Citation4,Citation5 The AUS is composed of silicone and consists of an inflatable narrow backed cuff, a pressure-regulating balloon (PRB), and a control unit that consists of a deflating pump, a refill resistor, and deactivating button. The AUS has not undergone any significant changes except for the addition of a 3.5 cm cuff introduced in 2009.Citation6,Citation7 After 4 decades of existence, there is substantial experience with the AUS. This article focuses on current perspectives regarding its use today.
Device
The AUS consists of three separate components whose tubing must be connected using connectors provided within the AMS accessory kit. The occlusive cuff ranges from 3.5 cm to 11 cm and most commonly is placed circumferentially at the bulbar urethra. The circumference of the urethra determines the cuff size. In certain situations, it is placed more distally as in transcorporal approaches. The cuff can even be placed at the bladder neck in rare instances, although this approach requires more extensive retropubic surgical dissection. The width of all cuff sizes when deflated is 2 cm. AMS introduced the narrow backed cuff in 1987Citation5,Citation8 in an attempt to decrease the rates of reoperation. The cuff may be coated with InhibiZone (American Medical Systems), which was approved by the US Food and Drug Administration in 2007, although presently there are no data showing that InhibiZone reduces infection rates.
The PRB transmits pressure to the occlusive cuff and comes in six pressure ranges from 41 cmH2O to 100 cmH2O in increments of 10 cmH2O. The most commonly used PRB pressure range is 61–70 cmH2O. The goal of pressure regulation is to provide the lowest amount of pressure that is sufficient for continence. Excessive pressure will increase ischemia to the urethral segment being occluded and may lead to urethral atrophy and erosion. The PRB may be placed in the space of Retzius, in a submuscular location,Citation9 or via a counter incision in a preperitoneal space. Some favor placement of the PRB in a high submuscular location, giving comparable functional outcomes in comparison to traditional placement within the space of Retzius, while avoiding associated complications of the latter and facilitating PRB placement in reoperative cases with a hostile abdomen.Citation10
The final component is the control unit or pump and consists of two parts. The lower part is a bulb that the patient squeezes to transfer fluid out of the compressive cuff to the PRB allowing micturition. The upper part contains the resistor valves and deactivation button. In men, the pump is placed within a subdartos pouch within the scrotum where it can be easily accessed by the patient. Proper manipulation of the pump requires a certain degree of manual dexterity by the patient, which should be assessed preoperatively.Citation11
Indications
The AUS is an effective treatment for intrinsic sphincter deficiency in men resulting from various etiologies, which may include prostate cancer treatment, transurethral resection of prostate, neurologic disease, trauma, or congenital anomalies. Prior to 1985, a significant proportion (17%–50%) of AUSs were placed for neurologic disease.Citation12 However since 1985, postprostatectomy incontinence (PPI) has been the most common indication, representing 39% to 69% of AUS placed through 2005.Citation8,Citation12 Indications for spinal cord injury are relatively narrow as thoracolumbar spinal cord injuries resulting in intrinsic sphincter deficiency are relatively rare. Most AUSs placed for spinal cord disease are in the pediatric population with myelodysplasia. Women currently account for <1% of AUS placements,Citation13 and the AMS 800 is not US Food and Drug Administration approved for use in women. This review focuses on AUS as it is most commonly used today, in men with SUI. In the era of robotic-assisted radical prostatectomies, the incidence of 12-month urinary incontinence rates varies between 4% and 31%.Citation14 However, only a portion of these patients will undergo AUS placement. Currently, ~11,500 AUSs are placed annually worldwide. In 2005, only 13% of US urologists performed AUS surgery, with only 4% considered high-volume surgeons, performing >20 per year.Citation12
Traditionally, a waiting period of at least 1 year following prostate cancer treatment prior to placing an AUS has been suggested. However, more recent expert opinion agrees that an AUS may be placed as early as 6 months following radical prostatectomy (RP), if SUI is severe, bothersome to the patient, and not improving with conservative treatment.Citation11 On the other hand, if there is ongoing improvement in SUI even 12 months after RP it is up to the surgeon’s discretion to delay surgical management of SUI. A cystoscopy prior to placement of an AUS is traditionally advised to look for any underlying urethral pathology that may complicate AUS placement or that may put the AUS at risk of subsequent damage. For example, up to 32% of patients have been found to have a vesicourethral anastomotic stricture on cystoscopy following RP.Citation15 Vesicourethral anastomotic strictures should be stable prior to AUS placement.
