Abstract
Aim
Polysubstance users represent the largest group of patients seeking treatment at addiction and rehabilitation clinics in Turkey. There is little knowledge about the structural brain abnormalities seen in polysubstance users. This study was conducted to examine the structural brain differences between polysubstance use disorder patients and healthy control subjects using voxel-based morphometry.
Methods
Forty-six male polysubstance use disorder patients in the early abstinence period and 30 healthy male controls underwent structural magnetic resonance imaging scans. Voxel-based morphometry analysis was performed to examine gray matter (GM) abnormality differences.
Results
Polysubstance use disorder patients displayed significantly smaller GM volume in the thalamus, temporal pole, superior frontal gyrus, cerebellum, gyrus rectus, occipital lobe, anterior cingulate cortex, superior temporal gyrus, and postcentral gyrus.
Conclusion
A widespread and smaller GM volume has been found at different regions of the frontal, temporal, occipital, and parietal lobes, cerebellum, and anterior cingulate cortex in polysubstance users.
Introduction
Approximately 40 years ago, there came great curiosity about the possible long-term effects of substances on the human brain.Citation1 Advances in neuroimaging and analysis methods have given researchers the opportunity to study the neurobiological correlates of the chronic and acute effects of alcohol and other substances.Citation2 Many studies have examined the structural brain differences in order to gain more insight into the brain regions that are relevant to the development and maintenance of addiction. A number of structural magnetic resonance imaging (MRI) studies have demonstrated that chronic alcohol and substance exposure is associated with morphological differences in several brain regions, especially the orbitofrontal cortex.Citation3 Most of the neuroimaging studies have been conducted in monoalcohol/substance users, while the majority of the treatment-seeking substance users consists of polysubstance users. Although numerous studies investigated the sole effects of each substance on the brain, no conclusive results are available concerning which specific brain regions are affected by single substance use.Citation4,Citation5 Substances from different classes may be combined by substance users to enhance the anticipated effects of each substance or to alleviate the adverse effects of other substances such as craving or withdrawal.Citation6 The combined use of three or more substances with the purpose of intoxication may have cumulative or synergistic adverse effects on the brain function via complicated interactions between substances.Citation7 Studies that explored brain structural differences using voxel-based morphometry (VBM) in polysubstance users are very limited. In 1998, Liu et alCitation8 first reported that individuals with a history of polysubstance use have smaller prefrontal and temporal lobes than control subjects. These volume abnormalities were found only in gray matter (GM), while no volumetric abnormalities were detected in the white matter. Liu et alCitation8 speculated that the observed abnormalities in the temporal lobe could be a consequence of prefrontal deficits due to the extensive interconnections between the temporal lobe and the prefrontal cortex. Schlaepfer et alCitation9 found smaller white matter volume percentages in only the frontal cortex in polysubstance users than in comparison to matched control subjects. Tanabe et al reported reduced medial orbitofrontal cortex GM volume in polysubstance-dependent individuals after prolonged abstinence. This volume loss was related to pathological decision making capacity.Citation10 Dalwani et alCitation11 reported reduced cortical GM volumes in the dorsolateral prefrontal cortex, the inferior frontal gyrus, and the cerebellum in male adolescents with substance and conduct problems. Grodin et al indicated that abstinent polysubstance users have a reduced GM in the right inferior temporal gyri, the medial frontal lobe, the superior frontal gyrus, the cingulate and paracingulate gyri, and the middle/superior frontal gyri. These volume alterations were not related to comorbid Axis I diagnoses such as anxiety disorders, depressive disorders, and posttraumatic stress disorders.Citation12 Mon et alCitation13 found that polysubstance users had a larger lobar white matter volume and smaller GM volume in the temporal lobe, thalamus, and lenticular nucleus than light drinkers and one-month-abstinent alcohol-dependent individuals. Tanabe et al differed from previous studies with the findings of insular cortex thinning but not orbitofrontal cortex (OFC) alterations regarding frontolimbic pathway involvement in behaviors related to polysubstance dependence. In their study, they concluded that the brain morphology in substance dependence was modulated by sex, with the result of larger insular cortex in male dependent subjects than female dependent subjects.Citation14
Although polysubstance users represent the largest group of individuals seeking treatment in Turkey, little is known about the structural brain abnormalities due to poly-substance use. In this volumetric MRI study, we assessed GM volume differences using whole-brain analysis by implementing VBM between male polysubstance users receiving inpatient treatment within a week of abstinence and healthy control subjects. We hypothesized that the polysubstance patients would display more widespread, extensive, and significant GM abnormalities than the control subjects.
