Abstract
Background
Vilazodone has been shown to reduce core symptoms of generalized anxiety disorder (GAD) in three randomized, double-blind, placebo-controlled trials. Since sexual dysfunction (SD) is not well characterized in GAD, a post hoc analysis of these trials was conducted to evaluate the effects of vilazodone on sexual functioning in GAD patients.
Materials and methods
Data were pooled from one fixed-dose trial of vilazodone 20 and 40 mg/day (NCT01629966) and two flexible-dose studies of vilazodone 20–40 mg/day (NCT01766401, NCT01844115) in adults with GAD. Sexual functioning was assessed using the Changes in Sexual Functioning Questionnaire (CSFQ). Outcomes included mean change from baseline to end of treatment (EOT) in CSFQ total score and percentage of patients shifting from SD at baseline (CSFQ total score ≤47 for males, ≤41 for females) to normal functioning at EOT. Treatment-emergent adverse events related to sexual functioning were also analyzed.
Results
A total of 1,373 patients were included in the analyses. SD at baseline was more common in females (placebo, 46.4%; vilazodone, 49%) than in males (placebo, 35.1%; vilazodone, 40.9%). CSFQ total score improvement was found in both females (placebo, +1.2; vilazodone, +1.6) and males (placebo, +2.1; vilazodone, +1.0), with no statistically significant differences between treatment groups. The percentage of patients who shifted from SD at baseline to normal sexual functioning at EOT was higher in males (placebo, 40.6%; vilazodone, 35.7%) than in females (placebo, 24.9%; vilazodone, 34.9%); no statistical testing was performed. Except for erectile dysfunction and delayed ejaculation in vilazodone-treated males (2.4% and 2.1%, respectively), no treatment-emergent adverse events related to sexual functioning occurred in ≥2% of patients in either treatment group.
Conclusion
Approximately 35%–50% of patients in the vilazodone GAD studies had SD at baseline. Vilazodone and placebo had similar effects on CSFQ outcomes in both females and males, indicating a limited adverse impact on sexual functioning with vilazodone.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Sexual complaints are a common problem in the US general population, with prevalence estimated at 31% in males and 43% in females.Citation1 These complaints are even more common in psychiatric illnesses. Sexual dysfunction (SD) has been reported in 40%–65% of patients with major depressive disorder (MDD).Citation2 The relationship between SD and MDD is bidirectional, with SD increasing the risk of depression and vice versa.Citation3 Complicating this clinical scenario are the unwanted sexual effects of serotonergic agents commonly used to treat depression and anxiety disorders. Approximately 35%–45% of antidepressant-treated patients experience global SD, with 60%–80% of patients reporting decreased functioning in a specific phase of the sexual cycle.Citation4
Among MDD patients, a population in which SD has been fairly well characterized,Citation5–Citation10 decreased desire, erectile difficulties, and delayed ejaculation are commonly reported in males; decreased desire, diminished arousal, and difficulty achieving orgasm are often reported in females. Comorbid depressive and anxiety disorders are often seen in clinical settings, and like depression symptoms, anxiety can be a prominent factor in the etiology of SD.Citation11 This is an important point to consider, since the estimated 12-month prevalence of anxiety disorders in the general population is 18% and the estimated lifetime prevalence is 29%.Citation12,Citation13 Although SD has been researched in some anxiety-related disorders (eg, social phobia, obsessive–compulsive disorder, posttraumatic stress disorder),Citation2 less is known about SD in patients with generalized anxiety disorder (GAD). One study, however, has shown SD to be present in 64% of GAD patients,Citation14 which the investigators defined as a total score ≥17 or any item score ≥5 on the Arizona Sexual Experience Scale.Citation15 This result was lower than the incidence of SD found in MDD patients (76%), but higher than was found in obsessive–compulsive disorder patients (50%).Citation14
