Abstract
The neurological disorder cerebral palsy (CP) is caused by unprogressive lesions of the immature brain and affects movement, posture, and the musculoskeletal system. Vibration therapy (VT) is increasingly used to reduce the signs and symptoms associated with this developmental disability. The purpose of this narrative review was systematically to appraise published research regarding acute and long-term effects of VT on functional, neuromuscular, and structural parameters. Systematic searches of three electronic databases identified 28 studies that fulfilled the inclusion criteria. Studies were analyzed to determine participant characteristics, VT-treatment protocols, effect on gross motor function (GMF), strength, gait, posture, mobility, spasticity, reflex excitability, muscle tone, mass, and bone strength within this population, and outcome measures used to evaluate effects. The results revealed that one acute session of VT reduces reflex excitability, spasticity, and coordination deficits. Subsequently, VT has a positive effect on the ability to move, manifested for GMF, strength, gait, and mobility in patients with CP. Effects persist up to 30 minutes after VT. Long-term effects of VT manifest as reduced muscle tone and spasticity occurring concomitantly with improved movement ability in regard to GMF, strength, gait, and mobility, as well as increased muscle mass and bone-mineral density. Posture control remained unaffected by VT. In conclusion, the acute and chronic application of VT as a nonpharmacological approach has the potential to ameliorate CP symptoms, achieving functional and structural adaptations associated with significant improvements in daily living. Even though further studies including adult populations validating the neuromuscular mechanisms underlying the aforementioned adaptations should be fostered, growing scientific evidence supports the effectiveness of VT in regard to supplementing conventional treatments (physiotherapy and drugs). Therefore, VT could reduce CP-associated physical disability and sensorimotor handicaps. Goals for patients and their caregivers referring to greater independence and improved safety may be achieved more easily and time efficiently.
Introduction
Cerebral palsy (CP) is an umbrella term used to classify individuals with unprogressive lesions of the immature brain.Citation1 CP is a disease with a prevalence of two cases per 1,000 live-born neonates, characterized by motor and mental dysfunction.Citation2 Individuals with CP experience a variety of concomitant health problems as a result of their diagnosis, including movement disorders, difficulty with motor planning and control, and cognitive impairments.Citation3 The level of severity is categorized by the Gross Motor Function Classification System (GMFCS), with values of I–V. Major motor impairments comprise increased cocontraction and joint stiffness,Citation4 muscle weakness,Citation5,Citation6 decreased strength and power,Citation4 restricted single- and multijoint range of motion (ROM),Citation7 and gait and balance deteriorations.Citation8–Citation10 In spastic CP, as an upper-motor-neuron lesion, hypertonicity can be observed, being composed of neural and secondary nonneural components, including muscle structure or connective tissues.Citation11,Citation12 The pathophysiology of sensorimotor dysfunction due to lesions in the immature brain of humans with CP is manifested as insufficient control of afferents,Citation13,Citation14 spinal hyperexcitability,Citation3,Citation15 increased stretch reflexes, and increased cocontraction of antagonistsCitation8,Citation16 concomitantly with diminished voluntary control of body movement.Citation6,Citation16
Interventions to counteract motor impairments in patients with CP range from drug administration and surgery to therapeutic exercise.Citation17,Citation18 Nonpharmacological nonoperative approaches comprise training modalities in the settings of neurorehabilitation conceptualized to stimulate the impaired sensorimotor system of patients with CP that may – due to plasticity of the brain and spinal cord – profit and ameliorate motor syndromes. Exercise interventions aim to improve development and function by capitalizing on the innate capacity of the neural system to change and adapt throughout the life span.Citation18 Moderate–intense exercises include resistance training and stretching, physiotherapy, and systematic locomotor or postural exercises.Citation19 Therefore, the severity of motor and mental dysfunction in CP patients significantly narrows the applicability of the aforementioned training modalities, which often require voluntary motor skills and solid cognitive understanding to follow instructions and perform the exercises. In particular, limited options exist for CP patients with GMFCS scores of IV and V. As a consequence, vibration therapy (VT) is an alternative easy-to-apply and time-efficient intervention that has recently moved into focus.Citation20
VT is a training modality that uses mechanical oscillations as an indirect stimulus to act on human neuromuscular structures.Citation21–Citation23 Vibration determinants refer to the frequency (number of complete cycles per second, 5–200 Hz), amplitude (vertical displacement 0.5–10 mm), and type of VT (sinusoidal vertical and side-alternating). Two distinct categories can be identified: focal vibration, which acts with regional emphasis directly on the muscle belly or a tendon,Citation21 and whole-body vibration (WBV), during which mechanical oscillations are transmitted indirectly through the entire body.Citation24 Disregarding the topographic distinctions and the stimulus specified for the aforementioned VT modalities, the feasibility and efficacy of both focal and WBV therapy are independent of subjects’ movement ability, health, and mental status. As a consequence, the clinical application of vibration in neurorehabilitation has emerged as a particularly valuable tool, as there are no decisive motor prerequisites or particular cognitive requirements.Citation25,Citation26
Therefore, in the last few decades, research to investigate the effect of VT on patients with the central nervous system disorder CP has increased considerably. Scientific objectives include acute and long-term effects of VT on gross motor function, strength, and gait and posture control, as well as the effects on bone and muscle, which are of high relevance for everyday life. Additionally, numerous experiments have been executed with emphasis placed on the sensorimotor system to evaluate mechanisms underlying the aforementioned functional adaptations to the vibratory stimulus: methodologies including electromyography coupled with electrophysiology have allowed assessment of the excitability of spinal and corticospinal pathways, as well as afferent reflex loops related to CP-induced spasticity. It is thus important to conduct a review to examine systematic evidence guiding clinical decision making.
The objective of this narrative review is to provide a systematic overview of the literature related to the immediate and chronic effects of VT in CP and outline the sensorimotor mechanisms and adaptations, their functional consequences in terms of movement control, and resulting structural changes for muscle and bone. In accordance with the close relationship between successful treatment of CP symptoms and clear comprehension of the underlying pathophysiology, natural history, and impact on patient performances, we further address the neuromuscular mechanisms underlying the effect of VT evaluated in the context of CP-deconditioning prevention or rehabilitation.
Methods
This study was a systematic review of the available literature reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and guidelines.Citation27
Search strategy
We performed an electronic database search on Medline/PubMed, Google Scholar, and Web of Knowledge. The keywords “cerebral palsy” AND “vibration” were included in our final Boolean search strategy. Results were limited to articles in English and German languages and studies published in the period from January 1900 to December 2017. Broad search terms were used to ensure that all articles with potential for inclusion in this review were evaluated. Furthermore, we scanned each article’s reference list in an effort to identify additional suitable studies for inclusion in the database.
Available data identified by the electronic search were extracted independently by two well-trained researchers to determine whether the article met our inclusion criteria. In cases of disagreement, a third author was consulted. If the title or abstract did not provide sufficient information to determine eligibility, the article was obtained to determine whether it met the criteria. The number of articles that were included or excluded and the reasons for exclusion are detailed in .
Eligible studies
The eligibility criteria for selection were human participants with a diagnosis of CP, intervention of vibration treatment, full-text original articles, studies with an experimental design, randomized controlled trials, controlled trials, cohort studies, case–control studies, pre–post studies, or case reports, no inconsistencies in “Methods” and “Results” sections, and complete data.