Methods
Study participants
Forty-six polysubstance use disorder patients who were under treatment in the addiction clinic of the Neuropsychiatry Istanbul Hospital between January 2013 and January 2014 and 30 healthy control subjects were enrolled in the study. We reviewed the medical records of 76 patients, and 30 patients were excluded due to a lack of sufficient data and a previous history of schizophrenia or bipolar disorder diagnoses. All participants were diagnosed of having three or more alcohol/substance dependence criteria based on Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV), by two independent psychiatrists. The exclusion criteria included having a major medical or neurological illnesses, a life time history of schizophrenia and bipolar disorder, and a history of traumatic head injury. The data derived from patient records included sociodemographic data, including the following parameters: sex, age, marital status, duration of education, age of first alcohol/substance use, duration of alcohol/substance use (years), duration of regular alcohol/substance use (years), and frequency of alcohol/substance use in the past year. MRI scans were acquired on day 7 after the last alcohol/substance use. Control subjects were recruited from the community and were excluded if they met any DSM-IV criteria for lifetime dependence of alcohol or any substances except nicotine. The study was approved by the ethical committee of Uskudar University. All participant provided written informed consent. All study participants were tobacco smokers and had undergone psychometric evaluation by a trained psychiatrist via a clinical interview that employed the NPIstanbul Addiction Interview Form and Beck Depression Inventory, Beck Anxiety Inventory, Hamilton Rating Scale for Depression, and the Brief Psychiatric Rating Scale.
Image acquisition
Imaging was performed using a 1.5 T MR scanner (Achieva, Philips Healthcare, Best, the Netherlands) with a SENSE-Head-8 coil at NPISTANBUL Neuropsychiatry Istanbul Hospital. A T1-weighted MPRAGE sequence was employed to achieve a high-resolution anatomical scan (repetition time =8.6, echo time =4, flip angle =8, voxel size 0.9375/0.9375/1.2 mm; slice spacing =1.2 mm, field of view =240 mm, and 125 slices).
VBM analyses
We examined between-group differences in GM volume by using the VBM toolbox. The relevant data were processed and examined using the Statistical Parametric Mapping 8 software package (Welcome Department of Imaging Neuroscience Group, London, UK; http://www.fil.ion.ucl.ac.uk/spm) and the VBM8 Toolbox (http://dbm.neuro.uni-jena.de/vbm.html) with the default preprocessing parameters. Adaptive nonlocal means (SANLM) and a classical Markov random field model were applied to the images to remove inhomogeneity and to increase the signal-to-noise ratio.Citation15,Citation16 Registration to the standard Montreal Neurological Institute (MNI) space was performed using a linear affine transformation and a nonlinear deformation using a high-dimensional diffeomorphic anatomical registration through exponentiated Lie algebra (DARTEL) normalization.Citation17 Subsequent analyses were performed on segmented GM images, which were multiplied by the nonlinear components derived from the normalization matrix to control for differences in total brain volume (modulated GM volumes). Sample homogeneity was checked using covariance matrix to identify potential outliers. To assess the quality of the normalization procedure, the normalized unsegmented images were visually inspected for gross artifacts. Finally, the segmented and modulated images were smoothed with an 8 mm full-width-half-maximum Gaussian kernel to explore volumetric differences in GM volume. Using the voxel of interest (VOI) method, the GM volumes were extracted by grouping the significant clusters as follows: thalamus, bilateral temporal lobes, superior frontal gyrus, cerebellum, anterior cingulate cortex (ACC), gyrus rectus, postcentral gyrus, and occipital cortex.
