Abstract
Background
Treatment-resistant depression (TRD) is common and potentially life-threatening in adults, and the benefits and risks of adjunctive aripiprazole in these patients remain controversial. Therefore, we conducted a meta-analysis of randomized controlled trials (RCTs) to assess the efficacy, acceptability, safety, and quality of life of adjunctive aripiprazole in patients with TRD.
Methods
RCTs published in PubMed, Web of Science, and Embase were systematically reviewed to evaluate the efficacy and safety profiles of TRD patients who were treated with adjunctive aripiprazole. The main outcome measures included response rate, remission rate, changes from baseline in Montgomery–Asberg Depression Rating Scale (MADRS), Clinical Global Impression-severity (CGI-S), Clinical Global Impression-improvement (CGI-I), 17-Item Hamilton Rating Scale for Depression (HAM-D17), Sheehan Disability scale (SDS), and Inventory of Depressive Symptomatology Self-Report Scale (IDS-SR), discontinuation due to adverse events, and adverse events. Risk ratio (RR) or weight mean difference with 95% confidence intervals (CIs) were pooled using a fixed-effects or random-effects model according to the heterogeneity among studies.
Results
A total of 8 RCTs involving 2,260 patients were included in this meta-analysis. Adjunctive aripiprazole was associated with a significantly higher remission rate (RR =1.64, 95% CI: 1.42 to 1.89; P<0.001) and response rate (RR =1.45, 95% CI: 1.13 to 1.87; P=0.004) than other treatments. Moreover, adjunctive aripiprazole had greater changes in MADRS score, CGI-S score, CGI-I score, HAM-D17 score, SDS score, and IDS-SR score. There were more patients treated with adjunctive aripiprazole who discontinued their treatments due to adverse events. The incidence of adverse events was significantly higher in the adjunctive aripiprazole group than in other treatment groups.
Conclusion
The adjunctive aripiprazole showed benefits in improving the response rate, remission rate, and the quality of life in patients with TRD. However, clinicians should interpret these findings with caution due to the evidence of potential treatment-related side effects.
Introduction
Major depressive disorder (MDD) is common in adults, which leads to disability, suicidality, and increased mortality.Citation1–Citation3 Although several available treatments have been applied for MDD over the past 2 decades, it is still a challenging illness that psychiatrists face.Citation3 Up to 50%–60% of patients do not achieve adequate response,Citation4 and two-thirds of them do not experience a timely remission.Citation5 This is of significant concern, since patients with partial response or residual symptoms have reduced functioning and a worse prognosis than these with remission.Citation4 In addition, patients who fail to achieve remission from MDD are more likely to have functional impairment and suicide.Citation6 These patients who did not respond adequately to the conventional antidepressant therapy are broadly defined as having treatment-resistant depression (TRD).Citation4 It is difficult to estimate the prevalence of TRD, but patients with TRD usually have poor long-term outcomes and increased risk of recurrence.Citation7 Therefore, there is need for additional treatment strategies for those patients with TRD.
A range of augmentation and combination strategies has been used to improve the response rate and remission rate in patients with inadequate antidepressant response.Citation8 These treatment options include mirtazapine, bupropion, and augmentation with lithium, second-generation antipsychotics, olanzapine/fluoxetine combination.Citation9,Citation10 Augmentation strategies involve the addition of a nonstandard agent to the treatment regimen.Citation11 One advantage of augmentation is that it eliminates the transition period between one antidepressant to another, thereby building on any partial response (20%–50% improvement).Citation12
Aripiprazole is a second atypical antipsychotic approved by the Food and Drug Administration for augmentation treatment of MDD. It is distinct from other antipsychotics acting as a partial agonist at dopamine D2, D3, and serotonin 5-HT1A receptors and as an antagonist at 5-HT2A receptors.Citation13–Citation15 In one 6-week prospective open-label multicenter study, adjunctive aripiprazole significantly reduced the Montgomery–Asberg Depression Rating Scale (MADRS) score by 14.0 points in patients with MDD who had inadequate antidepressant response.Citation16 Moreover, it also improved the response rate and remission rate by 52.3% and 39.8%, respectively.Citation16 There are several clinical trials that assessed the efficacy and safety of adjunctive aripiprazole in TRD patients; however, their results remain inconsistent. Thus, we conducted this meta-analysis of randomized controlled trials (RCTs) to evaluate the efficacy, acceptability, safety, and quality of life of adjunctive aripiprazole in the treatment of patients with TRD.