Outcomes
There are more than 400 articles that have been published regarding outcomes of the AUS; however, most of the literature are retrospective series, with heterogeneous groups and different definitions regarding improvement or success making direct comparison of studies difficult. Success rates vary widely between 61% and 100%.Citation16–Citation22 In a recent systematic review, social continence (£1 pad/d) was reported at 79% with follow-up ranging from 5 months to 192 months.Citation23 Dry rates varied between 4% and 86%. In a prospective study of 103 patients suffering from PPI, the dry rate was 57%.Citation21 Patients should be provided with realistic expectations, regarding their continence. They should be advised to expect an improvement in their continence, but not to be necessarily 100% dry. Many patients continue to wear a safety pad and are quite satisfied with such results.Citation24 Litwiller et alCitation25 found that patients with an AUS who leaked less than a teaspoon a day were satisfied and would recommend the surgery to a friend. Patients were more likely to be dissatisfied when they leaked more than a teaspoon per day. Generally, the AUS has good long-term outcomes and improves SUI sufficiently achieving a high patient satisfaction usually 80%–90%.Citation17,Citation25–Citation27
A significant proportion of patients suffer from SUI following radical cystectomy with neobladder (RC/NB). Studies report the incidence of SUI after RC/NB to be between 33% and 95%.Citation28,Citation29 Only few studies exist evaluating outcomes in patients with orthotopic neobladders who have undergone an AUS. In one of the largest series reported,Citation30 72% (21/29) of patients noted an improvement in SUI at a mean follow-up of 40 months. However, 60% of patients underwent a revision or explantation due to infection, erosion, device malfunction, or recurrent SUI, with erosion or infection being the most common reason. Most of the AUSs placed in this series were 4.5 cm cuffs with 61–70 cm balloons. Of these patients, 28% had been treated with radiotherapy. In comparison to PPI, these outcomes seem poorer. A small proportion of RC/NB patients perform clean intermittent catheterization. This is an additional important factor to consider prior to placing an AUS as it may possibly increase the risk of erosion secondary to catheterization trauma.
Complications
Despite the good outcomes achieved with the AUS, it does come with a substantial need for revision surgery. A candid discussion on the potential complications of the procedure should be discussed with the patient including infection, urinary retention, urethral atrophy, erosion, or device malfunction. The complication rates are generally low; however, the need for revision surgery increases with time.
Infection
As with any foreign body, infection can be a significant concern and mandates early recognition and explantation. An infection usually presents with scrotal erythema and induration at the site of the pump. It is advised to remove all components when patients present with concerns for an infected AUS, given the possibility of biofilm formation along the device.Citation11 The rate of infection in contemporary series are reported to be between 1% and 8%,Citation31–Citation35 with rates <2% at high volume centers.Citation8,Citation36,Citation37 Gram-positive organisms such as Staphylococcus aureus and Staphylococcus epidermidis account for the majority of infections, with methicillin resistance reported in 26% of organisms.Citation38 Gram-negative infections account for 26% of infections.Citation38 Perioperative antibiotics are routinely administered; however, there is no standardized antibiotic regimen and the choice of antibiotics is dependent on surgeon preference. Our recommendation is to provide both gram-positive and gram-negative coverage with consideration of coverage for methicillin-resistant Staphylococcus species.Citation11
Urinary retention
A subset of patients suffer from urinary retention. This most often is transient and may be due to postoperative urethral inflammation, which should typically resolve over the course of several days. In a large series, Linder et alCitation39 noted the rate of postoperative urinary retention to be 31%. Prolonged catheterization with an AUS is not recommended given that it may lead to erosion by compromising blood flow to the urethra. The smallest caliber catheter available ought to be used if needed (eg, 10 Fr or 12 Fr) and if needed for >48 hours, a suprapubic tube should be considered.Citation11,Citation34 If urinary retention persists for longer than a week, improper cuff sizing may be the cause, and the patient may benefit from undergoing a revision with cuff upsizing. Interestingly, urinary retention was found to be associated with adverse 6-month device survival and increased rates of erosion.Citation39
Urethral atrophy
Urethral tissue atrophy, which is among the most common complications following AUS implantation, may occur naturally following persistent cuff-induced urethral compression and ischemia. Urethral atrophy and erosion lie on a spectrum and are both most often secondary to the constant compression experienced by the urethra, erosion being the more severe complication. The urethra may lose tissue bulk circumferentially with time, and a cuff that was initially sized appropriately may become too large for adequate urethral coaptation. Patients who experience urethral atrophy often complain of recurrent incontinence as a late presentation. Use of narrow-backed cuffs was meant to provide persistent adequate coaptation as urethral tissue lost bulk over time, translating into a reduced revision rate.Citation24 Once atrophy occurs, various revision strategies, discussed in detail later, may be used to manage recurrent incontinence, including but not limited to downsizing the cuff, repositioning the cuff preferably more proximal, transcorporal cuff placement, and tandem cuff placement. Increasing the cuff pressure is not recommended as this leads to further ischemia and possibly urethral erosion.