Data synthesis and analysis
Three reviewers extracted the data from each of the studies independently and cross-checked the results to eliminate any errors. Key demographic characteristics of the participants, vibration-intervention protocols, and outcomes of each study were systematically collected. Articles were divided into categories based on the type of study design and vibration intervention the participants received, in order to compare the effectiveness of vibration in patients with CP. The categories longitudinal and cross-section study designs and focal and WBV with side-alternating, vertical vibration or “other” were used.
Quality of evidence
We used the PEDro scale to estimate the quality of evidence. This scale assesses the methodological quality of a study based on such criteria as concealed allocation, intention-to-treat analysis, and adequacy of follow-up. These characteristics make the PEDro scale a useful tool for assessing the quality of therapy and rehabilitation trials.Citation28 Methodological quality was independently assessed by two researchers. Studies were scored on the PEDro scale based on a Delphi listCitation29 that consisted of eleven items. One item on the PEDro scale (eligibility criteria) is related to external validity and is generally not used to calculate the method score, leaving a score range of 0–10.
Results
The search strategy identified 215 articles (71 PubMed, 55 Google Scholar, and 89 Web of Knowledge), including 131 duplicate publications. After screening both abstracts and titles, 49 full-text articles were retrieved. After reviewing the articles to determine whether they met all the inclusion criteria, 28 studies remained and were included in the review. shows details of the flow of studies throughout the review.
Study characteristics
The 28 studies were divided into two intervention categories: longitudinal studies (18), documenting training effects, and cross-sectional studies (10), documenting the acute effects of vibration. Defined subcategories contain training attributes referring to focal and WBV. A total of 21 studies had been executed with subjects under the age of 18 years, only seven studies experimented with adult volunteers. A general description of the 28 studies is presented in and , including information about the study design, participant demographics, intervention, dosing parameters, outcome measures, and results.
Methodological quality of included trials
The methodological quality of included trials assessed with the PEDro scale ranged from high to poor for the cross-sectional (total scores 3–6, average 5) and longitudinal studies (total scores 1–8, average 4). Detail for each item is provided in and . No article scored more than 8 (out of 10) on this scale. Not all the criteria on the PEDro scale were able to be satisfied in the selected studies. Items with considerably weak scores were identified as items associated with “subject allocation” and “blinding of participants and operators” (2, 3, 6, and 7). Most of the studies fulfilled criteria 4, 8, 10, and 11, indicating baseline comparability, and most subjects undertook the designated training program and outcome measures were reported.
Acute effects
Acute modulation refers to immediate changes manifested after acute vibration exposure. The definition of vibration exposure varies between singleCitation30–Citation33 and repetitive bouts of vibration.Citation15,Citation34–Citation37 The duration of the applied vibration varies: 3,Citation15 10,Citation37 and 30 secondsCitation31,Citation33 and 1,Citation32,Citation34,Citation36 3,Citation30 and 10 minutes.Citation35 Details of vibration application can be found in . The results are summarized and clustered by functional category ().
Functional modulation
Gross motor function
The effect of VT on gross motor function was assessed in three studies. Motor control was reported to be improved during vibration.Citation30,Citation31,Citation37 Cannon et al used focal vibration in three children with CP and documented a longer duration of head-erect behavior.Citation30 Eklund and Steen used focal vibration in a population of >200 patients with CP and noted in a descriptive approach increased movement speed, precision, and spontaneous movement of weak musculature concomitantly with improved awareness of the recognition of body parts and urge to move.Citation31 These adaptations were accompanied by improved pronunciation and feeding patterns, as well increased memory of motor acts and repeatability after breaks in VT. Improved kinesthesiaCitation31 occurred concomitantly with reduced stretch sensation of the vibrated muscle,Citation37 indicating vibration-induced modulations in the excitably of afferent pathways coupled with supraspinal changes involving brain structures.
Strength
Only minor evidence exists for acute effects of vibration on muscle strength, including differing paradigms and methodological approaches. Tupimai et al assessed the acute effect of oscillating WBV on time to complete “five-time sit to stand” in 12 children with spastic CP (GMFCS I–III), and found a trend toward a reduced duration to complete the task.Citation36 Eklund and Steen used a descriptive approach and reported increased voluntary muscle power of the vibrated muscle.Citation31 With emphasis on the upper back, neck, and head, Cannon et al documented longer duration of head-erect behavior, which may point toward increased strength.Citation30
Gait
In two studies, the effect of VT on gait was assessed. Using vertical WBV, Dickin et al found increased walking speed and elevated stride length accompanied by increased angular excursion in eight diplegic and hemiplegic patients with CP. No effects were shown for cadence, step length, or step and stride time.Citation34 Cheng et al found significantly increased distance covered in a predefined time-frame 6-minute walking test (6MWT) and reduced duration in a predefined distance (timed up and go) immediately after vertical WBV in 16 children with spastic diplegia or spastic quadriplegia.Citation38
Posture control and balance
Only limited results have been registered in regard to postural control. Tupimai et al assessed the acute effect of oscillating WBV on the control of upright posture in 12 children with spastic CP (GMFCS I–III) by means of the pediatric balance scale, and found no significant effects.Citation36
Mobility
Improved mobility after VT has been demonstrated by increased angular ROM in lower limb joints. Improvements were observed at ankleCitation34,Citation38 and knee joints.Citation32,Citation38 Krause et al assessed the acute effects of side-alternating WBV on active ROM, and found immediately increased ROM in the knee joint, whereas the ankle joint remained unaffected in 44 children with CP.Citation32 Using vertical WBV, Dickin et al found increased active ROM during walking in the ankle joints of eight diplegic and hemiplegic patients.Citation34 Likewise, Cheng et al found increased active ROM concomitant with increased Wartenberg relaxation-index scores after exposure to vertical WBV in the ankle and knee joint in 16 children with spastic CP.Citation38 Passive ROM remained unchanged. Evidently, this increase was manifested only in dynamic measurements, such as during walkingCitation34 or for active ROM,Citation39 but not in passive ROM.Citation34,Citation38 A possible explanation for these varying results might be found in the underlying neuromuscular modulation associated with the stretch-reflex excitability and hypertonicity.
Neuromuscular modulation
Independently of the classification of VT – including WBV and focal vibration – mechanical sinusoidal stimulation elicits a frequency- and amplitude-dependent succession of stretch reflexes.Citation21,Citation24 The responsiveness of secondary muscle-spindle endings to focal vibration has been manifested in animal modelsCitation40 and human subjects,Citation41–Citation44 and the expression “tonic vibration reflex” is commonly used to describe this neuromechanical coupling. Whereas focal vibration elicits stretch-reflex responses via afferent reflex circuitry, mainly in the vibrated muscle,Citation21,Citation42 WBV acts throughout the whole organism and encompasses the muscles of all body segments.Citation24,Citation45 Although less pronounced, this phenomenon has also been described to be valid for patients with CP.Citation37
Reflex excitability
Three studies assessed the effect of VT on reflex excitability and hypertonicity and consistently demonstrated vibration-induced inhibition. Leonard et al assessed spinal excitability, ie, afferent transmission in the α-motor-neuron pool, in six children with spastic CP during focal vibration to the Achilles tendon and found inhibition of H-reflex responses.Citation15 This inhibition occurred approximately 300 milliseconds after the onset of vibrationCitation15 and was maintained after vibration. Although the vibration-induced effects were of statistical significance, percentage changes were smaller compared to reference values obtained in a sample of healthy controls. Krause et al investigated the effect of side-alternating WBV on the stretch-reflex response and found decreased stretch-reflex amplitude and slightly delayed latency immediately after treatment.Citation32 Furthermore, Eklund and Steen used a descriptive approach including >200 subjects with CP and reported a reduction in hypertonicity in the vibrated muscles.Citation31 Taken together, the outcomes indicate that VT inhibits reflex activity addressing afferent pathways.