Methods
Search strategy
We conducted this meta-analysis in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis criteria.Citation17 A comprehensive search was conducted to identify relevant studies on the use of adjunctive aripiprazole in the treatment of TRD. PubMed, Embase, and Web of Science were searched for all studies published before October 7, 2017. The search was limited to human subjects and no language restriction was imposed. The search terms used were (“depressive disorder, treatment-resistant” [MeSH Terms]) OR (“depressive” [All Fields] AND “disorder” [All Fields] AND “treatment-resistant” [All Fields]) OR “(treatment-resistant depressive disorder” [All Fields]) OR (“treatment” [All Fields] AND “resistant” [All Fields] AND “depression” [All Fields]) OR (“treatment resistant depression” [All Fields]) AND (“aripiprazole” [MeSH Terms] OR “aripiprazole” [All Fields]). In addition, we also searched the reference lists of the included studies to identify other potentially eligible studies that we may have left out with our primary search.
Study selection
All clinical trials that were assessed for the efficacy and safety of adjunctive aripiprazole for TRD were considered eligible for analysis. The selection criteria applied were as follows: 1) study design: RCT; 2) population: adult patients diagnosed with TRD; 3) intervention: adjunctive aripiprazole; 4) comparison intervention: any type of control; 5) outcome measure: response rate, remission rate, mean change from baseline in MADRS, Clinical Global Impression-severity (CGI-S), Clinical Global Impression-improvement (CGI-I), 17-Item Hamilton Rating Scale for Depression (HAM-D17), Sheehan Disability scale (SDS), Inventory of Depressive Symptomatology Self-Report Scale (IDS-SR), discontinuations due to adverse events, and incidence of treatment-related adverse events.
Data extraction
Data were extracted from selected studies independently by 2 investigators (SL and HW) using a standardized data extraction method. We extracted the following data: first author, year of publication, number of patients in each group, baseline patient characteristics, and outcomes, including remission rate, response rate, mean change from baseline in MADRS, CGI-S, CGI-I, HAM-D17, SDS, IDS-SR, and treatment-related adverse events. The data were entered into a standardized Excel file and checked by a third investigator. Any disagreements between the 2 investigators were resolved by discussion and consensus.
Risk of bias and evidence grade assessment
We used the Cochrane risk-of-bias tool to assess the risk of bias of the included study.Citation18 Each study was assigned a value of low, unclear, or high risk of bias according to the following domains: random sequence generation; allocation concealment; blinding of participants and personnel to the study protocol; blinding of outcome assessment; incomplete outcome data; selective reporting; and other bias.Citation18 We also evaluated the quality of evidence for the outcome measures using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE).Citation19 Each outcome was classified as very low, low, moderate, or high quality of evidence. A summary table was prepared using the GRADE profiler (GRADEpro, version 3.6).
Statistical analysis
We estimated the risk ratio (RR) with 95% confidence intervals (CIs) for dichotomous outcomes, and weighted mean difference (WMD) with 95% CIs for continuous outcomes. We first tested the heterogeneity between the studies using I2 statistics, in which I2>50% indicated significant heterogeneity.Citation20 Whenever heterogeneity was found among the included studies, a random-effects modelCitation21 was used to pool the estimates; otherwise, a fixed-effects modelCitation22 was applied. We also conducted sensitivity analysis, subgroup analysis, and meta-regression to explore the potential sources of heterogeneity whenever significant heterogeneity was present. Publication bias was assessed by the Begg and MazumdarCitation23 and Egger et alCitation24 test. A P-value <0.05 was judged as statistically significant, except where otherwise specified. All statistical analyses were performed using STATA, version 12.0 (Stata Corporation, College Station, TX, USA).
Results
Identification of eligible studies
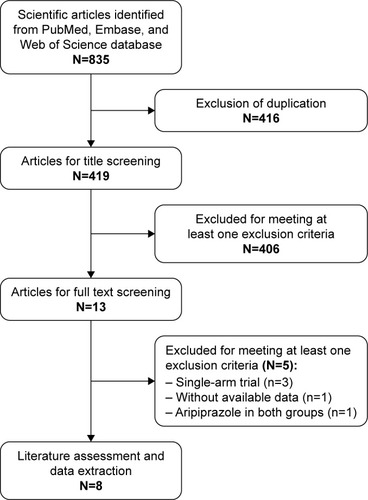
The initial search yielded 835 relevant publications, of which 416 were excluded because of duplicate studies. After reviewing the abstract and title, 406 were excluded because of various reasons (reviews, non-RCTs, or not relevant to our topics). Then 13 potentially relevant studies were identified for the full-text information analysis, and 5 were excluded because they were single-arm studies,Citation16,Citation25,Citation26 or did not provide available data,Citation27 or assigned aripiprazole in both groups.Citation28 Finally, 8 RCTsCitation29–Citation36 met the inclusion criteria and were included in this meta-analysis. The search flow chart is shown in .