Urethral erosion
Erosions may occur as either an early or a late postoperative complication. When an erosion presents within the first few months, it is likely that there was an unrecognized intraoperative urethral injury at the time of AUS placement.Citation34 Late erosions present at a median time of 19 months and at a rate of 5%–10%.Citation40 Over time constant cuff compression may cause urethral tissue atrophy and eventually erosion. Prior studies have shown that radiation-induced endarteritis, prior urethral surgery including urethroplasty and prior anti-incontinence surgery, coronary artery disease, and compromised urethral blood flow are all risk factors for erosion.Citation41,Citation42 Since both urethral atrophy and erosion may present with recurrent SUI, patients who present with a recurrence of symptoms should undergo a cystoscopy before undergoing any surgical intervention to rule out erosion as these two complications are managed differently. Eroded cuffs will be exposed to urine and are traditionally associated with infection. Some erosions, however, may not show signs of overt infection within the scrotum or perineum and may remain sterile for many years. These may go unnoticed for a long time.Citation43
When a patient experiences an erosion, the device should be explanted. A recent study found that the incidence of urethral stricture formation was significantly lower when patients underwent an in situ urethroplasty, which reapproximated the epithelial edges (38%), compared to patients treated solely with an indwelling Foley catheter (85%).Citation44 Furthermore, stricture development leads more intervening procedures prior to replacement of a new AUS, impacting a patient’s quality of life by delaying the restoration of continence. In this series, the average interval before AUS replacement was 9 months for those who underwent an in situ urethroplasty vs 17 months for those who received Foley catheter drainage only.Citation44 A new device may then be implanted after a 3-month healing period. Nocturnal deactivation has been suggested as a prevention strategy to avoid urethral atrophy and subsequent erosion. This may be a strategy to consider in motivated patients that have the manual dexterity and understanding of how the mechanism operates.
Radiation
It is worth mentioning that radiated patients may constitute a group of patients with an increased risk of complications. A significant proportion of patients with a history of locally advanced prostate cancer treatment undergo adjuvant radiotherapy. These patients should be advised that they might be at higher risk of needing revision surgery. Radiotherapy is associated with endarteritis and chronic vascular changes that may decrease urethral blood flow leading to spongiosal atrophy prior to AUS implantation.Citation11 Several series have found higher rates of erosion or complications necessitating revisions in patients with a prior history of radiation.Citation7,Citation17,Citation45,Citation46 Despite these risks, AUS implantation leading to satisfactory continence has been successfully performed in irradiated patients. Some surgeons prefer transcorporal cuff placement in such instances and others have suggested using a lower pressured PRB (51–60 cmH2O) and delayed activation at 6 weeks.Citation43
Mechanical failure
If patients develop recurrent or worsening SUI and are not found to have an erosion, it may be that the device has failed. Mechanical failures on average occur later than urethral atrophy, erosions, and infections.Citation47 Imaging such as an ultrasound of the PRB or a CT scan may confirm a loss of fluid within the system. However, imaging will not help determine the exact location of the leak. It is advised that if there is a mechanical failure of the device after 2 years, the whole device should be replaced. Higher rates of mechanical failure were noted in a series of revisions where patients did not have complete replacements but merely had their cuffs downsized.Citation48 Some have suggested that performing partial replacements of an AUS can lead to higher failure/leakage rates secondary to biofilm that builds on the in situ components if one uses the accessory quick connect kit rather than suture tie the connections.Citation49
Revisions
Although the durability of AUS has been well established, a significant percentage of patients undergo revisions for various reasons, including, erosion, infection, urethral atrophy, and mechanical failure. The proportion of AUS revision surgeries performed annually is ~24%–34%.Citation12 Generally, the reported revision rate is ~25%.Citation47 However, it is important to consider long-term outcomes as device survival rate diminishes with longer follow-up. More specifically, reported 5-year survival rates vary between 59% and 79%,Citation18,Citation36,Citation40,Citation47,Citation50,Citation51 10-year survival rates between 28% and 64%,Citation47,Citation50–Citation52 and 15-year survival rates between 15% and 41%.Citation47,Citation50 In the largest series of 1,082 AUS from a single institution with long follow-up of up to 15-years, the device survival rate at 5 years, 10 years, and 15 years was 74%, 57%, and 41%, respectively.Citation47 These numbers are quite significant, and based on these data, patients with a significant life expectancy (greater than a decade) have a high likelihood of needing to undergo a revision in their lifetime. Although there is a high revision rate with time, most series show that AUS revisions have comparable outcomes to initial AUS placement,Citation36,Citation53,Citation54 particularly if undergoing a revision for mechanical failure. Patients undergoing revisions for multiple erosions have compromised urethras and may be at higher risk of subsequent erosions.Citation42 Revision techniques include repositioning the cuff at a different location along the urethra (preferably more proximal if possible) and downsizing the cuff, using tandem cuffs or transcorporal cuffs. In the largest multi-institutional series of revisions, the mean time to revision was 28.9 months.Citation48 Additionally, patient satisfaction has been shown to be independent of the number of revisions, and studies have shown that up to 90% of patients undergoing revision had no change in satisfaction as long as they had a functional AUS.Citation24–Citation26
