Abstract
Benzodiazepines are one of the most prescribed medications as first-line treatment of anxiety, insomnia, and epilepsy around the world. Over the past two decades, advances in the neuropharmacological understanding of gamma aminobutyric acid (GABA)A receptors revealed distinct contributions from each subtype and produced effects. Recent findings have highlighted the importance of α1 containing GABAA receptors in the mechanisms of addiction and tolerance in benzodiazepine treatments. This has shown promise in the development of tranquilizers with minimal side effects such as cognitive impairment, dependence, and tolerance. A valium-like drug without its side effects, as repeatedly demonstrated in animals, is achievable.
Introduction
Benzodiazepines are a class of tranquilizers that enhance gamma aminobutyric acid (GABA)ergic transmission. They are seen ubiquitously in the modern health care system as >5% of the total adults in USA are prescribed benzodiazepines each year.Citation1 The major behavioral and psychoactive effects of benzodiazepines include anticonvulsive, sedative, muscle relaxant, and anxiolytic effects.Citation2 They are readily prescribed by physicians and are regarded as a frontline treatment for many common psychiatric disorders such as anxiety, obsessive-compulsive disorder, seizures, as well as a number of sleep disorders.Citation3
Benzodiazepines were discovered in 1955 by chemist Leo Sternbach and, when first introduced, were proposed as a promising replacement for barbiturates, another similar class of tranquilizers that also act on GABA.Citation4,Citation5
The medical use of barbiturates was prominent until the 1950s when serious side effects, such as high incidence of abuse, dependence, and overdose finally started to surface.Citation6
When benzodiazepines hit the market in the 1960s, they were thought to be the successor to barbiturates due to lower toxicity and side effects. Despite having a lower abuse profile, benzodiazepines still cause dependence after repeated use, which was not widely recognized until the 1980s.Citation7,Citation8
Many attempts to produce dependence and tolerance-free benzodiazepine drugs have been investigated in the past. The selective agonist zolpidem was marketed, as promising data showed reduced abuse potential.Citation9 However, these results did not translate in the clinic since zolpidem causes dependence after repeated use.Citation10
Studies have also attempted to investigate neuropharmacological mechanisms of benzodiazepines. At first, the benzodiazepine site was categorized into benzodiazepine subtype I and benzodiazepine subtype II, where traditional benzodiazepines bind to both, but triazolopyridazines (TPZs) have high affinity for only type I.Citation11 It was later found that TPZ was actually just a selective agonist for one of many subtypes that exist within the GABAA receptor family.Citation12
Progress in neuropharmacology has revealed various subtypes within the GABAA receptor family, as well as anatomical and pharmacological differences between them. Investigations have also been directed toward the addiction and tolerance mechanisms of benzodiazepines; their relationships with specific subtypes within the GABAA receptor family will be discussed in further depth.
Pharmacological targets of benzodiazepine
Benzodiazepines, although referred to as a positive allosteric modulator (PAM) of the GABAA receptor, does not actually enhance GABA’s binding to the receptor, like conventional PAMs. Benzodiazepines increase the frequency of chloride channel influx which hyperpolarizes the GABA receptor, resulting in increased inhibitory postsynaptic potential.Citation13,Citation14
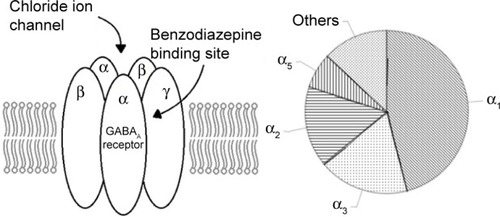
α1–6, β1–3, γ1–3, δ, ε, θ, and π make up the currently defined GABAA subunits in the human brain.Citation15 Classic benzodiazepines such as diazepam binds to α1, α2, α3, and α5 containing GABAA receptors.Citation16 α1 containing GABAA receptors are the most abundant subtype and can be found throughout the brain, while α2, α3, and α5 subtypes are more region specific.Citation17
Although numerous combinations exist, most GABAA receptors structurally contain two α, two β, and a single γ subunit surrounding a chloride ion channel as shown in .Citation18 The benzodiazepine site is located between the α and γ subunit.
Figure 1 The GABAA receptor and its approximate subtype composition (adapted from Wafford’s study).
Abbreviation: GABA, gamma aminobutyric acid.