Spasticity
After an acute bout of vibration, spasticity has been shown to be reduced in studies using clinical assessments,Citation35,Citation36,Citation38 measurements of muscle tone and hypertonicity,Citation31,Citation38 and stretch-reflex activity.Citation39 Clinical measures comprised the modified Ashworth Scale (MAS)Citation35,Citation36 and modified Tardieu Scale (MTS).Citation35 With the exception of Eklund and Steen, who found a decline in spasticity in all body segments, spasticity reduction was recorded in the lower extremities only.Citation31,Citation35,Citation36,Citation39 Using side alternating WBV, Park et al found improved MAS and MTS in 17 children with GMFCS levels IV–V.Citation35 Accordingly, Krause et al showed an immediate reduction in musculus soleus stretch-reflex response after VT in 44 children with CP.Citation32 Furthermore, Tupimai et al found a reduction in spasticity in the MAS of the hip adductor, quadriceps, hamstrings, and soleus muscle of the stronger leg as well as the soleus of the weaker leg after passive muscle stretching (PMS) combined with the application of oscillating WBV in 12 children with spastic CP (GMFCS levels I–III) in comparison to PMS alone.Citation36 Accordingly, Cheng et al found reduced MAS scores in knee extensors concomitant with increased Wartenberg pendulum-test relaxation-index scores after exposure to vertical WBV in 16 children with CP.Citation38
At the same time, such symptoms as seizures have not been shown to be worsened during the application of vibration.Citation30 Interestingly, the effects of reduced hypertonicity in response to VT were most prominent in subjects suffering from hypertonicity because of spasticity.Citation31 However, it has to be taken into account that vibration not only affects symptoms of spasticity, but – vice versa – also effects vibration transmission: in patients with spastic CP, higher MAS scores were correlated with reduced vibratory signal transmission to the femur. Therefore, vibration may be dampened at the distal femur in spasticity.Citation33
Neuromuscular coordination
In patients with CP, agonist-reflex muscle activation and antagonist-reflex muscle inhibition are reduced during voluntary movement compared to healthy controls.Citation15 As an underlying mechanism, the authors discussed pathological impairments in reciprocal inhibition during voluntary movements around the ankle.Citation15 In three studies, the effect of VT on neuromuscular coordination was assessed using electromyography. In addition to stretch-reflex inhibition, Krause et al demonstrated that the activation ratio of agonist:antagonist muscle is increased during maximal isometric voluntary contraction after side-alternating WBV.Citation32 Likewise, focal vibration applied to the anterior tibial tendon causes a reflex inhibition in the antagonistic triceps surae.Citation15 Eklund and Steen used a descriptive approach and reported increased spontaneous movement of weak muscles, as well as augmented movement speed and precision.Citation31 Taken together, the results indicate that vibration modulates not only agonist- but also antagonist-muscle activity.Citation15,Citation31,Citation32 This involves improvements in muscle coordination immediately after vibration.Citation32
Duration of effects
The acute effects of VT on muscle activation and functional performance have been demonstrated during and also following vibration. Time windows varied between investigations, showing sustained effects immediately,Citation39 as well as 1 (MAS) and 2 hours (MTS)Citation35 following the application of vibration. As reported by Eklund and Steen, VT causes increased memory of motor acts and improved repeatability after breaks.Citation31 Therefore, the period after VT is sufficiently long for therapists to teach the patient skilled movement after VT and benefit from reduced spasticity and hypertonicity in the skeletal muscle.Citation31
Long-term effects
Long-term adaptations refer to chronic changes manifested after repeated sessions of VT executed over weeks or months. The duration of applied vibration ranges from 30 secondsCitation46 to 1,Citation47–Citation49 3,Citation48,Citation50–Citation56 5,Citation57 9,Citation58,Citation59 and 10 minutes,Citation36,Citation60 with the number of sets ranging from one to six. A summary of methodological quality can be found in . Details of vibration application can be found in . In the following section, results are summarized and clustered by functional category ().
Table 1 Study quality on the PEDro scale: acute adaptations to vibration
Table 2 Study quality on the PEDro scale: long-term adaptations to vibration
Table 3 Acute adaptations to vibration
Table 4 Long-term adaptations to vibration
Table 5 Conclusive effects of vibration therapy
Functional adaptations
Gross motor function
The most frequently used assessment for gross motor function in CP is the Gross Motor Function Measure (GMFM). The GMFM is a semiquantitative, standardized, and validated assessment for motor function primarily developed for children with CP.Citation61,Citation62 The test consists of 88 items scored 0–3 points divided into five dimensions: A (laying and rolling), B (sitting), C (crawling), D (standing), and E (walking). The GMFM66 is the interval-scaled total score of the GMFM.
Effects of side-alternating WBV on gross motor function have been assessed in five longitudinal studies. Ibrahim et al found improvements in GMFM88 dimensions D (standing) and E (walking, running, and jumping) after 12 weeks of side-alternating WBV application in 15 children (30 total) with diplegic CP in comparison to 1-hour traditional physiotherapy.Citation59 Likewise, Stark et al found improvements in a modified version of the GMFM after 6 months of home-based side-alternating WBV application in addition to intensive, functional blocks of interval rehabilitation in 78 children with spastic diplegic or spastic quadriplegic CP.Citation55 In 2013, Stark et al found improved GMFM66 total scores and improvements in all GMFM88 dimensions (A–E) after the same intervention in 356 children with CP (GMFCS levels I–V).Citation56 However, other studies demonstrated no significant effects of WBV. Ruck et al found no difference in the GMFM88 dimensions D (standing) or E (walking, running, jumping) after 6 months of additional side-alternating WBV application to the conventional school physiotherapy program in ten children (20 total) with CP (GMFCS levels II–IV) in comparison to conventional physiotherapy alone.Citation52 Likewise, Stark et al found no additional effects for GMFM66 and Pediatric Evaluation of Disability Inventory scores after 14 weeks of home-based side-alternating WBV application beyond to the standard therapeutic protocol in 12 (total 24) young (12–24 months) children with CP (GMFCS levels II–IV) in comparison to standard of care only.Citation54
Two studies using vertical WBV documented positive effects on gross motor function. Ahlborg et al found improvement in GMFM88 dimensions D (standing) and E (walking, running, jumping) after eight weeks of vertical WBV application in seven children (14 total) with diplegic CP in comparison to resistance training,Citation49 while Yabumoto et al, in their case report of an 8-year-old boy with CP (GMFCS level III), found that GMFM88 dimensions C (sitting) and D (standing) improved after 5 weeks of vertical WBV application complementing conventional physiotherapy.Citation63 After 6 months of focal vibration, however, Reyes et al found no changes in GMFM scores among three groups – placebo vs 60 Hz vs 90 Hz vibration – in 20 children (total 61) with a first-neuron diagnosis (mixed with some second-neuron and other diseases).Citation57
Strength
The effects of side-alternating WBV on strength have been assessed in three longitudinal studies. el Shamy found increased knee-extension peak torque at an angle speed of 90°/second and 60°/second in isokinetic muscle testing after 3 months of side-alternating WBV application in 15 children (30 total) with diplegic CP (GMFCS levels I–II) in comparison to traditional physiotherapy (muscle stretching, strengthening, balance, and proprioceptive training).Citation51 Ibrahim et al found increased isometric knee-extensor muscle strength after 12 weeks of side-alternating WBV application in 15 children (30 total) with diplegic CP in comparison to 1 hour’s conventional physiotherapy.Citation59 Stark et al found improved muscle force and angle of verticality after 6 months of home-based side-alternating WBV application with intensive, functional blocks of interval rehabilitation in 78 children with spastic diplegic or spastic quadriplegic CP.Citation55
Three studies using vertical WBV documented different effects on strength in various paradigms. Ahlborg et al found increased concentric and eccentric work and peak torque in the weak leg at an angle speed of 90°/second in isokinetic muscle testing; both groups improved by 30°/second after 8 weeks of vertical WBV application in seven children (14 total) with diplegic CP in comparison to resistance training.Citation49 There were no differences between groups. Unger et al found an increased number of sit-ups executed in 1 minute after 4 weeks of vertical WBV application in addition to school physiotherapy in 27 children with CP (GMFCS levels I–III) in comparison to school physiotherapy alone.Citation46 Wren et al found no differences between vibration and standing for any of the muscle or strength variables after 6 months of microimpact vertical WBV application, with considerably smaller displacement amplitudes compared to the sham intervention (standing only) in 31 children (crossover design) with CP (all types, GMFCS levels I–V).Citation60 They found no correlation between compliance and outcome and similar results for all GMFCS levels. After 6 months of focal WBV, Reyes et al found an increase in muscle force for the muscle group vibrated with 60 Hz of focal vibration on the radius and femur (high frequency, low magnitude) compared to placebo and 90 Hz vibration in 20 children (total 61) with a first-neuron diagnosis (mixed with some second neuron and other diseases).Citation57 No changes were found for the lower limb.