Vilazodone is a selective serotonin-reuptake inhibitor and 5-HT1A-receptor partial agonist that is approved by the US Food and Drug Administration for the treatment of MDD in adults at the recommended dose of 20–40 mg/day.Citation15,Citation16 Based on findings from preclinical studies,Citation16 it has been hypothesized that activation of 5-HT1A receptors may offset the negative effects of increased serotonin action on sexual functioning. This hypothesis was consistent with results of a post hoc analysis of Arizona Sexual Experience Scale and Changes in Sexual Functioning Questionnaire (CSFQ)Citation17 data from three vilazodone studies in adult MDD patients, which included two double-blind and placebo-controlled trialsCitation18,Citation19 and a 52-week open-label extension study.Citation20 Results of this post hoc analysis indicated that vilazodone had a low adverse impact on sexual function in patients with MDD.Citation8
Since vilazodone had shown anxiolytic effects in rats,Citation21 and the 5-HT1A mechanism of vilazodone is also found in antianxiety medications, such as buspirone, vilazodone was subsequently investigated for the treatment of adults with GAD in three double-blind, randomized, placebo-controlled trials. Based on the primary end point, defined as change from baseline in the Hamilton Anxiety Rating Scale (HAM-A),Citation22 positive results with vilazodone versus placebo were reported in two flexible-dose trials (20–40 mg/day).Citation23,Citation24 In a fixed-dose trial of vilazodone 20 or 40 mg/day, a statistically significant difference from placebo in HAM-A total score change was found with the higher vilazodone dose, but not the lower dose.Citation25 The CSFQ was included as a safety measure in all the three GAD studies. In the individual studies, small and similar mean changes in CSFQ total score were seen in both vilazodone- and placebo-treatment groups, suggesting similar effects of treatment on sexual functioning in adults with GAD. Pooled CSFQ data from these trials were analyzed post hoc to further evaluate the effects of treatment on SD and better characterize SD in adults with GAD.
Materials and methods
Study design and patients
Post hoc analyses were conducted using data from the three positive 8-week clinical trials of vilazodone in adult patients with GAD (NCT01766401,Citation23 NCT01844115,Citation24 and NCT01629966Citation25). All vilazodone-dose groups were pooled for this analysis.
Detailed methods for the individual studies have previously been reported.Citation23–Citation25 In brief, they included patients 18–70 years of age who met Diagnostic and Statistical Manual of Mental Disorders, fourth edition – text revision criteria for GAD. At baseline, patients were required to have a HAM-A total score ≥20, with scores ≥2 on HAM-A items 1 (anxious mood) and 2 (tension), Clinical Global Impression – SeverityCitation26 score ≥4 (indicating at least moderate illness), and 17-item Hamilton Depression Rating Scale (HAM-D17)Citation27 total score ≤17. Typical exclusion criteria were applied, and included the following: an Axis I diagnosis other than GAD, nonresponse to adequate treatment trials of two or more selective serotonin-reuptake inhibitors or serotonin norepinephrine-reuptake inhibitors for GAD (≥8 weeks at the recommended dose), and suicide risk (past year attempt, score ≥3 on item 3 of the HAM-D17, or investigator judgment).
In the constituent studies, prospective assessment of sexual functioning was conducted at week 0 (baseline), 4, and 8 using the CSFQ, a self-report 14-item questionnaire that evaluates the effects of antidepressant treatment on five domains of sexual functioning: pleasure, desire/frequency, desire/interest, arousal/erection (males) or arousal/excitement (females), and orgasm/ejaculation (males) or orgasm/completion (females) ().Citation28 Global SD is defined as a CSFQ total score of ≤47 in males and ≤41 in females. SD in the CSFQ domains is defined using the following threshold scores: pleasure (≤4), desire/frequency (males ≤8, females ≤6), desire/interest (males ≤11, females ≤9), arousal/erection (≤13) or arousal/excitement (≤12), and orgasm/ejaculation (≤13) or orgasm/completion (≤11).
Figure 1 Items, domains, and scoring for the Changes in Sexual Functioning Questionnaire (CSFQ).
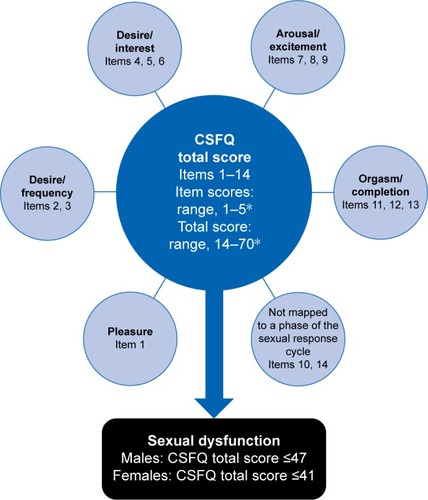
Screening, enrollment, and randomization of patients were independent of any measure or assessment of sexual functioning. No evaluations of sexual function status were performed before the baseline visit.