Roles of GABAA receptor α1, α2, α3, and α5 subunits in benzodiazepine pharmacology
GABAA subtype selective compounds and rodent models of subunit point mutation have provided promising data for identifying different subunit contributions toward each clinical effect. We will discuss clinical effects of sedation first, as it impairs the cognitive performance of benzodiazepine-prescribed patients.Citation20
We know that the α1 subtype plays a major role in sedation because α1(H101R) point mutated mice were resilient to sedative effects of benzodiazepines.Citation21 Benzodiazepines that possess sparing efficacy at α1 subtype such as L-838,417 act as a sedation-free anxiolytic in animal models.Citation21,Citation22 L-838,417 has seen popular use in research and is a partial agonist of α2, α3, and α5 containing GABAA receptors.Citation22 Other compounds with low or absent α1 subtype efficacy such as imidazenil, TPA123, and TPA023, are also shown to be sedation-free anxiolytics, revealing the importance of the α1 subtype in the mediation of sedation.Citation23,Citation24
Moreover, α1 subtype selective agonists such as zolpidem are unable to produce anxiolytic effects other than sedation.Citation25 The α5 inverse agonist, α5IA, improves memory without producing anxiety, increased awakeness, or proconvulsant effects in animals.Citation26 So far, we could conclude that anxiety is mediated by either α2 or α3 subtypes or both, while sedation is mediated by α1 containing GABAA receptors.
Because α5IA reversed alcohol-induced memory deficit when given prior to alcohol administration in humans, α5 subtype is thought to contribute toward amnesic effects.Citation26 This was further confirmed when the α5 subtype was found to be responsible for amnestic effects of GABAA receptor PAM etomidate, which correspond with earlier studies on the role of the α5 subtype in memory and its anatomical presence in the hippocampus.Citation27
However, it seems α5 is not the sole subtype that contributes to amnesic effects. α1 subtype full agonist zolpidem seemed to produce more memory and cognitive impairment compared to an equivalent dose of triazolam, an agonist of all benzodiazepine sites.Citation28 Given that zolpidem produces almost no efficacy at the α5 subtype, we can conclude that α1 subtype is also involved in amnesic effects of benzodiazepines.Citation28
In 2000, it was thought that α2 subtype solely mediated anxiolytic actions because only α2(H101R) point mutated mice were still anxious after diazepam treatment.Citation29 On the contrary, two studies 5 years later, the first study using TP003, an α3 subtype selective agonist, demonstrated that TP003 produced strong anxiolytic effects.Citation30 The second study used an α3 subtype inverse agonist α3IA to produce anxiogenic effects in mice, further confirming the role of α3 subtype in mediating anxiety.Citation31
Although α3IA’s weak inverse efficacy at α2 subtype could be argued to be anxiogenic, novel non-benzodiazepine compounds such as adipiplon (NG2-73) and SB-205,384 produced anxiolytic effects without any efficacy at the α2 subtype.Citation32
Moreover, it was shown by data that various novel benzodiazepine compounds with stronger efficacy at α3 than α2 subtype showed more anxiolytic effects. Löw et al’s study received several responses suggesting that the data from elevated plus maze in α2(H101R) mice could be more related and influenced by the motor activity mediated through α2 subtype than anxiolysis.Citation33,Citation34
This is important because almost all past models and reviews have categorized the anxiolytic effects of benzodiazepines solely from efficacy at the α2 subtype, some further suggesting novel benzodiazepine anxiolytics should be α2 selective.Citation35–Citation39 However, the most recent investigations on this matter suggest that both α2 and α3 subtypes mediate anxiety.Citation40,Citation41 Therefore, the design of subtype selective benzodiazepine anxiolytics should not be limited to α2 containing GABAA receptors. This paper will present a revised and comprehensive model of benzodiazepine pharmacology.
α5 subtype has also been shown to play a role in mediating anxiety and especially conditioned fear memory, as shown by α5 subtype inverse agonists, RY024, and α5(H105R) point mutated mice.Citation42,Citation43 However, the α5 subtype does not seem to play a major role in mediating anxiety as another α5 subtype inverse agonist, α5IA, does not produce anxiety as assessed by elevated plus maze.Citation44 α5 subtype also appears to play a minor role in anticonvulsive actions as proconvulsive as α5IA did not increase seizure thresholds as assessed by pentylenetetrazol.Citation44 As a result, the α5 subtype most probably mediates a narrow category of anxiety, such as conditioned fear, but could be clinically relevant.