Gait
For side-alternating WBV, Gusso et al found improved 6MWT after 20 weeks of side-alternating WBV application in 40 children with mild–moderate CP (GMFCS levels II–III).Citation48 Ibrahim et al also found improved 6MWT after 12 weeks of side-alternating WBV application in 15 children (30 total) with diplegic CP in comparison to 1-hour conventional physiotherapy.Citation59 Ko et al found improved gait speed and step width after 3 weeks of additional side-alternating WBV application to their conventional physiotherapy in 12 children (24 total) with diplegic or hemiplegic CP in comparison to physiotherapy alone.Citation58 Lee and Chon also found improvements in gait speed, stride length, cycle time, and increase in ankle dorsiflexion and plantar-flexion excursions after 8 weeks of additional side-alternating WBV application to conventional physiotherapy in 15 children (30 total) with spastic diplegic or quadriplegic CP in comparison to conventional physiotherapy alone.Citation50 Ruck et al found increased walking speed in the 10 m walking test after six months of additional side-alternating WBV application to the conventional school physiotherapy program in ten children (20 total) with CP (GMFCS levels II–IV) in comparison to conventional school physiotherapy alone.Citation52 Semler et al reported improved walking ability, prolonged walking distance, and improved agility to perform unassisted steps after 6 months of side-alternating WBV application in one child with CP.Citation53
The results for vertical WBV were comparable to those of side-alternating WBV. Unger et al found improved 1-minute walking test (1MWT) scores after 4 weeks of vertical WBV application in addition to school physiotherapy in 27 children with CP (GMFCS levels I–III) in comparison to physiotherapy alone.Citation46 Yabumoto et al found in their case report of an 8-year-old boy with CP (GMFCS level III) that the number of steps for the 5 m walking test decreased after 5 weeks of vertical WBV application complementing conventional physiotherapy.Citation63 Ahlborg et al found no difference in 6MWT and timed up-and-go test results after 8 weeks of vertical WBV application in seven children (14 total) with diplegic CP in comparison to resistance training.Citation49 In a case report, Camerota et al reported higher gait velocity and symmetry concomitant with shorter stance time and step length after focal vibration applied to the calf muscle in a triplegic boy with GMFCS level II.Citation64 Improvements in gait rhythm were accompanied by kinematic adaptations, characterized as increased functional ROM in joints of the lower extremities.
Posture control and balance
Using side-alternating WBV, el Shamy found increased anteroposterior and mediolateral postural stability after 3 months of application in 15 children (30 total) with diplegic CP (GMFCS levels I–II) in comparison to traditional physiotherapy (muscle stretching, strengthening, balance, and proprioceptive training).Citation51 Tupimai et al also found improved balance on the pediatric balance scale after 6 weeks of prolonged PMS combined with oscillating WBV application in 12 children (crossover) with spastic CP (GMFCS levels I–III) in comparison to PMS alone.Citation36 However, Ko et al found no difference in balance following 3 weeks of additional side-alternating WBV to their conventional physiotherapy in 12 children (24 total) with diplegic or hemiplegic CP in comparison to conventional physiotherapy alone.Citation58 Using vertical WBV, Unger et al found more upright posture after 4 weeks of vertical WBV application additionally to school physiotherapy in 27 children with CP (GMFCS levels I–III) in comparison to school physiotherapy alone.Citation46 In contrast, Ibrahim et al found no difference for walking balance after 12 weeks of vertical WBV application in 15 children (30 total) with diplegic CP in comparison to 1-hour conventional physiotherapy.Citation59
Mobility
Myśliwiec et al found increased ROM in the knee joint but no change in the hip joint after 4 weeks of vertical WBV application in three female adults with spastic CP.Citation47 Yabumoto et al found a trend in their case report of an 8-year-old boy with CP (GMFCS level III) for improved ROM after a 5-week intervention of vertical WBV application complementing conventional physiotherapy.Citation63 Furthermore, Camerota et al observed increased passive ROM in the ankle joint and increased functional mobility in joints of the lower extremities during gait in a 1-month follow-up after 3-day focal vibration applied to the muscle belly of the triceps surae in a child with triplegic CP (GMFCS level II).Citation64
Neuromuscular adaptations
Spasticity
The effects of side-alternating WBV on spasticity have been assessed in two longitudinal studies. Ibrahim et al found reduced spasticity of the knee extensor of the stronger leg on the MAS after 12 weeks of side-alternating WBV application in 15 children (30 total) with diplegic CP in comparison to 1-hour conventional physiotherapy.Citation59 Semler et al reported reduced spasticity after 6 months of side-alternating WBV application in one child with CP.Citation53 For vertical WBV, results were similar. Ahlborg et al found decreased spasticity in the stronger leg measured by the MAS in the knee extensors after 8 weeks of vertical WBV application in seven children (14 total) with diplegic CP in comparison to resistance training.Citation49 In their case report of an 8-year-old boy with CP (GMFCS level III), Yabumoto et al found that MAS did not change after 5 weeks of vertical WBV application complementing conventional physiotherapy.Citation63 Additionally, Tupimai et al found reduced spasticity on the MAS of the hip adductor, quadriceps, hamstrings, and soleus muscles of stronger and weaker legs after 6 weeks of PMS combined with oscillating WBV application in 12 children (crossover) with spastic CP (GMFCS levels I–III) in comparison to PMS alone.Citation36
Structural adaptations
Muscle
The effects of side-alternating WBV on muscle structure have been assessed in three longitudinal studies. Gusso et al found increased lean body (muscle) mass measured by dual X-ray absorptiometry (DXA) after 20 weeks of side-alternating WBV application in 40 children with mild–moderate CP (GMFCS levels II–III).Citation48 They also found improved muscle function on the chair-rise test (Leonardo mechanography). Stark et al also found increased lean body (muscle) mass measured by DXA after 6 months of home-based side-alternating WBV application in addition to intensive, functional blocks of interval rehabilitation in 78 children with spastic diplegic or spastic quadriplegic CP.Citation55 Lee and Chon found increased muscle thicknesses of the tibialis anterior and soleus in ultrasound measurements and no change in the gastrocnemii after 8 weeks of side-alternating WBV application in addition to conventional physiotherapy in 15 children (30 total) with spastic diplegic or quadriplegic CP in comparison to conventional physiotherapy alone.Citation50 Using vertical WBV, Unger et al found an increase in resting thickness of all four abdominal muscles (transversus abdominis, obliquus inter-nus, obliquus externus, rectus abdominis) after 4 weeks of VT in addition to school physiotherapy in 27 children with CP (GMFCS levels I–III) in comparison to school physiotherapy alone.Citation46