Characteristics of eligible studies
The main patient characteristics of the 8 included studies are presented in . All the included studies were well-performed, prospective RCTs. Clinical characteristics were matched for age, gender, and duration of current episode in each study. These studies were published between 2007 and 2016. Most of patients in these studies were white, black, and Asian patients. Among the 8 trials, 5 were conducted in USA,Citation29,Citation30,Citation32–Citation34 1 in China,Citation31 1 in Germany,Citation35 and 1 in Japan.Citation36 Of the included studies, 7 compared adjunctive aripiprazole with placebo,Citation29–Citation34,Citation36 whereas the remaining 1 compared arip-iprazole plus mirtazapine with mirtazapine monotherapy.Citation35 The dosage of aripiprazole ranged from 2 to 20 mg/day. The duration of follow-up ranged from 4 to 12 weeks, and most of the studies had a follow-up of 6 weeks.
Table 1 Baseline characteristics of patients in the trials included in the meta-analysis
Risk of bias and evidence of grade assessment
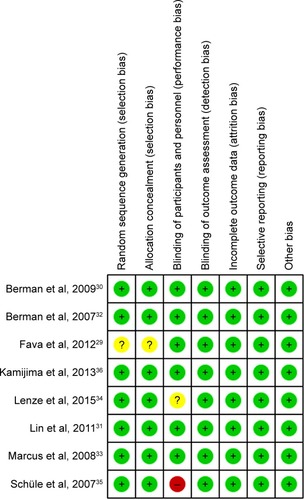
The overview of the risk of bias is summarized in . Overall, 5 studies were classified as being at low risk of bias, 2 being at unclear risk of bias, and 1 being at high risk of bias. The reason for high risk of bias was that this study was not conducted in a double-bind design, and participants and personnel were aware of the therapeutic schedule.Citation35 The reason for the unclear risk of bias was that the 2 studies did not adequately report the methods for random sequence generation or allocation concealment.Citation29,Citation34
The GRADE evidence profiles for these outcomes are shown in . The quality of evidence was high for remission rate, response rate, changes from baseline in MADRS, CGI-S, and CGI-I scores, discontinuation due to adverse events, and adverse events, and moderate for changes from baseline in HAM-D17, SDS, IDS-SR scores.
Table 2 GRADE evidence profile
Remission rate
All the included studies reported the data of remission rate.Citation29–Citation36 The remission rate in the adjunctive aripiprazole group and control group was 29.8% and 18.1%, respectively. The aggregated results suggested that adjunctive aripiprazole was associated with a significantly higher remission rate than the control (RR =1.64, 95% CI: 1.42 to 1.89; P<0.001) (). No evidence of heterogeneity was found among the included studies (I2=0.0%, P=0.995).
Figure 3 Forest plot showing the effect of adjunctive aripiprazole on remission rate.
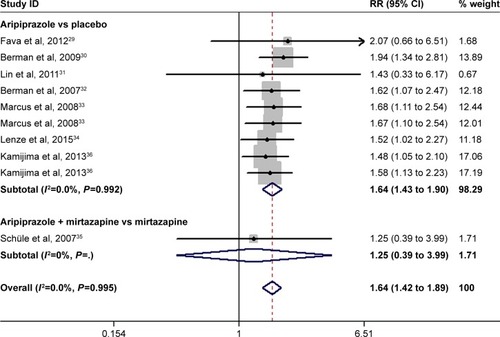
Subgroup analysis was conducted based on the control. The pooled results showed that adjunctive aripiprazole had a higher remission rate than placebo (RR =1.64, 95% CI: 1.43 to 1.90; P<0.001), but a comparable remission rate with mirtazapine alone when it was combined with mirtazapine (RR =1.25, 95% CI: 0.39 to 3.99; P=0.706) ().