Moreover, several models of GABAA subunit contribution in benzodiazepine pharmacology suggest anticonvulsive action only at α1 subtype.Citation36 TPA023 devoid of activity at the α1 subtype was still able to produce anticonvulsive effects, through α2 and possibly α3 subtype.Citation24
Diazepam was anticonvulsive in both α2(H101R) and α3(H126R) mice; however, more α2(H101R) mice convulsed after treatment toward pentylenetetrazol, revealing that the α2 subtype contributes more than α3 subtype in the anticonvulsant properties of benzodiazepines.Citation29
Most anticonvulsive actions of benzodiazepines are mediated by α1 as diazepam only mildly attenuated pentylenetetrazol-induced seizures in α1(H101R) mice. However, this data should not discourage anticonvulsant drug discovery that has no efficacy at α1 subtype. Recent investigations actually showed that the α1 subtype antagonist, α2, α3, and α5 subtype partial agonist imidazenil was actually more potent than diazepam at attenuating diisopropyl fluorophosphate-induced convulsions and neuronal damage.Citation46
Myorelaxation has been identified to involve α2/α3 subtype using respective point mutated mice; however, the action appears to be primarily mediated through α2 subunit because high doses were needed to induce myorelaxation in the α2(H101R) mice.Citation47 α1 and α5 subtypes might also contribute minorly toward myorelaxation because its respective selective antagonists are able to bluntly alleviate diazepam-induced myorelaxation.Citation48,Citation49
Not only does insufficient information support that α2 subtype exclusively mediates anxiety, other than a single study, current evidence for the anxiolytic effects of benzodiazepines also points toward a dual contribution from α2 and α3 subtypes.Citation29–Citation41 Therefore, in , anxiolytic effects were assigned to both α2/α3 containing GABAA receptors.
Table 1 Model of GABAA receptor subtypes and their contribution toward benzodiazepine’s psychopharmacological effects
In 2010, in a disappointing human trial on MRK-409, a GABAA receptor α2, α3, and α5 subtype partial agonist that promised to be a sedation-free anxiolytic in animals, demonstrated that its minor agonism on α1 subtype produced sedation in humans.Citation54 However, the question of whether other subunits were involved in sedation was quickly overthrown as the clinical results of TPA023B, published shortly after, as an α1 subtype antagonist and α2/α3 subtype partial agonist, produced a sedation-free anxiety suppressing profile,Citation50 demonstrating that any efficacy at α1 subtype could cause sedation in humans.
α5 subtype might be differentially distributed in different sexes. The selective α5 subtype allosteric modulator, SH-053-2′F-R-CH3, seemed to particularly impact female but not male mice at alleviating stress.Citation55 In humans, long-term benzodiazepine use inducing alterations in long-term memory was only significant in women.Citation56
Recent advances in novel clinical applications of benzodiazepines revealed densely populated α2 containing GABAA receptors within the dorsal root involved in relieving pain, in which its inhibitory currents are believed to contribute to nociception.Citation21 Furthermore, although α1 subtype agonists such as zolpidem and diazepam are efficacious for pain and neuropathy, α2, α3 and α5 subtype partial agonists seem to produce similar results without the sedative, amnesic, and addictive properties of α1 subtype agonists.Citation57,Citation58
Mechanism of benzodiazepine addiction
Benzodiazepine-induced activation of mesolimbic dopamine pathway was observed for the first time in 2009. Benzodiazepine indirectly acts upon the dopaminergic neurons in the ventral tegmental area (VTA), a brain region that plays a major role in addiction and reward.Citation59
Both the selective α1 subtype agonist zolpidem as well as diazepam were able to modulate glutamatergic transmission upon dopamine neurons in the VTA. This is groundbreaking because zolpidem has a significantly higher affinity toward α1 (Ki =20 nM) containing GABAA subtypes than α2 and α3 (Ki =400 nM) subtypes, and is almost inactive at α5.Citation60 This suggests that α1 containing GABAA receptors unquestionably play a role in addiction.