Tandem cuffs
In an effort to improve efficacy and continence, some have used tandem cuffs with the thought that increasing resistance over a greater area may improve continence. According to American Medical Systems, ~15% of AUS are placed as tandem cuffs.Citation55 Brito et alCitation56 was the first to describe successful tandem cuff placement with success reported at 95%. The idea behind tandem cuffs was that using two cuffs would lead to increased resistance to leakageCitation57 without increasing pressure on a single segment of the urethra. Despite initial enthusiasm and favorable continence outcomes from several groups following tandem cuffs for patients with severe PPI, urethral atrophy or prior failed single cuff placement,Citation56–Citation60 subsequent longer follow-up demonstrated a higher risk of complications when using tandem cuffs.Citation18 Additionally, the authors of a cadaver study did not find a significant difference in retrograde leak point pressure for single vs tandem cuffs. They did find an association between urethral circumference and retrograde leak point pressure, which favors proximal cuff placement.Citation61 In reviewing this literature, one must keep in mind that most of these seriesCitation56,Citation57,Citation59,Citation60,Citation62 are retrospective in nature and not randomized. Therefore, a selection bias in choosing patients who underwent tandem cuff placement likely exists. It is likely that two cuffs do provide greater continence, but the benefit may be not worth the cost of increased complications.
Transcorporal
Transcorporal cuff placement was developed in an attempt to improve continence in patients with recurrent incontinence secondary to erosion, subcuff urethral atrophy, inadequate urethral coaptation, or for patients undergoing revisions where more proximal placement could not be achieved.Citation63 Initial success for transcorporal placement was reported at 84%. The advantages of transcorporal placement include avoiding a difficult distal dorsal urethral dissection near the corpora, which may result in a thin urethra, as well as the additional bulk provided by the tunica. In a prospective series of transcorporal AUS placement, dry or socially continent rates were reported to be 76% at median follow-up of 20 months.Citation64
There is a concern that transcorporal cuff placement affects erectile dysfunction. However, most patients undergoing transcorporal cuff placement have undergone prostate cancer treatment and already have some degree of erectile dysfunction at baseline.Citation20,Citation64–Citation67 Despite this concern, a small series did report that the majority of patients maintain their erectile function even after transcorporal cuff placement if they had it to begin with, although the numbers are small, 5/6 (83%).Citation64
AUS following sling placement
The outcomes of primary AUS placement are comparable to those performed after prior sling placement. Following sling surgery, recurrence of incontinence ranges from 20% to 35%.Citation68 An AUS may be placed following a bone-anchored sling, transobturator sling, or quadratic sling. Transobturator slings may be left in place when placing the AUS. Usually, the transobturator sling cannot be seen during the AUS placement, and the AUS may simply be placed around the bulbar urethra via a perineal incision. In order to place an AUS after a bone-anchored sling, the sling must be incised and dissected off the bulbospongiosus muscle to expose the bulbar urethra or the AUS may be placed more distally via a transscrotal approach.Citation69 Finally, the quadratic sling can easily be identified through the perineum and can be incised and dissected from the bulbospongiosus muscle unveiling the unscarred urethra deep to it and allowing placement of the AUS.Citation68 AUS outcomes following sling placement are high, with success rates reported to be 79%–83%.Citation70,Citation71 Complication rates appear to be similar to initial AUS placement.
New inventions
Despite an increase in surgical options for PPI, including slings, bulking agents, and stem cell therapy, the AUS remains the gold standard. Given the difficulties with implanting the device, as well as the significant rate for revisions, there has been a push for the development of new devices. In recent years, there have been new inventions; however, none have yet been approved for use in the US. Most of these new devices attempt to simplify implantation by decreasing the number of connecting parts. Additionally, many of these devices are developed to allow in situ adjustment of the pressure cuff since constant urethral pressure is the likely cause of urethral atrophy and subsequent erosion.