Response rate
Seven studies reported the data of response rate.Citation29–Citation33,Citation35,Citation36 The response rate in the adjunctive aripiprazole group and control group was 43.1% and 31.3%, respectively. Pooled results showed that adjunctive aripiprazole group was associated with a significant greater response rate than other treatments (RR =1.45, 95% CI: 1.13 to 1.87; P=0.004) (). The test for heterogeneity was significant (I2=80.4%, P<0.001). Therefore, we conducted sensitivity to explore the potential source of heterogeneity. When we excluded the trial with the smallest sample size,Citation31 the overall estimation changed slightly (RR =1.46, 95% CI: 1.01 to 2.12; P=0.032), and the heterogeneity was still present (I2=85.8%, P<0.001). When we excluded the study with outlier,Citation35 the pooled result altered slightly (RR =1.56, 95% CI: 1.01 to 2.35; P=0.035), but the heterogeneity was still present (I2=86.3%, P<0.001). When we excluded the other studies individually, the overall combination and heterogeneity did not change substantially.
Figure 4 Forest plot showing the effect of adjunctive aripiprazole on response rate.
Abbreviations: CI, confidence interval; RR, risk ratio.
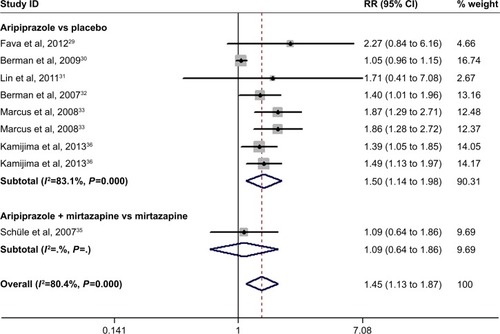
Subgroup analysis was conducted based on the control. The results showed that adjunctive aripiprazole had a higher response rate than placebo (RR =1.50, 95% CI: 1.14 to 1.98; P=0.004), but a comparable response rate with mirtazapine alone when it was combined with mirtazapine (RR =1.09, 95% CI: 0.64 to 1.86; P=0.749) ().
The change from baseline in MADRS score
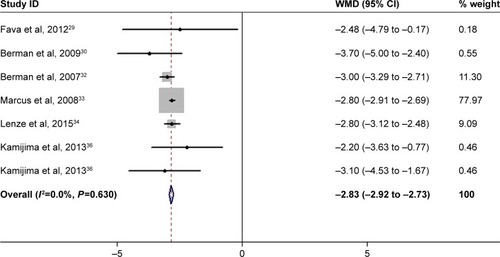
Six studies reported the data of changes from baseline in MADRS score.Citation29,Citation30,Citation32–Citation34,Citation36 The pooled results suggested that the mean change in MADRS score was significantly greater in patients receiving adjunctive aripiprazole than in those treated with adjunctive placebo (WMD =−2.83, 95% CI: −2.92 to −2.73; P<0.001) (). There was no significant heterogeneity among the included studies (I2=0.0%, P=0.630).
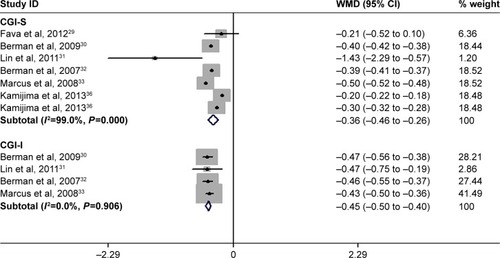
The changes from baseline in CGI-I and CGI-S scores
Six studies reported the data of changes from baseline in CGI-S and CGI-I scores.Citation29–Citation33,Citation36 The aggregated results of these studies demonstrated that adjunctive aripiprazole had greater decrease in CGI-S score (WMD =−0.36, 95% CI: −0.46 to −0.26; P<0.001) and CGI-I score (WMD =−0.45, 95% CI: −0.50 to −0.40; P<0.001) than adjunctive placebo (). There was significant heterogeneity among the included studies for the CGI-S score. Therefore, we conducted sensitivity analysis. When we excluded the trail conducted by Lin et alCitation31 the overall estimation did not change substantially (WMD =−0.44, 95% CI: −0.51 to −0.36; P<0.001), and the heterogeneity was still present (I2=97.1%, P<0.001). Further exclusion of any single study did not change the pooled estimation and heterogeneity substantially.
Figure 6 Forest plot showing the effect of adjunctive aripiprazole on changes from baseline in CGI-I and CGI-S scores.
Abbreviations: CGI-I, Clinical Global Impression-improvement; CGI-S, Clinical Global Impression-severity; CI, confidence interval; WMD, weight mean difference.