Statistical analysis
The two groups were compared using the voxel-wise independent-sample t-test as implemented in the Statistical Parametric Mapping second-level model. The clusters were considered to be significant if they survived a family-wise error rate (FWE) correction at P-level of 0.05 (cluster forming threshold =20 voxels). To identify the associations between structural abnormalities and substance use profiles, we performed VOI analyses on cerebral tissues for which differences between groups were identified. Later, these clusters were combined to form eight VOIs (as detailed in the “VBM analyses” section). To assess the correlations between GM volumes in these VOIs and the duration of polysubstance use, we used simple correlation analyses without any correction for multiple comparisons. The GM volumes in these areas were extracted using the MarsBaR toolboxCitation18 and transferred to Statistical Package for the Social Sciences (SPSS) for Windows, Version 11.5 (SPSS Inc., Chicago, IL, USA) for further analysis.
Results
Forty-six male participants with polysubstance dependence (mean age =27.39±7.11 years) and 30 males without any known neuropsychiatric problems (mean age =29.07±5.57 years) were included in the study. The demographic variables are described in . The age difference between groups was not significant (P=0.27). The substance use profiles of the patients with polysubstance dependence are described in .
Table 1 Sociodemographic characteristics of the participants
Table 2 Lifetime alcohol/substance dependence diagnosis of polysubstance use disorder patients
In the voxel-wise whole-brain analysis, significant group differences were observed in a number of brain areas, including the thalamus, the bilateral temporal lobes, the ACC, the left cerebellum, and the occipital areas (). In these areas, the polysubstance user group had significantly smaller GM volumes. However, no significant clusters were detected in a contrast analysis exploring areas that have greater GM volumes in the polysubstance use group.
Table 3 Talairach coordinates for regions of lower GM volume
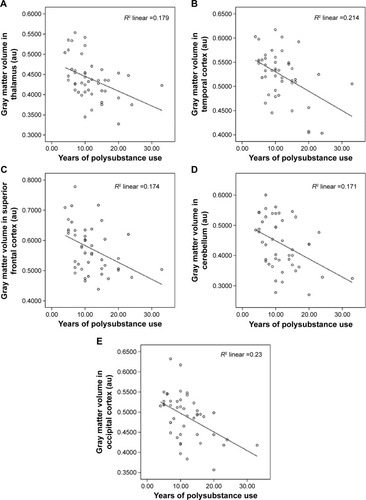
A number of significant correlations between brain GM volumes and the years of total polysubstance use were found. In correlation analyses, 17.9% of the variability in the thalamus GM volume alteration is accounted for by the years of total polysubstance use (r=−0.424, P=0.002), 21.4% of the variability in the temporal cortex volume alteration is accounted for by the years of total polysubstance use (r=−0.463, P=0.001), 17.4% variability in the superior frontal cortex GM volume alteration is accounted for by the years of polysubstance use (r=−0.417, P=0.004), 17.1% of the variability in the cerebellum GM volume alteration is accounted for by the years of polysubstance use (r=−0.414, P=0.032), 3.5% of the variability in gyrus rectus GM volume alteration is accounted for by the years of polysubstance use (r=−0.187, P=0.212), 4.4% of the variability in the postcentral gyrus GM volume alteration is accounted for by the years of polysubstance use (r=−0.209, P=0.163), 23.0% of the variability in the occipital cortex GM volume alteration is accounted for by the years of polysubstance use (r=−0.480, P=0.001), 10.9% of the variability in the ACC GM volume alteration is accounted for by the years of polysubstance use (r=−0.331, P=0.025). No statistically significant correlations were observed between the Beck Depression Inventory, Beck Anxiety Inventory, Hamilton Rating Scale for Depression, and Brief Psychiatric Rating Scale scores and the GM volumes (P>0.05) among poly-substance users.