FlowSecure
An adjustable AUS named the FlowSecure (Sphinx Medical, Bellshill, Scotland) has been undergoing trials predominately in the UK. Developed in 2006, it functions similarly to the AUS in that it has a PRB, pump, and cuff; however, the FlowSecure has an additional “stress-relieving balloon” and comes as a one-piece device.Citation72,Citation73 The idea of the FlowSecure is to decrease the magnitude of constant urethral pressure in the hope that this will decrease the erosion rates. It has two separate balloons, one that keeps the cuff inflated at low pressures and another which increases pressure to the cuff in response to an increase in intra-abdominal pressure.Citation74 The “stress relieving balloon” allows the cuff to rest at a lower baseline pressure exerted by the PRB by providing intermittent increases in pressure to the urethra that is administered when there is an increase in intra-abdominal pressure. Additionally, the pressure of the FlowSecure can be adjusted by injecting or removing saline transscrotally through the pump, allowing individualization of the pressure according to the individual patient’s SUI severity. Although initial results in nine patients appeared promising,Citation72 in another study of 100 patients who underwent placement of the FlowSecure, 28% underwent explantation of the device for early and late infections, perforation of the pump at pressurization and mechanical failure.Citation75 An additional disadvantage is that patients require multiple pressurization procedures, typically three before reaching an adequate pressure.Citation73
Periurethral constrictor
The periurethral constrictor (PUC; Silimed, Rio de Janeiro, Brazil) was initially designed in 1996 for pediatric patients. However, its use has been reported in adults with PPI. The PUC is an adjustable hydraulic system that includes the constrictor connected to a valve that is placed subcutaneously in the lower abdomen where it can be accessed and punctured with a needle. Patients do not need to mechanically pump the PUC and typically must void abdominally. Although initial reports were not as robust,Citation76 the most recent report of 62 patients with at least 18-month follow-up indicates a continence rate of 79%.Citation77
Zephyr
The Zephyr or ZSI 375 is an AUS produced by Zephyr Surgical Implants (Geneva, Switzerland), a Swiss–French company. It is a hydraulic-based system that is implanted as a single unit via two separate incisions. The cuff is placed via a perineal incision, and the pump is placed in a subdartos pouch within the scrotum. The pump is adjustable in situ so that urethral pressure may be adjusted as needed to improve continence. In a series of 34 patients with SUI, “social continence” was achieved in 94.2%. Two devices (5.8%) were explanted because of infection. Longer follow-up is needed to determine the durability of the device and the rates of explantation due to urethral atrophy or erosion.Citation78
Tape mechanical occlusive device
A new artificial sphincter, the Tape Mechanical Occlusive Device (TMOD; GT Urological, Minneapolis, MN, USA) is currently under development.Citation79 Rather than rely on a hydraulic mechanism such as the AMS 800, TMOD uses a spring-loaded mechanism to apply circumferential pressure around the urethra. This device is also a one-piece device that should facilitate implantation. The control is an ON and OFF switch, which patients should find easier to control than the pump of the current AMS 800. At this time, the TMOD has only been studied in a canine model and human cadavers. These studies have demonstrated that the device provides occlusive pressure when activated in the 50–80 cmH2O range. Thus, far it has proven to be technically feasible and biocompatible. Live human clinical trials are to follow.
Various new devices are under development. None are currently approved for use in the US. Most of the devices at this time require further investigation, need to be implanted in a greater number of patients, and need longer follow-up to determine their durability and long-term rates of complications. An advantage of most of the new devices is that they are purportedly less expensive than the AMS 800.
Conclusion
Despite the wide variation in results and heterogeneity of studies, one can surmise that the AUS has been a highly effective surgical solution for many patients suffering from moderate to severe SUI and has significantly improved the quality of life for many patients. However, given the current mechanism inherent to the function of the AUS which is via urethral compression, concerns for urethral atrophy and erosion arise from potentially decreased perfusion of the affected urethral segment. Additionally, with time, mechanical failure of the device may occur. Therefore, a significant percentage of patients will require a revision, which increases with longer follow-up. Various techniques and strategies have been developed over the years to successfully handle these clinical scenarios. While we continue to await the outcomes of newer devices under development, the AMS 800 model currently remains the gold standard.
Disclosure
The authors report no conflicts of interest in this work.