Post hoc analyses
Analyses were conducted in the pooled CSFQ-analysis population, defined as all randomized patients who took one or more doses of double-blind study drug and had a baseline and one or more available postbaseline CSFQ assessments. Each CSFQ-based analysis was conducted in patients from the CSFQ-analysis population who had available assessments that were appropriate for that analysis. For treatment-emergent adverse events (TEAEs) that were related to sexual functioning, analyses were conducted in the pooled safety population, defined as all randomized patients who had received one or more doses of the double-blind study drug.
The effects of treatment on sexual function were evaluated based on least squares mean (LSM) change from baseline to week 8 in CSFQ total score in all males and females, as well as in males and females categorized by age (≤50 or >50 years), baseline sexual functioning status (with or without SD), and HAM-A response. The LSM change from baseline to week 8 in CSFQ domain scores was also analyzed in all males and females included in the CSFQ-analysis population, as well as in males and females categorized by age. Least squares mean differences (LSMDs) between treatment groups and P-values for CSFQ total and domain-score changes were analyzed using a mixed-effect model for repeated measures, with treatment group, pooled study center, sex, visit, and treatment-group-by-visit interaction as fixed effects and the baseline value and baseline-value-by-visit interaction as covariates.
The percentage of patients whose sexual functioning changed from SD status at baseline (ie, CFSQ total score of ≤47 in males and ≤41 in females) to normal functioning (ie, CSFQ total score ≥48 in males and ≥42 in females) at the end of double-blind treatment were analyzed descriptively. Descriptive statistics were also used to analyze the percentage of patients who shifted from normal sexual functioning at baseline to SD status at end of treatment. The incidence of TEAEs related to the sexual function in the pooled safety population was analyzed descriptively.
Results
Patient characteristics
The pooled CSFQ-analysis population consisted of 1,373 patients (placebo, n=577; vilazodone, n=796). Demographic characteristics were similar between treatment groups (). In the placebo and vilazodone groups, baseline SD was seen in 35% and 40.9% of males and 46.4% and 49% of females, respectively (). Mean baseline CSFQ total scores were lower in females than in males.
Table 1 Patient demographics (CSFQ-analysis population)
Table 2 Baseline sexual functioning characteristics (CSFQ-analysis population)
CSFQ total score changes
For change in baseline to week 8 in CSFQ total score, no significant differences between vilazodone and placebo were detected in males of any age, males categorized by age (≤50 or >50 years), females of any age, or females categorized by age (≤50 or >50 years) (). LSMDs for CFSQ total score change in younger and older males (−0.9 and −1.1, respectively; both P>0.05) were similar to the LSMD found in all males (−1.1; P=0.0705). LSMDs in younger and older females (0.4 and 0.3, respectively; both P>0.05) were also similar to the LSMD found in all females (0.4; P=0.3746).
Table 3 CSFQ total and domain score changes in males and females (CSFQ-analysis population)
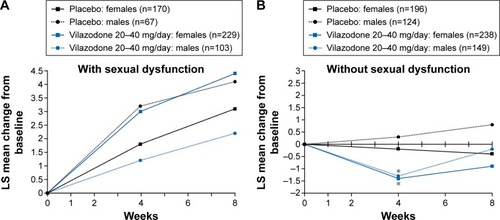
Among males and females with SD at baseline, mean improvements in CSFQ total scores were found with both vilazodone and placebo (), with no statistically significant differences between treatment groups at weeks 4 or 8. Among patients without SD at baseline, small decreases (<1.5 points) in mean CSFQ total scores were seen in all patient groups, except for a slight increase (<0.5 points) in placebo-treated males (). At week 4, in males and females without SD at baseline, the mean decrease in CSFQ total score was significantly greater in the vilazodone group compared with the placebo group; however, the difference between treatment groups was not significant at week 8.
Figure 2 CSFQ total score change from baseline to week 8 in patients with (A) or without (B) baseline sexual dysfunction (CSFQ-analysis population).
Abbreviations: CSFQ, Changes in Sexual Functioning Questionnaire; LS, least squares.