Discussion
The main finding of this study is that a widespread and extensive smaller GM volume has been found at different regions of the frontal, temporal, occipital, and parietal lobes, cerebellum, and ACC among polysubstance users. Smaller GM volumes notably in the frontal cortex (superior frontal, gyrus rectus),Citation8,Citation11 ACC,Citation12 thalamus,Citation13 temporal cortex (temporal pole, superior temporal gyrus),Citation12 and cerebellumCitation11 were consistent with previous studies. The smaller GM volumes in the thalamus, superior temporal gyrus, and ACC in the poly-substance users are shown in . Smaller GM volumes in the occipital lobe and parietal lobe (postcentral gyrus) among polysubstance users are one of the new findings of this study. Smaller GM volumes in the occipital lobe have been reported previously among cocaine abusers,Citation19 and smaller GM volumes in the postcentral gyrus have been reported previously in stimulant drug-dependentCitation20 and heroin-dependentCitation19 patients. The significant correlations between GM changes in the thalamus, temporal cortex, superior frontal cortex, cerebellum, and occipital cortex and years of polysubstance use are shown in .
Figure 1 Voxel-based morphometric analysis of polysubstance users (n=46) and healthy control patients (n=30).
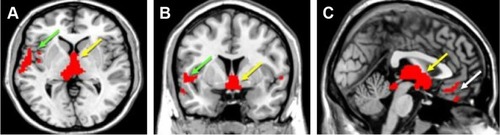
Figure 2 Correlations between years of polysubstance use and GM volumes in the (A) thalamus, (B) temporal cortex, (C) superior frontal cortex, (D) cerebellum, and (E) occipital cortex (n=46).
Only a few studies have investigated the structural brain abnormalities associated with the combined use of substances such as cannabis, synthetic cannabis, alcohol, opiate, cocaine, methamphetamine, and other psychostimulants. In this study, we aimed to examine GM volumes using the VBM in whole-brain analyses. Previous studies that assessed structural abnormalities among polysubstance users have some methodological differences regarding neuroimaging analysis techniques and patients’ characteristics. Most of these previous studies of polysubstance users have focused on a single regionCitation10 or the major lobes, especially the frontal cortexCitation8,Citation13 rather than performing a whole-brain analysis. One of the previous studies used a semi-automated procedure,Citation8 while in another study, automated procedures were usedCitation10 to perform volumetric assessments.
Because a limited number of studies have been performed and because methodological differences and heterogeneity of patient characteristics exist among those studies, the published results are variable. Therefore, it is not possible to attribute the observed volume differences to the use of a single substance. The question of whether the combined use of more than two substances is the cause or the result of these brain structural abnormalities remains unclear. While the unique effect of each substance on the brain is well known, no clear evidence is available concerning the consequences of the interactions between two or more substances. Neuropathological studies suggest that smaller GM volumes could be caused by the shrinkage of neuronal cell bodies or the loss of axonal and/or dendritic processes.Citation20,Citation21 Chronic alcohol consumption activates some inflammatory mediators that may cause inflammatory injury, cell death, and neurodegeneration.Citation22 Dietary changes and the pattern and history of alcohol use may cause differences in brain volume. The anti-inflammatory effects of cannabinoids (CBs) via CB2 receptors may have a neuroprotective effect on the thalamic and midbrain structure.Citation12 Stimulant (cocaine, 3,4-methylenedioxy-methamphetamine, methamphetamine) use could mask the GM reduction due to inflammation-induced brain volume enlargement.Citation23 Although 3,4-methylenedioxy-methamphetamine has less neurotoxic effects than methamphetamine, structural abnormalities among stimulant users may have been derived from stimulant-mediated neurotoxicity.Citation24 Chronic opiate exposure may cause altered dendritic spine density and neuronal apoptosis leading to a smaller GM volume.Citation25
Abstinence is the other confounding factor for sparse results. Each study employed a different abstinence periods. Mon et alCitation13 carried out their study among outpatients after a 1-month-abstinence period. Another study included 2-year-abstinent patients as the study sample.Citation14 The substance abstinence period is an important factor in determining how much the brain can repair itself during the period of prolonged abstinence and is related to vulnerability, which presents itself with relapses. This study was conducted in the early abstinence period during residential treatment in the psychiatry service.