Bone
The effects of side-alternating WBV on bone structure have been assessed in three longitudinal studies. Gusso et al found increased bone-mineral content (BMC) measured by DXA after 20 weeks of side-alternating WBV application in 40 children with mild–moderate CP (GMFCS levels II–III).Citation48 They also found increased bone-mineral density (BMD) on peripheral quantitative computed tomography measurement. Stark et al found improved BMC measured by DXA after six months of home-based side-alternating WBV application in addition to intensive, functional blocks of interval-rehabilitation in 78 children with spastic diplegic or spastic quadriplegic CP.Citation55 However, Ruck et al did not detect a positive treatment effect on bone (measured by DXA) after 6 months of additional side-alternating WBV application to a conventional school physiotherapy program in ten children (20 total) with CP (GMFCS levels II–IV) in comparison to conventional school physiotherapy alone.Citation52
Using vertical WBV, Wren et al found an increase in cortical bone area and moments of inertia measured by computed tomography, but no difference in vertebral cancellous bone density after 6 months of microimpact WBV application compared to a sham intervention (standing only) in 31 children with CP (all types, GMFCS levels I–V).Citation60 They also found no correlation between compliance and outcome and similar results for all GMFCS levels. After applying focal vibration on the radius and femur (high frequency, low magnitude), Reyes et al found an increase in BMD at the ultradistal radius for the group vibrated with 60 Hz after 6 months of VT compared to placebo and 90 Hz vibration in 20 children (total 61) with a first-neuron diagnosis (mixed with some second-neuron and other diseases).Citation57 They also found an increase in BMC at the ultradistal radius for the group vibrated with 60 Hz (20 children) and the group with 90 Hz (16 children).
Duration of effects
The duration of vibration-induced effects on a long-term scale has not been studied to date. Investigations including a follow-up after termination of VT are missing.
Use of vibration treatment in patients with CP
Among the different intervention protocols, the analysis revealed no apparent dosage-dependency of VT. The great variation in VT protocols makes it difficult to highlight a clear favorite setting with efficiency beyond the others. Vibration has been applied at different levels: 5–50 Hz for WBV and reaching peak frequencies for focal vibration at 60–200 Hz. Amplitude utilized in the studies was 1–6 mm for WBV and much smaller for focal vibration: 0.3–0.5 mm. Smaller amplitude with increasing frequency is usually chosen for VT ( and ). In contrast to studies executed in samples of healthy subjects,Citation21–Citation23 protocols for patients with CP were often composed individually using personalized, progressively augmented dosages, instead of universal intervention settings. For acute and longitudinal studies using a WBV protocol, one to six sets of VT were applied with durations between 30 seconds and 10 minutes in static and dynamic conditions: standing upright with stance support, knees extended or flexed at 10°, 30°, or 50°, sitting and four-point position on knees and hands, dynamic squats and semisquats, lateral shifting and tilting, hip and lumbar extensions, and sit-up variations. Also, tilt tables at angles of 10°–50° have been used to apply WBV when the subjects could not stand independently or even with support. Focal vibration was simply applied to the muscle belly of interest or to the tendon attached to it. With increasing VT-treatment analysis, it became apparent that dosage depended on the severity level of the motor dysfunction in patients with CP. Simple static exercises coupled with low-frequency and low-amplitude VT were utilized for patients with high GMFCS levels (IV–V, lower functioning), whereas training settings for low GMFCS levels (I–III, higher functioning) contained high-frequency and high-amplitude VT concomitant with dynamic exercises.
Effect of age differences between children and adults
The majority of the studies cited (21) were executed in subjects aged 3–17 years. Seven cross-sectional experimentsCitation15,Citation30,Citation31,Citation33,Citation35,Citation37,Citation38 and 14 longitudinal trialsCitation46,Citation50–Citation60,Citation63,Citation64 elaborated the efficiency of VT in children, whereas only threeCitation32,Citation34,Citation36 cross-sectional and fourCitation36,Citation47–Citation49 longitudinal studies included adult volunteers older than 17 years. In the last few decades, scientific debate in the field of neurorehabilitation has consistently concluded that training regimes commonly achieve higher efficiency in children compared to adolescent patient groups.Citation65,Citation66 The child’s immature brain and spinal cord have higher plasticity potential, learning curves for children are steeper, and functional recovery after brain injuries is faster.Citation65–Citation67 Although VT demonstrably has a positive and age-independent influence on neuromuscular, functional, and structural factors associated with the disease-related deficits in patients with CP, we expect that VT will be particularly efficient in children, due to their advantage of neuroplasticity, and that VT may initiate long-term developmental effects that may persist into adulthood.Citation65,Citation66
Feasibility and side effects
VT can be applied regardless of subjects’ dysfunctional movement ability, health, and mental status with the help of a second person, and is thus appreciated as a feasible exercise modality providing a wide range of dosages tailored to individual requirements ( and ). Therefore, its neurorehabilitative application has emerged as a valuable alternative to other interventions, as there are no decisive motor prerequisites or particular cognitive requirements.Citation25,Citation26 Nevertheless, a few studies have documented adverse effects, including fatigue,Citation52 pain,Citation52 panic, and torsion spasms due to suddenly applied vibration.Citation31 These side effects should be considered in the context of clinical VT and investigated further.
Although no study has been executed to establish the safety and feasibility of VT, there are major factors mentioned in the aforementioned manuscripts to be considered. First, VT is a machine-based therapy and thus requires hardware, installation, and supervision.Citation20,Citation54 Second, a specialist should guide each training session, secure the patient, and adjust vibration determinants.Citation24,Citation54,Citation80 In particular, patients with high GMFCS levels (IV–V, lower functioning) may need help to activate (WBV) or fix (focal vibration) the device and adjust individual settings using an appropriate amplitude and frequency.Citation24,Citation80 Coupled with the high mass of WBV devices and transfer of mechanical oscillations to surroundings (floor, wall), the material and additional manpower necessary may be a bias for the application of VT in the clinical and home-based setting.