References
- LandefeldCSBowersBJFeldADNational Institutes of Health state-of-the-science conference statement: prevention of fecal and urinary incontinence in adultsAnn Intern Med2008148644945818268289
- MarklandADGoodePSReddenDTBorrudLGBurgioKLPrevalence of urinary incontinence in men: results from the national health and nutrition examination surveyJ Urol201018431022102720643440
- FoleyFEAn artificial sphincter; a new device and operation for control of enuresis and urinary incontinenceJ Urol194758425025920266239
- LeibovichBCBarrettDMUse of the artificial urinary sphincter in men and womenWorld J Urol19971553163199372584
- LightJKReynoldsJCImpact of the new cuff design on reliability of the AS800 artificial urinary sphincterJ Urol199214736096111538439
- HudakSJMoreyAFImpact of 3.5 cm artificial urinary sphincter cuff on primary and revision surgery for male stress urinary incontinenceJ Urol201118651962196621944140
- SimhanJMoreyAFSinglaN3.5 cm artificial urinary sphincter cuff erosion occurs predominantly in irradiated patientsJ Urol2015193259359725106901
- ElliottDSBarrettDMMayo Clinic long-term analysis of the functional durability of the AMS 800 artificial urinary sphincter: a review of 323 casesJ Urol19981594120612089507835
- MoreyAFCefaluCAHudakSJHigh submuscular placement of urologic prosthetic balloons and reservoirs via transscrotal approachJ Sex Med201310260361023216955
- SinglaNSiegelJASimhanJDoes pressure regulating balloon location make a difference in functional outcomes of artificial urinary sphincter?J Urol2015194120220625711196
- BiardeauXAharonySAUS Consensus GroupCampeauLCorcosJArtificial urinary sphincter: report of the 2015 consensus conferenceNeurourol Urodyn201635suppl 2S8S2427064055
- LeeRTeAEKaplanSASandhuJSTemporal trends in adoption of and indications for the artificial urinary sphincterJ Urol200918162622262719375102
- FicarraVNovaraGRosenRCSystematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomyEur Urol201262340541722749852
- CrivellaroSMorlaccoABodoGSystematic review of surgical treatment of post radical prostatectomy stress urinary incontinenceNeurourol Urodyn Epub2015923
- ComiterCVDobberfuhlADThe artificial urinary sphincter and male sling for postprostatectomy incontinence: which patient should get which procedure?Investig Clin Urol2016571313
- RamsayAKGranitsiotisPConnIGThe use of the artificial urinary sphincter in the West of Scotland: a single centre 10-year experienceScott Med J2007522141717536635
- WalshIKWilliamsSGMahendraVNambirajanTStoneARArtificial urinary sphincter implantation in the irradiated patient: safety, efficacy and satisfactionBJU Int200289436436811872025
- O’ConnorRCLyonMBGuralnickMLBalesGTLong-term followup of single versus double cuff artificial urinary sphincter insertion for the treatment of severe postprostatectomy stress urinary incontinenceUrology2008711909318242372
- ImamogluMATuygunCBakirtasHYigitbasiOKiperAThe comparison of artificial urinary sphincter implantation and endourethral macroplastique injection for the treatment of postprostatectomy incontinenceEur Urol200547220921315661416
- AaronsonDSElliottSPMcAninchJWTranscorporal artificial urinary sphincter placement for incontinence in high-risk patients after treatment of prostate cancerUrology200872482582718752838
- MottetNBoyerCChartier-KastlerEBen NaoumKRichardFCostaPArtificial urinary sphincter AMS 800 for urinary incontinence after radical prostatectomy: the French experienceUrol Int199860suppl 22529 discussion 359607555
- O’ConnorRCNanigianDKPatelBNGuralnickMLEllisionLMStoneARArtificial urinary sphincter placement in elderly menUrology200769112612817270633
- Van der AaFDrakeMJKasyanGRPetrolekasACornuJNYoung Academic Urologists Functional Urology GroupThe artificial urinary sphincter after a quarter of a century: a critical systematic review of its use in male non-neurogenic incontinenceEur Urol201363468168923219375
- GousseAEMadjarSLambertMMFishmanIJArtificial urinary sphincter for post-radical prostatectomy urinary incontinence: long-term subjective resultsJ Urol200116651755175811586217
- LitwillerSEKimKBFonePDWhiteRWStoneARPost-prostatectomy incontinence and the artificial urinary sphincter: a long-term study of patient satisfaction and criteria for successJ Urol19961566197519808911369
- MontagueDKArtificial urinary sphincter: long-term results and patient satisfactionAdv Urol2012201283529022536227
- MontagueDKAngermeierKWPaoloneDRLong-term continence and patient satisfaction after artificial sphincter implantation for urinary incontinence after prostatectomyJ Urol2001166254754911458065
- SantucciRAParkCHMayoMELangePHContinence and urodynamic parameters of continent urinary reservoirs: comparison of gastric, ileal, ileocolic, right colon, and sigmoid segmentsUrology199954225225710443720