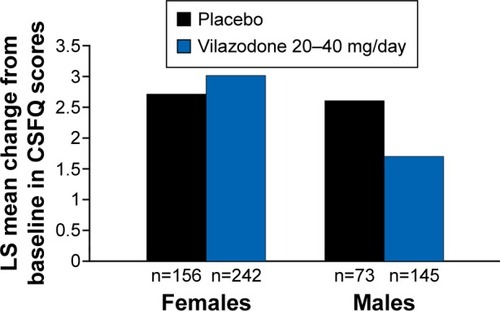
The percentage of patients with a HAM-A response was 39.7% (229 of 557) for placebo and 48.6% (387 of 796) for vilazodone. In HAM-A responders at week 8, mean improvements in CSFQ total score were found with both placebo (males, +2.6; females, +2.7) and vilazodone (males, +1.7; females, +3) (), with no significant differences between treatment groups in either males or females.
Figure 3 CSFQ total score change from baseline to week 8 in patients with HAM-A response (CSFQ-analysis population).
Abbreviations: CSFQ, Changes in Sexual Functioning Questionnaire; HAM-A, Hamilton Anxiety Rating Scale; LS, least squares.
CSFQ domain-score changes
In males and females from both treatment groups, mean baseline scores were lower than SD thresholds in each of the five CSFQ domains (). Older males and females (>50 years) had lower mean baseline scores across the CSFQ domains than younger patients (≤50 years), suggesting worse baseline sexual function in the older subgroups.
After 8 weeks of double-blind treatment, mean CSFQ domain scores generally remained above the score cutoff for SD (ie, no SD) in males and females from both treatment groups. In older males, a small mean worsening with vilazodone was found in desire/frequency, desire/interest, and orgasm/ejaculation (LSM change −0.0, −0.1, and −0.0 points, respectively). However, no statistically significant difference between vilazodone and placebo was found in any CSFQ domain in older or younger males. Older and younger females had mean improvements with both placebo and vilazodone in all CSFQ domains, with no statistically significant differences between treatment groups in any domain.
Changes in sexual function status
In both treatment groups, >80% of males and females who had normal sexual function at baseline maintained their normal status until the end of double-blind treatment (). In both males and females with SD at baseline, approximately one-third of vilazodone-treated patients improved to normal sexual function status at the end of double-blind treatment. In males with baseline SD, the percentage of patients improving to normal function was lower with vilazodone (35.7%) than placebo (40.6%). However, in females with baseline SD, the percentage of patients improving to normal function was greater with vilazodone (34.9%) than with placebo (24.9%). No statistical testing between treatment groups was conducted for any of these outcomes.
Table 4 Changes in sexual function status during DB treatment (CSFQ-analysis population)
Adverse events related to sexual function
In the pooled safety population (placebo, n=621; vilazodone, n=854), TEAEs related to sexual function were more common with vilazodone than with placebo (). Decreased libido was the most common sexual function TEAE in the overall population and in vilazodone-treated female patients; eight male patients (four in each treatment group) also reported a decrease in libido. The most common sexual function TEAEs for vilazodone-treated males were erectile dysfunction (2.4%) and delayed ejaculation (2.1%). No other sexual function TEAE occurred in >2% of patients in either treatment group.
Table 5 Sexual function adverse events (safety population)
Discussion
The results of this post hoc analysis, conducted using data pooled from three randomized and placebo-controlled trials of vilazodone 20–40 mg/day, indicated that vilazodone had similar effects to placebo on sexual functioning in adults with GAD. In male and female patients, mean changes in CSFQ total and domain scores from baseline to end of double-blind treatment were small in both treatment groups, with no statistically significant differences between vilazodone and placebo. When analyzed by age-groups, no significant differences were found in older patients (males and females >50 years) or younger patients (males and females ≤50 years). These findings were not unexpected, since a previous post hoc analysis in adult MDD patients also showed vilazodone to have minimal impact on sexual functioning.Citation8
One aim of this post hoc analysis was to characterize SD in GAD, since little is known in this area, especially compared to MDD. At baseline, global SD (CSFQ total score ≤47 for males or ≤41 for females) was found in both treatment groups, with a higher percentage in females (placebo, 46.4%; vilazodone, 49%) than in males (placebo, 35.1%; vilazodone, 40.9%). Consistent with a previous report of SD in GAD patients,Citation14 these incidences of SD in vilazodone GAD studies were lower than the results from MDD vilazodone studies (68% of females, 50% of males).Citation8 Nonetheless, the number of GAD patients with baseline SD in this report represents a sizable portion of the overall pooled GAD study population.