The changes from baseline in HAM-D17, SDS, and IDS-SR scores
Six studies reported the data of changes from baseline in HAM-D17, SDS, and IDS-SR.Citation30–Citation33,Citation35,Citation36 The pooled estimation showed that, compared with adjunctive placebo, adjunctive aripiprazole was associated with greater changes from baseline in HAM-D17 (WMD =−1.70, 95% CI: −2.18 to −1.22; P<0.001), SDS (WMD =−0.50, 95% CI: −0.54 to −0.46; P<0.001), and IDS-SR scores (WMD =−1.57, 95% CI: −1.81 to −1.32; P<0.001).
Discontinuations due to adverse events
Six studies reported the data of discontinuations due to adverse events.Citation30–Citation34,Citation36 The rates of discontinuation due to adverse events in the adjunctive aripiprazole and adjunctive placebo groups were 3.7% and 1.7%, respectively. Pooled estimation suggested that, adjunctive aripiprazole resulted in a significantly higher rate of discontinuation due to adverse events than placebo (RR =2.12, 95% CI: 1.23 to 3.67; P=0.007). The test for heterogeneity was not significant (I2=0.0%, P=0.810).
Adverse events
All the studies reported the data of adverse events.Citation29–Citation36 Overall, the incidence of adverse events in the adjunctive aripiprazole and adjunctive placebo groups was 67.9% and 53.6%, respectively. The aggregated results showed that adjunctive aripiprazole had a significantly higher incidence of adverse events than the adjunctive placebo (RR =1.24, 95% CI: 1.17 to 1.33; P<0.001). The test for heterogeneity was not significant (I2=0.0%, P=0.699).
Compared with adjunctive placebo, adjunctive aripiprazole induced a significantly higher incidence of constipation (RR =2.33, 95% CI: 1.21 to 4.50; P=0.011), fatigue (RR =1.68, 95% CI: 1.04 to 2.70; P=0.033), akathisia (RR =4.47, 95% CI: 1.77 to 11.28; P=0.002), insomnia (RR =2.19, 95% CI: 1.35 to 3.54; P=0.001), restlessness (RR =4.51, 95% CI: 2.36 to 8.63; P<0.001), and blurred vision (RR =4.05, 95% CI: 1.68 to 9.75; P=0.002) ().
Table 3 Summary of the RRs with 95% CIs of adverse events
Meta-regression analysis
We first conducted univariate meta-regression analyses for each of the following variables: duration of follow-up, dosage of aripiprazole, sample size, and the control agent. The results showed that there was no significant association of effect size with these variables for the response rate (duration of follow-up, P=0.258; dosage of aripiprazole, P=0.165; sample size, P=0.762; control agent, P=0.544). This suggested that these variables were not significant and independent predictors for heterogeneity.
Publication bias
We used the Egger’s and Begg’s tests to assess the publication bias, and the results showed that there was no evidence of publication bias (Egger’s test: t=−0.66, P=0.532; Begg’s test: Z=0.62, P=0.536).
Discussion
The objective of this meta-analysis was to evaluate the efficacy, acceptability, safety, and quality of life of adjunctive aripiprazole in the treatment of patients with TRD. Our meta-analysis suggested that adjunctive aripiprazole was associated with a significantly higher remission rate and response rate than other treatments. In addition, adjunctive aripiprazole had greater changes in the scores of MADRS, CGI-S, CGI-I, HAM-D17, SDS, and IDS-SR. There were more patients treated with adjunctive aripiprazole who discontinued their studies due to adverse events, and more patients treated with adjunctive aripiprazole who experienced adverse events than those with other treatments. These results help to clarify the risk–benefit profiles of adjunctive aripiprazole for clinicians in the treatment of TRD patients.
There have been 2 published systematic review and meta-analysis of augmentation agents for TRD patients.Citation37,Citation38 In these studies, the authors evaluated the efficacy, acceptability, and tolerability of several augmentation agents for TRD patients, including aripiprazole, bupropion, buspirone, lamotrigine, lithium, methylphenidate, olanzapine, pindolol, quetiapine, risperidone, and thyroid hormone. Their results suggested that these antipsychotic augmentations have proven efficacious in reducing the depressive symptoms, and aripiprazole also showed benefits in improving the quality of life in TRD patients.Citation37,Citation38 Our study spends on the prior studies in providing more significant evidence for the use of adjunctive aripiprazole in TRD. First, the present meta-analysis had a more enlarged sample sizes than the previous analysis, which enhanced the statistical power to assess this effect. In this meta-analysis, we included 8 RCTs involving 2,260 patients, whereas in the previous 2 meta-analysis, there were only 4 RCTs of 1,317 patients focusing on the adjunctive aripiprazole. Second, we also conducted subgroup analysis based on control agent to evaluate the impact of these factors on the overall estimates, which was not analyzed in the previous meta-analysis.Citation37,Citation38 Third, in this study, we also evaluated the effects of adjunctive aripiprazole on the changes from baseline in the scores of MADRS, CGI-S, CGI-I, HAM-D17, SDS, and IDS-SR, which were not performed in the previous meta-analysis.Citation37,Citation38 The enlarged sample size has increased the statistical power to provide more reliable effect estimates, and the additional analysis provided more comprehensive information for the clinical physicians.