- HautmannREde PetriconiRGottfriedHWKleinschmidtKMattesRPaissTThe ileal neobladder: complications and functional results in 363 patients after 11 years of followupJ Urol19991612422427 discussion 427–4289915417
- VainribMSimma-ChiangVBoydSDGinsbergDAPotential risk factors and outcomes of artificial urinary sphincter placement after radical cystectomy and orthotopic neobladder urinary diversionNeurourol Urodyn20133271010101323595916
- de CogainMRElliottDSThe impact of an antibiotic coating on the artificial urinary sphincter infection rateJ Urol2013190111311723313209
- BordenaveMRoupretMTaksinLRésultats à long terme du traitement de l’incontinence urinaire masculine par implantation de sphincter artificiel urinaire (AMS 800) en position bulbaire: expérience monocentrique [Long-term results of the treatment of urinary incontinence with bulbar implantation of artificial urinary sphincter in men: a single-center experience]Prog Urol2011214277282 French21482403
- MontagueDKAngermeierKWPostprostatectomy urinary incontinence: the case for artificial urinary sphincter implantationUrology20005512410654883
- SuarezOAMcCammonKAThe artificial urinary sphincter in the management of incontinenceUrology201692141926845050
- HajivassiliouCAA review of the complications and results of implantation of the AMS artificial urinary sphincterEur Urol199935136449933793
- RajGVPetersonACTohKLWebsterGDOutcomes following revisions and secondary implantation of the artificial urinary sphincterJ Urol200517341242124515758761
- RajGVPetersonACWebsterGDOutcomes following erosions of the artificial urinary sphincterJ Urol2006175621862190 discussion 219016697836
- MageraJSJrElliottDSArtificial urinary sphincter infection: causative organisms in a contemporary seriesJ Urol200818062475247818930496
- LinderBJPiotrowskiJTZiegelmannMJRiveraMERangelLJElliottDSPerioperative complications following artificial urinary sphincter placementJ Urol2015194371672025776908
- LaiHHHsuEITehBSButlerEBBooneTB13 years of experience with artificial urinary sphincter implantation at Baylor College of MedicineJ Urol200717731021102517296403
- BrantWOEricksonBAElliottSPRisk factors for erosion of artificial urinary sphincters: a multicenter prospective studyUrology201484493493825109562
- McGeadyJBMcAninchJWTruesdaleMDBlaschkoSDKenfieldSBreyerBNArtificial urinary sphincter placement in compromised urethras and survival: a comparison of virgin, radiated and reoperative casesJ Urol201419261756176125014577
- SinglaNSinglaAKReview of single-surgeon 10-year experience with artificial urinary sphincter with report of sterile cuff erosion managed nonsurgicallyUrology201585125225625530393
- RozanskiATTauschTJRamirezDSimhanJScottJFMoreyAFImmediate urethral repair during explantation prevents stricture formation after artificial urinary sphincter cuff erosionJ Urol2014192244244624512955
- BatesASMartinRMTerryTRComplications following artificial urinary sphincter placement after radical prostatectomy and radiotherapy: a meta-analysisBJU Int2015116462363325601072
- RavierEFassi-FehriHCrouzetSGeletAAbidNMartinXComplications after artificial urinary sphincter implantation in patients with or without prior radiotherapyBJU Int2015115230030724731208
- LinderBJRiveraMEZiegelmannMJElliottDSLong-term outcomes following artificial urinary sphincter placement: an analysis of 1082 cases at mayo clinicUrology201586360260726135815
- EswaraJRChanRVetterJMLaiHHBooneTBBrandesSBRevision techniques after artificial urinary sphincter failure in men: results from a multicenter studyUrology201586117618026142602
- KavoussiLNovickACPartinAWPetersCACampbell-Walsh Urology10th edPhiladelphia, PAElsevier Saunders2011
- LeonPChartier-KastlerERoupretMAmbrogiVMozerPPheVLong-term functional outcomes after artificial urinary sphincter implantation in men with stress urinary incontinenceBJU Int2015115695195724958004
- VennSNGreenwellTJMundyARThe long-term outcome of artificial urinary sphinctersJ Urol20001643 pt 1702706 discussion 706–70710953129
- KimSPSarmastZDaignaultSFaerberGJMcGuireEJLatiniJMLong-term durability and functional outcomes among patients with artificial urinary sphincters: a 10-year retrospective review from the University of MichiganJ Urol200817951912191618353376
- LaiHHBooneTBComplex artificial urinary sphincter revision and reimplantation cases – how do they fare compared to virgin cases?J Urol2012187395195522264456
- LinderBJde CogainMElliottDSLong-term device outcomes of artificial urinary sphincter reimplantation following prior explantation for erosion or infectionJ Urol2014191373473824018241