Deficits in different phases of the sexual functioning cycle may vary across psychiatric disorders. Decreased libido is the most commonly reported problem in depressed patients, although problems with arousal, erectile dysfunction, and absent or delayed orgasm are also prevalent.Citation29 In social phobia, SD may be related to performance anxiety, with premature ejaculation and impaired sexual satisfaction seen in males and impaired arousal, desire, activity, and satisfaction noted in femalesCitation30–Citation33 High levels of sexual aversion and phobic avoidance have been noted in patients with panic disorder and obsessive–compulsive disorder,Citation11,Citation34 while erectile dysfunction and premature ejaculation have been reported in combat veterans with posttraumatic stress disorder.Citation35 In the current post hoc analysis, mean scores at baseline were lower than SD thresholds for all CSFQ domains in both males and females, which largely explains the high levels of global SD seen in the vilazodone GAD studies.
Since sexual functioning can be negatively affected by age-related medical conditions, such as menopause, hypertension, benign prostatic hyperplasia, heart disease, and type 2 diabetes,Citation36–Citation39 analyses were conducted in male and female patients categorized by age (≤50 or >50 years). In comparison to younger females, older females in both treatment groups had lower mean CFSQ total and domain scores at baseline; in both age groups, mean baseline CSFQ scores were lower than SD thresholds. These findings suggest that female GAD patients – and older female patients in particular – may have decrements across a range of sexual functions that may need to be considered when discussing and choosing treatment options. In males, mean CSFQ total scores at baseline met the threshold for global SD in the older subgroup but not in the younger subgroup. However, CSFQ domain scores in both age groups were lower than the SD thresholds. These results suggest that while overall concerns about sexual functioning may need to be discussed with older male GAD patients, all male patients with GAD may benefit from more targeted questions regarding specific sexual problems.
Given the prevalence of global SD found in this study population, along with the low baseline CSFQ domain scores found in both males and females, the potentially adverse effects of pharmacologic agents on sexual functioning need to be considered when treating patients with GAD. However, since sexual difficulties may also be linked to the core anxiousness experienced by GAD patients, choosing a medication that effectively reduces anxiety symptoms is also an important consideration. In patients who experienced a HAM-A response in the vilazodone GAD studies, mean improvements in CSFQ total score were found with both vilazodone and placebo, although there were no statistically significant differences between the treatment groups. At best, these changes suggest modest improvements in sexual functioning in treatment responders. More importantly, however, they indicate that male and female GAD patients who responded to vilazodone did not have concurrent worsening in sexual functioning. In other words, these results indicate that symptom improvements with vilazodone may outweigh potential negative serotonergic effects, such that patients may achieve anxiety relief without experiencing diminished sexual functioning.
Since underlying sexual difficulties associated with GAD could have been a confounding factor in evaluating the effects of vilazodone on sexual functioning, subgroup analyses were conducted in males and females with or without baseline SD. In both treatment groups, greater mean improvements in CSFQ total score occurred in males and females with baseline SD than in patients without baseline SD, which may reflect a ceiling effect in the patients without baseline SD. By week 8, it seems some vilazodone-treated patients were experiencing therapeutic effects on SD, while others may have already experienced tolerance to these effects over time. For example, approximately 35% of male and female patients with SD at baseline switched to normal function status after 8 weeks of vilazodone treatment. In patients without SD at baseline, there was a significant mean worsening in CSFQ total score with vilazodone at week 4, but no significant difference from placebo was detected at week 8.
Although a greater percentage of patients with normal sexual functioning at baseline met SD criteria after treatment with vilazodone compared to placebo, >80% of patients in both treatment groups retained normal sexual functioning during the studies. One caveat to consider, however, is that these analyses were based on categorical shifts, but the categories themselves included a wide range of sexual symptom severity. For example, a male patient who had a CSFQ total score of 48 at baseline and a total score of 46 at the end of treatment would have been counted as a shift from normal functioning to SD; however, this <3 points change may not have been clinically meaningful.Citation40 The results thus need to be interpreted carefully. From this perspective, the shift analyses generally suggest that although some GAD patients without baseline SD may experience treatment-emergent SD with vilazodone, there is also a subset of patients with GAD-associated SD who may experience clinically meaningful improvements after acute treatment with vilazodone.