Discussion
For decades, vibratory stimuli have been considered an auspicious exercise modality in neurorehabilitation. The goal of this evidence-based data search was to determine the beneficial outcomes of VT in the CP population. A thorough review of the currently available data demonstrates that VT elicits numerous desirable acute and long-term effects (). Despite the bias of a limited article quantity of fair study quality, findings outlined that one session of VT reduced reflex excitability,Citation15,Citation32 hypertonicity,Citation31 spasticity,Citation35,Citation36,Citation38 and coordination deficitsCitation15,Citation31,Citation32 and subsequently had a positive influence on GMFM,Citation30,Citation31,Citation37 strength,Citation30,Citation31,Citation36 gait,Citation34,Citation38 and mobilityCitation32,Citation34,Citation38 in adults and children with CP (). A condensed statement of the chronic effects of VT based on a considerable number of randomized controlled trials of fair study quality would include significant benefits in regard to spasticity,Citation36,Citation49,Citation53 GMFM,Citation49,Citation55,Citation56,Citation59,Citation63 muscle strength,Citation46,Citation49,Citation51,Citation55,Citation59,Citation60 gait,Citation46,Citation48–Citation50,Citation52,Citation53,Citation58,Citation59,Citation63,Citation64 and mobility,Citation63,Citation64 as well as muscle massCitation48,Citation50,Citation55 and BMD.Citation48,Citation52,Citation55,Citation57,Citation60 Acute and long-term effects of VT on posture control were inconsistent and remained insignificant.Citation36,Citation46,Citation51,Citation58,Citation59
Figure 2 Overview of results.
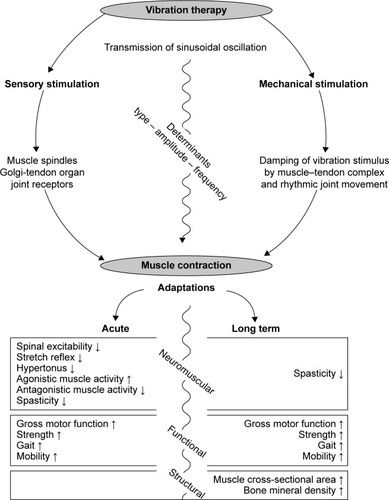
The duration of the aforementioned effects varies greatly: acute effects were demonstrated during,Citation30,Citation31,Citation37 immediately after,Citation32 and up to 2 hours after VT.Citation35 Using VT as an intervention, training periods varied from 3 weeksCitation58 to 6 months.Citation52,Citation53,Citation55–Citation57,Citation60 Therefore, VT is a training method that is on one hand used as a prerequisite of succeeding interventions (ie, strength training, stretching, and physiotherapy). Greater motor control might be enabled for the tasks selected (ie, squats, walking) being executed immediately following VT. On the other hand, VT may be used as a repetitive training method itself, enabling greater motor performance after a minimum intervention period of 3 weeks.
From neuromuscular effects to motor function and musculoskeletal tissue
Although minor evidence points toward causal relationships among the effects of VT on neuromuscular, functional, and structural components of the human body, which allow unambiguous chronological reasoning underlying the benefits of VT in CP populations, we would like to propose a conceptual model based on the pathophysiology and symptomatic of CP: while the region and magnitude of the brain injury differ greatly among patients with CP,Citation3 they consistently show deficits in their neuromuscular control.Citation6,Citation16 Impaired muscle functionCitation6,Citation16 is a common phenomenon, due to the cerebral lesion that subsequently hinders regular movement,Citation68–Citation71 resulting in deficient stimuli for muscle and bone growth.
We think that its specific inhibitory impact at the spinal level of the neuromuscular system might represent the origins of VT benefits. Outlined as gradually reduced spinal excitability after 300 milliseconds of VTCitation15 and a persistent inhibition of reflex responses,Citation32 VT might counteract disease-related spasticityCitation35,Citation36,Citation38 and hypertonicity.Citation31 As a consequence, factors interfering with precise movement execution are diminished for a certain period during and after VT, which may lead to greater synergisticCitation30–Citation32 and antagonistic muscle coordinationCitation32 associated with an increase in muscle strength,Citation31,Citation36 angular ROM,Citation32 kinesthesia,Citation31 gait,Citation34,Citation38 and mobility.Citation32,Citation34,Citation38 Additionally, any kind of exercise executed in a sensible time frame during or after VT is carried out with greater precision,Citation31 increased muscular activation intensities,Citation30 and resulting forces,Citation30,Citation31,Citation36 which CP patients could not reach without the support of VT.Citation30,Citation51,Citation59 However, those mechanical forces acting on the human body are known to be essential for growth or at least the homeostasis of musculoskeletal tissues. Therefore, we expect significantly augmented myogenic and osteogenic stimuli in response to VT, causing muscle hypertrophyCitation48,Citation50,Citation55 and increased BMC and BMD,Citation48,Citation52,Citation55,Citation57,Citation60 which is important to improve the fragile and deficient health status of patients with CP.Citation72,Citation73 The logical effect chain outlined in this paragraph can help in understanding the prerequisites and broad range of VT efficiency associated with CP.
Using VT in clinical settings
In comparison to other exercise modalities, VT depicts a possible intervention that can be applied in multiple settings of neurorehabilitation. In particular, the following benefits emphasize its uniqueness among conventional intervention methods in CP. First, demands in regard to motor activation are low, while great acute and long-term performance enhancements can be achieved. Therefore, reflex-induced contraction and relaxation of muscles are realized passively, with the VT device being fixated to the body (focal vibration) or the subject sitting or standing on top of the vibration platform (WBV). Second, illustrated by numerous different devices, possible training applications are manifold, considering topographic preferences in regard to particular body segments. Third, the execution of training tasks does not require great cognitive ability of the subject (easy to learn) and can be easily integrated into conventional therapy sessions, because of its time-efficiency and task variability (easy to apply). After all, adding vibration to conventional therapy triggers neuromuscular modulation via spinal reflex circuitry, which offers great potential in settings of neurorehabilitation.
Limitations
The research that is available on VT in patients with CP is limited and difficult to compare, due to the heterogeneous methodological approaches and multiple outcome measures. The study quality is satisfying, but not certified as good or excellent ( and ). Only a small number of studies considered adult subjects, and no study was executed to establish the safety and feasibility of VT. Furthermore, three other factors limit the scope of the results obtained in this systematic review. First, CP populations differ tremendously in their classification,Citation3 affected brain areas, and handicapped body regions,Citation74 as well as their responsiveness to nonpharmacological interventions.Citation20,Citation25,Citation75 Subject variability is a major bias in clinical trials,Citation57 and it is thus expected that the severity of motor and mental dysfunction significantly influences the feasibility and efficiency of exercises, including VT.Citation20 Although scientific evidence clearly points out the benefits in regard to neural control of skeletal muscles, daily motion, and structural adaptations of muscle and bone tissue, conclusive statements are restricted to particular sub-populations within the CP community. Second, the health and performance status of patients with CP is influenced by clinical measures applied apart from VT. These entail drugs administered with differing dosages (ie, baclofen, diazepam, tizanidine),Citation76 Botox injections,Citation77 and surgery on connective tissue (ie, lengthening of the muscle–tendon complex, joint relocation)Citation78 and nerves in the spinal cord (ie, rhizotomy).Citation79 Although the benefits of clinical treatments are not included in this review, their impact on spasticity and movement biomechanics, as well as adverse effects entailing seizures and hypotension, most probably influence the effects of VT exercises.Citation76–Citation78 Therefore, the effectiveness of VT can hardly be interpreted independently of clinical treatments applied with considerable interindividual differences. Third, VT determinants varied among studies. While the sensorimotor stimulation is identical for all types of VT, the frequency and amplitude – outlined as the major determinants in regard to muscle activation and training intensityCitation24,Citation80 – have been used arbitrarily within a wide range from low to high. Therefore, while the success of VT has been outlined clearly in the aforementioned experiments, systematic judgment on the choice of VT parameters requires further investigation.