- ChertackNChaparalaHAngermeierKWMontagueDKWoodHMFoley or fix: a comparative analysis of reparative procedures at the time of explantation of artificial urinary sphincter for cuff erosionUrology20169017317826743390
- BritoCGMulcahyJJMitchellMEAdamsMCUse of a double cuff AMS800 urinary sphincter for severe stress incontinenceJ Urol199314922832858426402
- KabalinJNAddition of a second urethral cuff to enhance performance of the artificial urinary sphincterJ Urol19961564130213048808859
- KowalczykJJSpicerDLMulcahyJJErosion rate of the double cuff AMS800 artificial urinary sphincter: long-term followupJ Urol19961564130013018808858
- DiMarcoDSElliottDSTandem cuff artificial urinary sphincter as a salvage procedure following failed primary sphincter placement for the treatment of post-prostatectomy incontinenceJ Urol20031704 pt 11252125414501735
- O’ConnorRCGerberGSAvilaDChenAABalesGTComparison of outcomes after single or DOUBLE-CUFF artificial urinary sphincter insertionUrology200362472372614550451
- MankaMGWrightEJDoes use of a second cuff improve artificial urinary sphincter effectiveness? Evaluation using a comparative cadaver modelJ Urol201519461688169126165585
- KowalczykJJSpicerDLMulcahyJJLong-term experience with the double-cuff AMS 800 artificial urinary sphincterUrology19964768958978677584
- GuralnickMLMillerETohKLWebsterGDTranscorporal artificial urinary sphincter cuff placement in cases requiring revision for erosion and urethral atrophyJ Urol2002167520752078 discussion 207911956443
- WiedemannLCornuJNHaabETranscorporal artificial urinary sphincter implantation as a salvage surgical procedure for challenging cases of male stress urinary incontinence: surgical technique and functional outcomes in a contemporary seriesBJU Int201311281163116824053170
- BlahMCaremelRSibertLBugelHGrisePTraitement de l’incontinence urinaire masculine par sphincter urinaire artificiel avec manchette intracaverneuse [Treatment of male urinary incontinence by artificial urinary sphincter with intracavernous cuff]Prog Urol2008182114119 French18396239
- LeeDZafirakisHShapiroAWestneyOLIntermediate outcomes after transcorporal placement of an artificial urinary sphincterInt J Urol201219986186622571275
- MageraJSJrElliottDSTandem transcorporal artificial urinary sphincter cuff salvage technique: surgical description and resultsJ Urol2007177310151019 discussion 1019–102017296400
- ComiterCSurgery for postprostatectomy incontinence: which procedure for which patient?Nat Rev Urol2015122919925558839
- ComiterCVSurgery insight: surgical management of postprostatectomy incontinence – the artificial urinary sphincter and male slingNat Clin Pract Urol200741161562417982438
- ChristineBKnollLDTreatment of recurrent urinary incontinence after artificial urinary sphincter placement using the AdVance male slingUrology20107661321132420709374
- AbdouACornuJNSebePThérapie de sauvetage par implantation d’un sphincter artificiel urinaire après échec de bandelette Advance™ pour incontinence urinaire après prostatectomie: une expérience monocentrique [Salvage therapy with artificial urinary sphincter after advance male sling failure for post-prostatectomy incontinence: a first clinical experience]Prog Urol20122211650656 French22999090
- KnightSLSusserJGreenwellTMundyARCraggsMDA new artificial urinary sphincter with conditional occlusion for stress urinary incontinence: preliminary clinical resultsEur Urol200650357458016720077
- VakalopoulosIKampantaisSLaskaridisLChachopoulosVKoptsisMToutziarisCNew artificial urinary sphincter devices in the treatment of male iatrogenic incontinenceAdv Urol2012201243937222567002
- CraggsMDChaffeyNJMundyARA preliminary report on a new hydraulic sphincter for controlling urinary incontinenceJ Med Eng Technol199115258621875383
- RodriguezDAAscanioEFVicensVAGarcia-MontesFFour years experience with the FlowSecure Artificial Urinary Sphincter. Problems and SolutionsProceedings of the 41st Annual Meeting of the International Continence Society (ICS ‘11)Glasgow, UKAugust 2011
- SchiaviniJLDamiaoRde Resende JuniorJADornasMCCruz Lima da CostaDSBarrosCBTreatment of post-prostate surgery urinary incontinence with the periurethral constrictor: a retrospective analysisUrology20107561488149220399484
- IntroiniCNaselliAZaninettaGSafety and efficacy of periurethral constrictor implantation for the treatment of post-radical prostatectomy incontinenceUrology20127951175117822546396
- StaermanFG-LlorensCLeonPLeclercYZSI 375 artificial urinary sphincter for male urinary incontinence: a preliminary studyBJU Int20131114 pt BE202E20622937774
- MalaebBSElliottSPLeeJAndersonDWTimmGWNovel artificial urinary sphincter in the canine model: the tape mechanical occlusive deviceUrology201177121121621067799