In this meta-analysis, we found that the adjunctive aripiprazole was associated with a significantly higher remission rate and response rate than the control. These findings were consistent with results of the previous controlled studies.Citation37,Citation38 In the double-blind, placebo-controlled study of Berman RM,Citation30 349 patients with inadequate response were randomly assigned to the adjunctive aripiprazole (n=177, 20 mg/day) group or adjunctive placebo (n=172) group.Citation30 At 14 weeks, the remission rates in the 2 groups were 36.8% and 18.9%, and response rates were 87% and 83%, respectively.Citation30 This indicated that adjunctive aripiprazole exhibited significantly better efficacy than placebo in the remission rate and response rate. Similarly, in another randomized, double-blind, placebo-controlled trial,Citation33 the authors also reported superior effects of adjunctive aripiprazole over placebo.Citation33 In that study, 381 patients were randomized to adjunctive aripiprazole (n=191, starting dose 5 mg/day, dose adjustments 2–20 mg/day, mean endpoint dose of 11.0 mg/day) or adjunctive placebo (n=190).Citation33 They were treated with these adjunctive agents for 8 weeks. At the endpoint, patients who received the adjunctive aripiprazole had significantly greater remission rate (25.4% vs 15.2%) and response rate (32.4% vs 17.4%) than those treated with adjunctive placebo.Citation33
In contrast to their positive results, Lin et al reported a comparable effect of adjunctive aripiprazole with placebo.Citation31 In that study, 21 and 20 patients were assigned to the aripiprazole group and placebo group, respectively.Citation31 They received 2.5 mg/day aripiprazole for 10 weeks.Citation31 At the endpoint, the remission rate and response rate in the aripiprazole group were 71.4% and 85.7%, compared with 50% and 50% in the placebo group, respectively.Citation31 However, the differences between them were not significant. The authors attributed the negative results to the small sample size. At 4 weeks, the remission rate and response rate were significantly different, but these benefit effects were not observed at the 6 weeks because of the high dropout rate.Citation31
According to this study, the change from baseline in MADRS score was significantly greater in the adjunctive aripiprazole group than that in the placebo group. This result was inconsistent with reports of the previous studies.Citation30,Citation31 Kamijima et alCitation36 conducted a randomized, double-blind, placebo-controlled study (ADMIRE study), which assessed the efficacy and safety of a fixed dose (3 mg/day) and flexible dose (3–15 mg/day) schedule of adjunctive aripiprazole in Japanese patients.Citation36 In that study, 286 patients were randomly assigned to the adjunctive treatment with flexible-dose aripiprazole group (n=194), fixed-dose aripiprazole group (n=197), and placebo group (n=195).Citation36 After the 8 week treatment, patients who received either the adjunctive fixed dose or flexible dose of aripiprazole achieved a significantly greater reduction from baseline in the MADRS score (−10.5 and −9.6, respectively) than those treated with adjunctive placebo (−7.4).Citation36
Whereas in another double-blind, placebo-controlled study conducted by Fava et al,Citation29 they also found a greater improvement of adjunctive aripiprazole in MADRS score, but this change was not significantly different when compared with the placebo.Citation29 In that study, 255 patients were randomized to adjunctive treatment with aripiprazole 2 mg/day or placebo, with a 2:3:3 randomization ratio to drug/drug (aripiprazole 2 mg/day in Phase I; 5 mg/day in Phase II), placebo/placebo (placebo in both phases), and placebo/drug (placebo in Phase I; aripiprazole 2 mg/day in Phase II).Citation29 The MARDS mean change for aripiprazole 2 mg/day decreased by 8.5 points in Phase I