Prospects
This systematic review depicts an overview of the evidence regarding the potential and limitations of VT for humans with CP. For clinicians and therapists, the identification of specific fields of application while simultaneously considering VT-induced side effects might be useful for designing evidence-based VT interventions. Outstanding issues include the effect of VT on posture control and balance and evidence-based justification of the mechanisms. Even though further high-quality research is needed – especially in regard to the acute effects of VT and its efficiency in adults – the potential and possible benefits of VT in neurorehabilitation shall be pointed out. After all, enhancing quality of life for people with CP should be the main aim being implemented by an interdisciplinary approach to high-quality scientific research. Thereby, based on the manuscripts included in this data research, it became apparent that VT helps patients with CP to master daily life challenges by the facilitation of movement quality.
Disclosure
The authors report no conflicts of interest in this work.
References
- Krägeloh-MannICansCCerebral palsy updateBrain Dev200931753754419386453
- Yeargin-AllsoppMBraunKNDoernbergNSBenedictREKirbyRSDurkinMSPrevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaborationPediatrics2008121354755418310204
- RosenbaumPPanethNLevitonAA report: the definition and classification of cerebral palsy April 2006Dev Med Child Neurol Suppl200710981417370477
- PoonDMHui-ChanCWHyperactive stretch reflexes, co-contraction, and muscle weakness in children with cerebral palsyDev Med Child Neurol200951212813519018843
- ElderGCKirkJStewartGContributing factors to muscle weakness in children with cerebral palsyDev Med Child Neurol200345854255012882533
- StackhouseSKBinder-MacleodSALeeSCVoluntary muscle activation, contractile properties, and fatigability in children with and without cerebral palsyMuscle Nerve200531559460115779003
- WangXWangYGait analysis of children with spastic hemiplegic cerebral palsyNeural Regen Res20127201578158425657696
- BergerWQuinternJDietzVPathophysiology of gait in children with cerebral palsyElectroencephalogr Clin Neurophysiol19825355385486177498
- BergerWCharacteristics of locomotor control in children with cerebral palsyNeurosci Biobehav Rev19982245795829595572
- RoseJWolffDRJonesVKBlochDAOehlertJWGambleJGPostural balance in children with cerebral palsyDev Med Child Neurol2002441586311811652
- Bar-OnLMolenaersGAertbeliënESpasticity and its contribution to hypertonia in cerebral palsyBiomed Res Int2015201531704725649546
- SheeanGMcGuireJRSpastic hypertonia and movement disorders: pathophysiology, clinical presentation, and quantificationPM R20091982783319769916
- BrouwerBAshbyPAltered corticospinal projections to lower limb motoneurons in subjects with cerebral palsyBrain1991114Pt 3139514072065257
- BrouwerBSmitsECorticospinal input onto motor neurons projecting to ankle muscles in individuals with cerebral palsyDev Med Child Neurol19963897877968810710
- LeonardCTMoritaniTHirschfeldHForssbergHDeficits in reciprocal inhibition of children with cerebral palsy as revealed by H reflex testingDev Med Child Neurol199032119749842269407
- Milner-BrownHSPennRDPathophysiological mechanisms in cerebral palsyJ Neurol Neurosurg Psychiatry1979427606618479900
- KriggerKWCerebral palsy: an overviewAm Fam Physician20067319110016417071
- AisenMLKerkovichDMastJCerebral palsy: clinical care and neurological rehabilitationLancet Neurol201110984485221849165
- RyanJMCassidyEENoorduynSGO’ConnellNEExercise interventions for cerebral palsyCochrane Database Syst Rev20176CD01166028602046
- VerschurenOKetelaarMTakkenTHeldersPJGorterJWExercise programs for children with cerebral palsy: a systematic review of the literatureAm J Phys Med Rehabil200887540441717993987
- SouronRBessonTMilletGYLapoleTAcute and chronic neuromuscular adaptations to local vibration trainingEur J Appl Physiol2017117101939196428766150
- CochraneDJVibration exercise: the potential benefitsInt J Sports Med2011322759921165804
- RittwegerJVibration as an exercise modality: how it may work, and what its potential might beEur J Appl Physiol2010108587790420012646
- RitzmannRGollhoferAKramerAThe influence of vibration type, frequency, body position and additional load on the neuro-muscular activity during whole body vibrationEur J Appl Physiol2013113111122538279
- SaquettoMCarvalhoVSilvaCConceiçãoCGomes-NetoMThe effects of whole body vibration on mobility and balance in children with cerebral palsy: a systematic review with meta-analysisJ Musculoskelet Neuronal Interact201515213714426032205
- Sa-CaputoDCCosta-CavalcantiRCarvalho-LimaRPSystematic review of whole body vibration exercises in the treatment of cerebral palsy: brief reportDev Neurorehabil201619532733325826535
- MoherDLiberatiATetzlaffJAltmanDGPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementAnn Intern Med20091514264269W6419622511
- HigginsJPGreenSCochrane Handbook for Systematic Reviews of Interventions. Version 4.2.6LondonCochrane Collaboration2006
- OlivoSAMacedoLGGadottiICFuentesJStantonTMageeDJScales to assess the quality of randomized controlled trials: a systematic reviewPhys Ther200888215617518073267
- CannonSERuesJPMelnickMEGuessDHead-erect behavior among three preschool-aged children with cerebral palsyPhys Ther1987678119812042956613
- EklundGSteenMMuscle vibration therapy in children with cerebral palsyScand J Rehabil Med19691135375406722
- KrauseASchönauEGollhoferAAlleviation of motor impairments in patients with cerebral palsy: acute effects of whole-body vibration on stretch reflex response, voluntary muscle activation and mobilityFront Neurol2017841628861038
- SinghHWhitneyDGKnightCASite-specific transmission of a floor-based, high-frequency, low-magnitude vibration stimulus in children with spastic cerebral palsyArch Phys Med Rehabil201697221822326392035
- DickinDCFaustKAWangHFrameJThe acute effects of whole-body vibration on gait parameters in adults with cerebral palsyJ Musculoskelet Neuronal Interact2013131192623445911
- ParkCParkESChoiJYChoYRhaDWCorrection: immediate effect of a single session of whole body vibration on spasticity in children with cerebral palsyAnn Rehabil Med201741472272328971060
- TupimaiTPeungsuwanPPrasertnooJYamauchiJEffect of combining passive muscle stretching and whole body vibration on spasticity and physical performance of children and adolescents with cerebral palsyJ Phys Ther Sci2015281713
- TardieuGTardieuCLespargotARobyABretMDCan vibration- induced illusions be used as a muscle perception test for normal and cerebral-palsied children?Dev Med Child Neurol19842644494566479464
- ChengHYJuYYChenCLChuangLLChengCHEffects of whole body vibration on spasticity and lower extremity function in children with cerebral palsyHum Mov Sci201539657225461434