In these post hoc analyses, older males and females generally had worse CSFQ domain scores at baseline than younger patients. Small mean improvements in all CSFQ domain scores were found with vilazodone in older females, as well as in younger females and males. In older males, mean increases (improvements) were found in the domains of pleasure and arousal/erection; no change or very small mean decreases (−0.0 to −0.1) were found in the remaining domains of desire/frequency, desire/interest, and orgasm/ejaculation. However, it is important to emphasize that none of the vilazodone results in the sex-by-age analyses were statistically different from placebo, suggesting that this drug was not associated with worsening in any sexual functioning domain, regardless of sex or age.
AE reporting provides additional information on sexual issues that can arise during treatment, although the reported incidences may be lower than actual rates, since patients may be hesitant to discuss matters related to sexual function voluntarily.Citation41,Citation42 In this post hoc analysis, TEAEs related to sexual functioning were more common in patients treated with vilazodone than those treated with placebo, and more common in vilazodone-treated males than vilazodone-treated females. Decreased libido was the most common sexual function TEAE in vilazodone-treated female patients; four male placebo- and four male vilazodone-treated patients also reported decreased libido. The most common sexual function TEAEs for vilazodone-treated males were erectile dysfunction (2.4%) and delayed ejaculation (2.1%). By definition, the sexual function TEAEs reported here represent new or worsening conditions. Therefore, sexual problems that existed at baseline and subsequently resolved or improved are not included, making it difficult to compare TEAEs with changes in sexual function that were systematically assessed using a quantitative survey, such as the CSFQ.
Limitations of these analyses include their post hoc nature and lack of an active control; results should be interpreted accordingly. The exclusion of patients with concurrent psychiatric disorders limits the ability to generalize the results of this pooled study population to patients with comorbid MDD or significant depressive symptoms. Additionally, the constituent studies were not designed or powered to evaluate sexual function as a primary objective. For this reason, statistically significant between-group differences (or lack thereof) may not necessarily reflect clinical significance. Future studies that characterize SD in GAD in a broader patient population should be conducted to improve the clinical management of this disorder.
Conclusion
More than one-third of patients in the vilazodone GAD studies had SD at baseline, underscoring the importance of identifying sexual problems in patients with GAD. In clinical settings, patients may be uncomfortable talking about sexual difficulties, and the management of core anxiety symptoms may be a more pressing issue for the health care provider. However, sexual health is an important component of a patient’s overall well-being, and treatment-related SD can reduce medication adherence.Citation4 Therefore, inquiring about specific changes in sexual functioning should be incorporated into the overall treatment strategy. Because SD can encompass a range of issues, from lack of desire/interest to inability to perform, specific questions may need to be asked to pinpoint the types of problems a patient might be experiencing. One way to broach this potentially sensitive subject may be to explain the possible adverse effects that various medications may have on sexual functioning. Regardless of the method of inquiry, SD in GAD remains an underrecognized clinical problem that warrants ongoing attention and discussion.
In the GAD studies that were included in the current post hoc analyses, changes in CSFQ total and domain scores after 8 weeks of double-blind treatment showed no significant differences between vilazodone 20–40 mg/day and placebo. One advantage of using the CSFQ in clinical trials is that the instrument is sex-specific, with established criteria for SD in both males and females. The CSFQ analyses presented in this report indicate that vilazodone was not associated with increased sexual deficits in either males or females, regardless of age. Moreover, among treatment responders (defined as patients with ≥50% improvement in anxiety symptoms based on HAM-A total score change from baseline), vilazodone was associated with modest improvements in SD; these effects were particularly noticeable among females who responded to treatment. Patients with global SD at baseline also experienced improvements in sexual functioning, with approximately 35% of males and females with baseline SD shifting to normal sexual functioning after 8 weeks of vilazodone treatment. TEAEs related to sexual functioning were observed with vilazodone in some patients, more frequently in males than in females. Although these potential negative effects need to be considered, the results of this post hoc analysis suggest that the negative impact of vilazodone on sexual functioning is generally limited in adults with GAD, which is consistent with the effects seen in patients with MDD.Citation8,Citation40 Such information, along with findings from other antidepressant trials,Citation6,Citation43 may help clinicians select medications with efficacy and safety profiles that are appropriate for each individual patient.
Acknowledgments
Writing assistance and editorial support for the preparation of this manuscript were provided by Carol Brown, MS, and Mildred Bahn, MA, of Prescott Medical Communications Group, Chicago, IL, a contractor of Forest Research Institute (Allergan affiliate). Funding support was received from Forest Laboratories LLC, an Allergan affiliate (Jersey City, NJ). Forest Laboratories was involved in the study design, collection (via contracted clinical investigator sites), analysis, and interpretation of data, and the decision to present these results.