and 5.8 points in Phase II, compared with 8.1 points in Phase I and 3.3 points in Phase II for placebo, respectively.Citation36 The difference between the 2 groups was not significant (weighted difference =−1.51, P=0.0649).Citation36 When the data were pooled from both phases, the mean MADRS score of adjunctive aripiprazole was 1.51 points more than placebo; however, the difference was still not statistically significant (P=0.0649).Citation36 The authors thought that the interpretation for these negative results were complex. Since the placebo response rate in Phase I was in line with that in the previous positive studies, the robust effects of adjunctive aripiprazole 2–15 mg/day in other trials suggested that the efficacy of low-dose aripiprazole 2 mg/day was minimal.Citation36 Despite the dosage of aripiprazole was raised to 5 mg/day in the second phase, the aripiprazole–placebo difference was only 4.3%, suggesting that the low-dose aripiprazole might not be efficacious, and the 5 mg/day aripiprazole only presented the minimal-effect benefits.Citation36
With regard to the safety profile, our results suggested that patients treated with adjunctive aripiprazole experienced a significantly higher incidence of adverse events. Moreover, there were more patients treated in the adjunctive aripiprazole group who discontinued the study due to adverse events than those in the placebo group. The most commonly occurring adverse events with aripiprazole included akathisia, constipation, fatigue, insomnia, restlessness, blurred vision, diarrhea, nausea, and fatigue. Most of these adverse events were generally mild-to-moderate in severity. Akathisia was the most common adverse event with adjunctive aripiprazole. In this study, adjunctive aripiprazole was associated with a 3.47-fold greater likelihood of akathisia than the placebo (RR =4.47, 95% CI: 1.77 to 11.28; P=0.002). This result was consistent with the finding of the study conducted by Lenze et al.Citation34 In that study, there was a significantly higher rate of akathisia with adjunctive aripiprazole than with placebo (26.7% vs 12.2%).Citation34 Moreover, akathisia resulted in a temporary increase in suicidal thoughts in 3 patients treated with aripiprazole and trial discontinuation in another 1 patient.Citation34 Thus, health care professionals should be aware of these adverse effects of aripiprazole and adjust dose or potentially switch treatment.
There were several limitations in this meta-analysis. First, our analysis was based on 8 RCTs, and some of them had a relatively small sample size (n<100). Overestimation of the treatment effect is more likely in smaller trials when compared with larger trials. Second, some of the subgroup analysis was based only on 3–4 studies; thus, conclusion about the remission rate and response rate of adjunctive aripiprazole should be interpreted with caution. Third, we found considerable heterogeneity across the studies in our meta-analysis. It was not surprising given the differences in the study population, duration of the treatment, dosage of aripiprazole, and the definitions of TRD and response. These factors account for the heterogeneity and could affect our results. Fourth, it should be noted that all the included trials were sponsored by pharmaceutical companies; thus, we could not rule out the existence of possible bias that was brought by the inherent conflict of interest.
Conclusion
The present meta-analysis suggested that adjunctive aripiprazole significantly exhibited benefit effects in improving the response rate, remission rate, and the quality of life in patients with TRD. However, clinicians should interpret these findings cautiously in light of the evidence of potential treatment-related side effects. More large-scale, well-designed RCTs are needed to verify our findings.
Disclosure
The authors report no conflicts of interest in this work.