- KrauseAGollhoferAFreylerKJablonkaLRitzmannRAcute corticospinal and spinal modulation after whole body vibrationJ Mus-culoskelet Neuronal Interact2016164327338
- GregoryJEProskeUThe responses of muscle spindles in the kitten to stretch and vibrationExp Brain Res19887336066143224670
- FallonJBMacefieldVGVibration sensitivity of human muscle spindles and Golgi tendon organsMuscle Nerve2007361212917471568
- BurkeDHagbarthKELöfstedtLWallinBGThe responses of human muscle spindle endings to vibration of non-contracting musclesJ Physiol19762613673693135840
- DesmedtJEGodauxEMechanism of the vibration paradox: excitatory and inhibitory effects of tendon vibration on single soleus muscle motor units in manJ Physiol1978285197207154563
- LanceJWThe reflex effects of muscle vibrationProc Aust Assoc Neurol1966449565966202
- ZaidellLNMilevaKNSumnersDPBowtellJLWoloschakGEExperimental evidence of the tonic vibration reflex during whole-body vibration of the loaded and unloaded legPLoS One2013812e8524724386466
- UngerMJelsmaJStarkCEffect of a trunk-targeted intervention using vibration on posture and gait in children with spastic type cerebral palsy: a randomized control trialDev Neurorehabil2013162798823477461
- MyśliwiecAKuszewskiMSauliczEThe influence of vibration on the quality of gait in women with cerebral palsyInt J Disabil Hum Dev2015142125129
- GussoSMunnsCFCollePEffects of whole-body vibration training on physical function, bone and muscle mass in adolescents and young adults with cerebral palsySci Rep201662251826936535
- AhlborgLAnderssonCJulinPWhole-body vibration training compared with resistance training: effect on spasticity, muscle strength and motor performance in adults with cerebral palsyJ Rehabil Med200638530230816931460
- LeeBKChonSCEffect of whole body vibration training on mobility in children with cerebral palsy: a randomized controlled experimenter-blinded studyClin Rehabil201327759960723411791
- el-ShamySMEffect of whole-body vibration on muscle strength and balance in diplegic cerebral palsy: a randomized controlled trialAm J Phys Med Rehabil201493211412124434887
- RuckJChabotGRauchFVibration treatment in cerebral palsy: a randomized controlled pilot studyJ Musculoskelet Neuronal Interact2010101778320190383
- SemlerOFrickeOVezyroglouKStarkCSchoenauEPreliminary results on the mobility after whole body vibration in immobilized children and adolescentsJ Musculoskelet Neuronal Interact200771778117396011
- StarkCHerkenrathPHollmannHEarly vibration assisted physiotherapy in toddlers with cerebral palsy: a randomized controlled pilot trialJ Musculoskelet Neuronal Interact201616318319227609033
- StarkCNikopoulou-SmyrniPStabreyASemlerOSchoenauEEffect of a new physiotherapy concept on bone mineral density, muscle force and gross motor function in children with bilateral cerebral palsyJ Musculoskelet Neuronal Interact201010215115820516632
- StarkCSemlerODuranIIntervallrehabilitation mit häuslichem Training bei Kindern mit ZerebralpareseMonatsschr Kinderheilkd20131617625632
- ReyesMLHernandezMHolmgrenLJSanhuezaEEscobarRGHigh-frequency, low-intensity vibrations increase bone mass and muscle strength in upper limbs, improving autonomy in disabled childrenJ Bone Miner Res20112681759176621491486
- KoMSSimYJKimDHJeonHSEffects of three weeks of whole-body vibration training on joint-position sense, balance, and gait in children with cerebral palsy: a randomized controlled studyPhysiother Can20166829910527909356
- IbrahimMMEidMAMoawdSAEffect of whole-body vibration on muscle strength, spasticity, and motor performance in spastic diplegic cerebral palsy childrenEgypt J Med Hum Genet2014152173179
- WrenTALeeDCHaraREffect of high-frequency, low-magnitude vibration on bone and muscle in children with cerebral palsyJ Pediatr Orthop201030773273820864862
- RussellDJRosenbaumPLCadmanDTGowlandCHardySJarvisSThe gross motor function measure: a means to evaluate the effects of physical therapyDev Med Child Neurol19893133413522753238
- MichaelisUGross Motor Function Measure (GMFM66 and GMFM88) User’s Manual, 2nd editionDev Med Child Neurol201557121188
- YabumotoTShinSWatanabeTWhole-body vibration training improves the walking ability of a moderately impaired child with cerebral palsy: a case studyJ Phys Ther Sci20152793023302526504349
- CamerotaFGalliMCellettiCQuantitative effects of repeated muscle vibrations on gait pattern in a 5-year-old child with cerebral palsyCase Rep Med2011201135912621826147
- DamianoDLRehabilitative therapies in cerebral palsy: the good, the not as good, and the possibleJ Child Neurol20092491200120419525491
- JohnstonMVClinical disorders of brain plasticityBrain Dev2004262738015036425
- BergerMSPittsLHLovelyMEdwardsMSBartkowskiHMOutcome from severe head injury in children and adolescentsJ Neurosurg19856221941993968558
- ChenJWoollacottMHLower extremity kinetics for balance control in children with cerebral palsyJ Mot Behav200739430631617664172
- DonkerSFLedebtARoerdinkMSavelsberghGJBeekPJChildren with cerebral palsy exhibit greater and more regular postural sway than typically developing childrenExp Brain Res2008184336337017909773
- TuzsonAEGranataKPAbelMFSpastic velocity threshold constrains functional performance in cerebral palsyArch Phys Med Rehabil20038491363136813680575
- WoollacottMHShumway-CookAPostural dysfunction during standing and walking in children with cerebral palsy: what are the underlying problems and what new therapies might improve balance?Neural Plast2005122–3211219 discussion 263–27216097489
- ShortlandAMuscle deficits in cerebral palsy and early loss of mobility: can we learn something from our elders?Dev Med Child Neurol200951Suppl 4596319740211
- HoulihanCMStevensonRDBone density in cerebral palsyPhys Med Rehabil Clin N Am200920349350819643349
- KentRMCerebral palsyBarnesMPGoodDCHandbook of Clinical Neurology: Neurological Rehabilitation110AmsterdamElsevier2013443459
- NaroALeoARussoMBreakthroughs in the spasticity management: are non-pharmacological treatments the future?J Clin Neurosci201739162728262404
- KitaMGoodkinDEDrugs used to treat spasticityDrugs200059348749510776831
- GrahamHKAokiKRAutti-RamoIRecommendations for the use of botulinum toxin type A in the management of cerebral palsyGait Posture2000111677910664488
- AbelMFDamianoDLPannunzioMBushJMuscle-tendon surgery in diplegic cerebral palsy: functional and mechanical changesJ Pediatr Orthop199919336637510344322
- FasanoVABroggiGBarolat-RomanaGSguazziASurgical treatment of spasticity in cerebral palsyChilds Brain197845289305657884
- AbercrombyAFAmonetteWELayneCSMcFarlinBKHinmanMRPaloskiWHVariation in neuromuscular responses during acute whole-body vibration exerciseMed Sci Sports Exerc20073991642165017805098