Grodin et alCitation12 indicated that alcohol-dependent patients have smaller GM volumes than alcohol-dependent patients with polysubstance use regarding the anti-inflammatory effects of CBs. Ersche et al reported that stimulant-dependent individuals and their nondependent siblings have smaller GM volumes in the left postcentral gyrus, superior temporal gyrus, and posterior insula than the control subjects. Ersche et alCitation4 speculated that an individual’s predisposition to addiction may be mediated by brain abnormalities. Liu et al speculated that smaller GM volumes in the frontal lobe of substance users may reflect developmental hypoplasia. On the other hand, those authors pointed out that a smaller GM volume in the frontal lobe may reflect the chronic effects of substance use.Citation8 ACC dysfunctions have been associated with stress-induced craving, poor inhibitory control, impaired insight, and less motivation to change.Citation26 Stimulant users have been reported to have less cortical GM and a larger striatal volume than control subjects.Citation24,Citation27 Although most of our patients were stimulant users (n=40), we did not find any larger striatal volumes in patients compared to the controls.
As a result, the type and characteristics of the substances used, the duration of alcohol/substance use, interactions of the different substances, the structural differences present before the initial use, the effects of occasional/recreational use, and the abstinence period can all affect the structural brain volumes. It is not easy to come up with direct conclusion about the causality of these brain structural abnormalities within this heterogeneous sample. There is long way to solve the puzzle whether these brain abnormalities are caused by the toxic or degenerative effects of the substances, or these structural abnormalities predispose certain individuals to illicit substance use.
The results reported in this study should be considered in light of certain limitations. First, the heterogeneity and the modest sample size (n=46) might be considered as a limitation. Future structural neuroimaging studies in more homogeneous polysubstance populations would provide more insights into the brain abnormalities that occur in polysubstance users. Our neuroimaging study reveals extensive smaller GM volumes at specific brain regions in male polysubstance users receiving treatment in comparison to the controls. Second, we only included male subjects to eliminate any confounding factors associated with sex differences. Therefore, the results of this study may not be generalizable to female and treatment-naïve substance users. It remains unclear to what extent structural changes are due to the ongoing illness process and to what extent to medication and how different psychotropic medications affect neuroimaging measures. Another limitation of our study is that we did not screen the participants for DSM-IV Axis II disorders. We did not assess mental diseases other than the schizophrenia and bipolar disorders that could affect the structural abnormalities. Subclinical symptoms such as psychotic and affective features were not taken into account. Scanning the subjects a week after the last alcohol use may be other confounding factor. We did not assess the cognitive functions that might be correlated with smaller GM volumes among polysubstance users. Prospective studies will help to clarify whether some correlations exist between GM volumes and cognitive functions. It is well known that smoking has substantial effects on brain volumeCitation28 especially smaller volume in the left thalamus, medial frontal cortex, and ACC.Citation29 Although all of our participants were tobacco smokers, we did not take the history of smoking status, which might present as a limitation of the study. Severity of nicotine dependence and the starting age of smoking may affect the brain volumes. Furthermore, we did not take alcohol use into account that did not reach to the level of addiction or abuse among both patients and healthy controls. The severity of nicotine dependence and alcohol use may present potential confounding factors in this study.
Conclusion
This study provided evidence for the existence of smaller GM volumes in polysubstance use disorder patients. The results of this study may help to improve prevention and treatment programs for polysubstance users. Recognizing the structural abnormalities as a cause or the consequences of the polysubstance use may help to detect vulnerable individuals and encourage them to seek treatment. Results of our study would help to destigmatize substance user patients in the society and even among mental health professionals as it would strengthen the concept of addiction as a brain disease, not character or will power defect. The relationship between specific brain volumes and clinical prognosis should be investigated in further longitudinal studies.