References
- WhitefordHADegenhardtLRehmJGlobal burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010Lancet201338299041575158623993280
- CallahanCMKroenkeKCounsellSRIMPACT InvestigatorsTreatment of depression improves physical functioning in older adultsJ Am Geriatr Soc200553336737315743276
- GalloJJMoralesKHBognerHRLong term effect of depression care management on mortality in older adults: follow-up of cluster randomized clinical trial in primary careBMJ2013346f257023738992
- FavaMDiagnosis and definition of treatment-resistant depressionBiol Psychiatry200353864965912706951
- TrivediMHRushAJWisniewskiSRSTAR*D Study TeamEvaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practiceAm J Psychiatry20061631284016390886
- NemeroffCBThe burden of severe depression: a review of diagnostic challenges and treatment alternativesJ Psychiatr Res2007413–418920616870212
- SackeimHAThe definition and meaning of treatment-resistant depressionJ Clin Psychiatry200162Suppl 161017
- FavaMRushAJCurrent status of augmentation and combination treatments for major depressive disorder: a literature review and a proposal for a novel approach to improve practicePsychother Psychosom200675313915316636629
- MaustDTOslinDWThaseMEGoing beyond antidepressant monotherapy for incomplete response in nonpsychotic late-life depression: a critical reviewAm J Geriatr Psychiatry2013211097398623567381
- CooperCKatonaCLyketsosKA systematic review of treatments for refractory depression in older peopleAm J Psychiatry2011168768168821454919
- LamRWKennedySHGrigoriadisSCanadian Network for Mood and Anxiety Treatments (CANMAT)Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III. PharmacotherapyJ Affect Disord2009117Suppl 1S26S4319674794
- FleurenceRWilliamsonRJingYA systematic review of augmentation strategies for patients with major depressive disorderPsychopharmacol Bull2009423579019752841
- BurrisKDMolskiTFXuCAripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptorsJ Pharmacol Exp Ther2002302138138912065741
- JordanSKoprivicaVChenRTottoriKKikuchiTAltarCAThe antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptorEur J Pharmacol2002441313714012063084
- ShapiroDARenockSArringtonEAripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacologyNeuropsychopharmacology20032881400141112784105
- JonDIKimDHSeoHJAugmentation of aripiprazole for depressed patients with an inadequate response to antidepressant treatment: a 6-week prospective, open-label, multicenter studyClin Neuropharmacol201336515716124045606
- MoherDLiberatiATetzlaffJAltmanDGPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementBMJ2009339b253519622551
- HigginsJPAltmanDGGotzschePCCochrane Statistical Methods GroupThe cochrane collaboration’s tool for assessing risk of bias in randomised trialsBMJ2011343d592822008217
- GuyattGHOxmanADVistGEGRADE Working GroupGRADE: an emerging consensus on rating quality of evidence and strength of recommendationsBMJ2008336765092492618436948
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- DerSimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- MantelNHaenszelWStatistical aspects of the analysis of data from retrospective studies of diseaseJ Natl Cancer Inst195922471974813655060
- BeggCBMazumdarMOperating characteristics of a rank correlation test for publication biasBiometrics1994504108811017786990
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- FabrazzoMPerrisFMonteleonePEspositoGCatapanoFMajMAripiprazole augmentation strategy in clomipramine-resistant depressive patients: an open preliminary studyEur Neuropsychopharmacol201222213213621784621
- NaylorJCKiltsJDBradfordDWA pilot randomized placebo-controlled trial of adjunctive aripiprazole for chronic PTSD in US military Veterans resistant to antidepressant treatmentInt Clin Psychopharmacol201530316717425647451
- KaneriyaSHRobbins-WeltyGASmagulaSFPredictors and moderators of remission with aripiprazole augmentation in treatment-resistant late-life depression: an analysis of the IRL-GRey Randomized Clinical TrialJAMA Psychiatry201673432933626963689
- YoshimuraRKishiTHoriHComparison of the efficacy between paroxetine and sertraline augmented with aripiprazole in patients with refractory major depressive disorderProgress Neuropsychopharmacol Biol Psychiatry2012392355357
- FavaMMischoulonDIosifescuDA double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy (ADAPT-A Study)Psychother Psychosom2012812879722286203
- BermanRMFavaMThaseMEAripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressantsCNS Spectr200914419720619407731
- LinCHLinSHJangFLAdjunctive low-dose aripiprazole with standard-dose sertraline in treating fresh major depressive disorder: a randomized, double-blind, controlled studyJ Clin Psychopharmacol201131556356821869699
- BermanRMMarcusRNSwaninkRThe efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled studyJ Clin Psychiatry200768684385317592907
- MarcusRNMcQuadeRDCarsonWHThe efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled studyJ Clin Psychopharmacol200828215616518344725
- LenzeEJMulsantBHBlumbergerDMEfficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trialLancet2015386100112404241226423182
- SchüleCBaghaiTCEserDMirtazapine monotherapy versus combination therapy with mirtazapine and aripiprazole in depressed patients without psychotic features: a 4-week open-label parallel-group studyWorld J Biol Psychiatry20078211212217455104
- KamijimaKHiguchiTIshigookaJADMIRE StudyGroupAripiprazole augmentation to antidepressant therapy in Japanese patients with major depressive disorder: a randomized, double-blind, placebo-controlled study (ADMIRE study)J Affect Disord2013151389990524074484
- ZhouXRavindranAVQinBComparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: systematic review and network meta-analysisJ Clin Psychiatry2015764e487e49825919841
- ZhouXKeitnerGIQinBAtypical antipsychotic augmentation for treatment-resistant depression: a systematic review and network meta-analysisInt J Neuropsychopharmacol20151811pyv06026012350