Disclosure
AC acknowledges that she has received grants from Auspex Pharmaceuticals, Forest Research Institute, Genomind, and Palatin Technologies; advisory board fees/consultant fees from Forest Laboratories (Allergan affiliate), Lundbeck, Naurex, Otsuka, Palatin Technologies, S1 Biopharmaceuticals Inc, and Sprout Pharmaceuticals; royalties/copyright from Ballantine Books/Random House, Changes in Sexual Functioning Questionnaire, and Guilford Publications; and shares/restricted stock units from Euthymics and S1 Biopharmaceuticals Inc. SD and CG acknowledge a potential conflict of interest as employees of Forest Research Institute, an Allergan affiliate. XT acknowledges a potential conflict of interest as a former employee of Forest Research Institute, an Allegan affiliate. AR acknowledges a potential conflict of interest as a former employee of Prescott Medical Communications, a contractor of Forest Research Institute, an Allergan affiliate. The authors report no other conflicts of interest in this work.
References
- LaumannEOPaikARosenRCSexual dysfunction in the United States: prevalence and predictorsJAMA1999281653754410022110
- WaldingerMDPsychiatric disorders and sexual dysfunctionHandb Clin Neurol201513046948926003261
- AtlantisESullivanTBidirectional association between depression and sexual dysfunction: a systematic review and meta-analysisJ Sex Med2012961497150722462756
- ClaytonAHThe impact of antidepressant-associated sexual dysfunction on treatment adherence in patients with major depressive disorderCurr Psychiatr Rev201394293301
- ClaytonAHReddySFochtKMusgnungJFayyadRAn evaluation of sexual functioning in employed outpatients with major depressive disorder treated with desvenlafaxine 50 mg or placeboJ Sex Med201310376877622905811
- ClaytonAKornsteinSPrakashAMallinckrodtCWohlreichMChanges in sexual functioning associated with duloxetine, escitalopram, and placebo in the treatment of patients with major depressive disorderJ Sex Med200744 Pt 191792917627739
- ClaytonAHCroftHAHandiwalaLAntidepressants and sexual dysfunction: mechanisms and clinical implicationsPostgrad Med20141262919924685972
- ClaytonAHKennedySHEdwardsJBGallipoliSReedCRThe effect of vilazodone on sexual function during the treatment of major depressive disorderJ Sex Med201310102465247623216998
- ClaytonAHLocklearJCSvedsäterHMcIntyreRSSexual functioning in patients with major depressive disorder in randomized placebo-controlled studies of extended release quetiapine fumarateCNS Spectr201419218219624067192
- CasperRCRedmondDEJrKatzMMSchafferCBDavisJMKoslowSHSomatic symptoms in primary affective disorder: presence and relationship to the classification of depressionArch Gen Psychiatry19854211109811043863548
- KaplanHSAnxiety and sexual dysfunctionJ Clin Psychiatry198849Suppl21253170497
- KesslerRCChiuWTDemlerOMerikangasKRWaltersEEPrevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey ReplicationArch Gen Psychiatry200562661762715939839
- KesslerRCBerglundPDemlerOJinRMerikangasKRWaltersEELifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey ReplicationArch Gen Psychiatry200562659360215939837
- KendurkarAKaurBMajor depressive disorder, obsessive-compulsive disorder, and generalized anxiety disorder: do the sexual dysfunctions differ?Prim Care Companion J Clin Psychiatry200810429930518787674
- McGahueyCAGelenbergAJLaukesCAThe Arizona Sexual Experience Scale (ASEX): reliability and validityJ Sex Marital Ther2000261254010693114
- OlivierBChanJSSnoerenEMDifferences in sexual behaviour in male and female rodents: role of serotoninCurr Top Behav Neurosci20118153621374021
- ClaytonAHMcGarveyELClavetGJPiazzaLComparison of sexual functioning in clinical and nonclinical populations using the Changes in Sexual Functioning Questionnaire (CSFQ)Psychopharmacol Bull19973347477539493487
- KhanACutlerAJKajdaszDKA randomized, double-blind, placebo-controlled, 8-week study of vilazodone, a serotonergic agent for the treatment of major depressive disorderJ Clin Psychiatry201172444144721527122