Acknowledgments
The authors thank the patients and healthy volunteers for participation and Sedat Aydin for technical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
- GrantIMohnsLChronic cerebral effects of alcohol and drug abuseSubst Use Misuse1975105883920
- Lingford-HughesAHuman brain imaging and substance abuseCurr Opin Pharmacol200551424615661624
- GoldsteinRZVolkowNDDysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implicationsNat Rev Neurosci2011121165266922011681
- ErscheKDWilliamsGBRobbinsTWBullmoreETMeta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilienceCurr Opin Neurobiol201323461562423523373
- AbéCMonADurazzoTCPenningtonDLSchmidtTPMeyerhoffDJPolysubstance and alcohol dependence: unique abnormalities of magnetic resonance-derived brain metabolite levelsDrug Alcohol Depend20131301–3303723122599
- ConnorJPGulloMJWhiteAKellyABPolysubstance useCurr Opin Psychiatry201427426927524852056
- LicataSCRenshawPFNeurochemistry of drug actionAnn N Y Acad Sci2010118714817120201852
- LiuXMatochikJACadetJLLondonEDSmaller volume of pre-frontal lobe in polysubstance abusers: a magnetic resonance imaging studyNeuropsychopharmacology19981842432529509492
- SchlaepferTELancasterEHeidbrederRDecreased frontal white-matter volume in chronic substance abuseInt J Neuropsychopharmacol20069214715316004619
- TanabeJTregellasJRDalwaniMMedial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individualsBiol Psychiatry200965216016418801475
- DalwaniMSakaiJTMikulich-GilbertsonSKReduced cortical gray matter volume in male adolescents with substance and conduct problemsDrug Alcohol Depend20111182–329530521592680
- GrodinENLinHDurkeeCAHommerDWMomenanRDeficits in cortical, diencephalic and midbrain gray matter in alcoholism measured by vbm: effects of co-morbid substance abuseNeuroimage Clin2013246947624179800
- MonADurazzoTCAbeCStructural brain differences in alcohol-dependent individuals with and without comorbid substance dependenceDrug Alcohol Depend201414417017725263262
- TanabeJYorkPKrmpotichTInsula and orbitofrontal cortical morphology in substance dependence is modulated by sexAJNR Am J Neuroradiol20133461150115623153869
- CuadraMBCammounLButzTCuisenaireOThiranJ-PComparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain imagesIEEE Trans Med Imaging200524121548156516350916
- ManjónJVCoupéPMartí-BonmatíLCollinsDLRoblesMAdaptive non-local means denoising of MR images with spatially varying noise levelsJ Magn Reson Imaging201031119220320027588
- AshburnerJA fast diffeomorphic image registration algorithmNeuroimage20073819511317761438
- BrettMAntonJLValabregueRPolineJBRegion of interest analysis using the marsbar toolbox for SPM 99Neuroimage2002162S497
- GardiniSVenneriAReduced grey matter in the posterior insula as a structural vulnerability or diathesis to addictionBrain Res Bull2012872–320521122178355
- ErscheKDJonesPSWilliamsGBTurtonAJRobbinsTWBullmoreETAbnormal brain structure implicated in stimulant drug addictionScience2012335606860160422301321
- O’SullivanSKendallDCannabinoid activation of peroxisome proliferator-activated receptors: potential for modulation of inflammatory diseaseImmunobiology2010215861161619833407
- VallésSLBlancoAMPascualMGuerriCChronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytesBrain Pathol200414436537115605983
- MackeySPaulusMAre there volumetric brain differences associated with the use of cocaine and amphetamine-type stimulants?Neurosci Biobehav Rev201337330031623253945
- BermanSO’NeillJFearsSBartzokisGLondonEDAbuse of amphetamines and structural abnormalities in the brainAnn N Y Acad Sci20081141119522018991959
- SeifertCLMagonSSprengerTReduced volume of the nucleus accumbens in heroin addictionEur Arch Psychiatry Clin Neurosci2015265863764525467383
- LiCRSinhaRInhibitory control and emotional stress regulation. Neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addictionNeurosci Biobehav Rev200832358159718164058
- ThompsonPMHayashiKMSimonSLStructural abnormalities in the brains of human subjects who use methamphetamineJ Neurosci200424266028603615229250
- MoralesAMLeeBHellemannGO’NeillJLondonEDGray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamineDrug Alcohol Depend2012125323023822445480
- LiaoYTangJLiuTChenXHaoWDifferences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry studyAddict Biol201217697798020731627