- RickelsKAthanasiouMRobinsonDSGibertiniMWhalenHReedCREvidence for efficacy and tolerability of vilazodone in the treatment of major depressive disorder: a randomized, double-blind, placebo-controlled trialJ Clin Psychiatry200970332633319284933
- RobinsonDSKajdaszDKGallipoliSWhalenHWamilAReedCRA 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorderJ Clin Psychopharmacol201131564364621869687
- AdamecRBartoszykGDBurtonPEffects of systemic injections of vilazodone, a selective serotonin reuptake inhibitor and serotonin 1A receptor agonist, on anxiety induced by predator stress in ratsEur J Pharmacol20045041–2657715507223
- HamiltonMThe assessment of anxiety states by ratingBr J Med Psychol1959321505513638508
- GommollCForeroGMathewsMVilazodone in patients with generalized anxiety disorder: a double-blind, randomized, placebo-controlled, flexible-dose studyInt Clin Psychopharmacol201530629730626291335
- DurgamSGommollCForeroGEfficacy and safety of vilazodone in patients with generalized anxiety disorder: a randomized, double-blind, placebo-controlled, flexible-dose trialJ Clin Psychiatry2016
- GommollCDurgamSMathewsMA double-blind, randomized, placebo-controlled, fixed-dose phase III study of vilazodone in patients with generalized anxiety disorderDepress Anxiety201532645145925891440
- GuyWThe clinician global severity and impression scalesECDEU Assessment Manual for PsychopharmacologyRockville (MD)National Institute of Mental Health1976218222
- HamiltonMA rating scale for depressionJ Neurol Neurosurg Psychiatry196023566214399272
- KellerAMcGarveyELClaytonAHReliability and construct validity of the Changes in Sexual Functioning Questionnaire short-form (CSFQ-14)J Sex Marital Ther2006321435216234225
- KennedySHRizviSSexual dysfunction, depression, and the impact of antidepressantsJ Clin Psychopharmacol200929215716419512977
- BodingerLHermeshHAizenbergDSexual function and behavior in social phobiaJ Clin Psychiatry2002631087487912416596
- FigueiraIPossidenteEMarquesCHayesKSexual dysfunction: a neglected complication of panic disorder and social phobiaArch Sex Behav200130436937711446198
- HeimbergRGBarlowDHPsychosocial treatments for social phobiaPsychosomatics198829127373277216
- LearyMRDobbinsSESocial anxiety, sexual behavior, and contraceptive useJ Pers Soc Psychol1983456134713546663447
- MonteiroWONoshirvaniHFMarksIMLelliottPTAnorgasmia from clomipramine in obsessive-compulsive disorder: a controlled trialBr J Psychiatry19871511071123315086
- LetourneauEJSchewePAFruehBCPreliminary evaluation of sexual problems in combat veterans with PTSDJ Trauma Stress19971011251329018683
- Al-AzzawiFBitzerJBrandenburgUTherapeutic options for postmenopausal female sexual dysfunctionClimacteric201013210312019958161
- HoekstraTLesman-LeegteILuttikMLSexual problems in elderly male and female patients with heart failureHeart201298221647165222875738
- MironeVSessaAGiulianoFCurrent benign prostatic hyperplasia treatment: impact on sexual function and management of related sexual adverse eventsInt J Clin Pract20116591005101321718399
- WylieKKenneyGSexual dysfunction and the ageing maleMaturitas2010651232720015601
- ClaytonAHGommollCChenDNunezRMathewsMSexual dysfunction during treatment of major depressive disorder with vilazodone, citalopram, or placebo: results from a phase IV clinical trialInt Clin Psychopharmacol201530421622326039688
- LandenMHogbergPThaseMEIncidence of sexual side effects in refractory depression during treatment with citalopram or paroxetineJ Clin Psychiatry200566110010615669895
- MontejoALLlorcaGIzquierdoJARico-VillademorosFIncidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. Spanish Working Group for the Study of Psychotropic-Related Sexual DysfunctionJ Clin Psychiatry200162Suppl 3102111229449
- JacobsenPLMahableshwarkarARPaloWAChenYDragheimMClaytonAHTreatment-emergent sexual dysfunction in randomized trials of vortioxetine for major depressive disorder or generalized anxiety disorder: a pooled analysisCNS Spectr Epub20151117