However, in the same study, diazepam had a significantly higher effect than zolpidem upon VTA; could there be more subtypes than just α1 subtype in the role of addiction? The role of α5 subtype in addiction was ruled out because α5 subtype inverse agonists were unable to prevent self-administration of ethanol.Citation61 Another study demonstrated that while α2, α3, and α5 point mutated mice showed clear preference toward drinking water contaminated with midazolam, α1(H101R) mice did not show any bias between water and midazolam solution,Citation62 implying that α1 containing GABAA receptors are required for addictive behaviors associated with benzodiazepines. Furthermore, α1 subtype inactive compounds such as TPA023 show almost no abuse properties.Citation60
Recently, opposing evidence has shown that α2 and α3 subtypes might also be implicated in the addiction of benzodiazepines. After α2 subtype within the nucleus accumbens (NAcc) are knockdown in mice, midazolam were no longer reinforcing. Implying that the α2 subtype is at least in part involved in the reinforcing effects of benzodiazepines.Citation63 Rhesus monkeys with a history of benzodiazepine use, but not cocaine use, have been shown to self-administer α1 subtype inactive compound L-838,417 and the α3 subtype selective agonist TP003.Citation64 These findings hint that the α3 containing GABAA receptors could be reinforcing in experienced benzodiazepine users. The experienced rhesus monkeys had been previously treated with numerous different benzodiazepines for around 6 months.Citation64
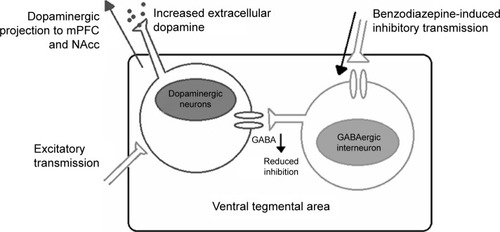
The α1 containing GABAA receptors expressed within the VTA favor its inhibitory projection toward GABA interneurons.Citation62,Citation65 With interneurons being responsible for the inhibitory control over dopaminergic neurons of the VTA, inhibiting the inhibition of dopaminergic neurons results in free firing of the dopaminergic neurons. This results in increased dopamine levels as shown in .Citation62,Citation65 This finding is in congruence with the lack of midazolam self-administration in α1(H101R) mice.Citation62 It seems that α1 subtype should be completely avoided since even partial agonists at this site could still produce sedation and addiction as seen in bretazenil and MRK-409.Citation54,Citation66
Figure 2 Mechanism of benzodiazepines at VTA.
Abbreviations: VTA, ventral tegmental area; GABA, gamma aminobutyric acid; NAcc, nucleus accumbens; mPFC, medial prefrontal cortex.
The increased AMPA/NMDA ratio in VTA has been shown in another GABAA PAM gaboxadol; however, it does not produce reinforcing effects.Citation67 Whether gaboxadol has another mechanism to avert addiction is unclear, but more regions than VTA could be involved. Selective α1 subtype antagonist βCCt and 3-PBC were shown to be able to prevent the reinforcing effects of alcohol.Citation68,Citation69 α1 subtype antagonists effectively blocking addictive behaviors in mice indicate that α1 containing GABAA receptors are highly involved and most likely contribute most or all of the reinforcing effects of GABAergic and benzodiazepine site acting substances. Furthermore, infusions of α1 subtype antagonist into the ventral pallidum and extended amygdala, which accommodates a large amount of α1 containing GABAA receptors, resulted in a reduction in ethanol-induced addictive behaviors.Citation68,Citation70
Opioid mechanisms are associated with reward pathways and a study has shown the competitive opioid receptor antagonist naloxone attenuating the addictive properties of benzodiazepines.Citation71 However, more studies opposing the above study have been published. Interestingly, naloxone is able to block anxiolytic and sedative properties of benzodiazepines.Citation72 More recent studies showed that opioid peptides are involved in benzodiazepine’s anxiolytic rather than addictive effects.Citation73
The role of nicotinic acetylcholine receptor (nAChR), especially the α4β2 subtype, has been highlighted in drug addiction.Citation74 Since nAChR is a major modulator of GABA release in regions such as the thalamus, hippocampus, and VTA,Citation68,Citation75 there is surprisingly less research in this direction. Is there really no link between acetylcholine and benzodiazepine addiction?
nAChRs, especially the α4β2 subtype, are upregulated after chronic exposure to drugs such as nicotine.Citation76 This phenomenon is also seen in naloxone-induced morphine withdrawal, alcohol withdrawal, and what we’re interested in: flumazenil-induced benzodiazepine withdrawal.Citation77,Citation78
Most interestingly, this acetylcholine release was not seen in the partial agonists imidazenil and abecarnil, which has no efficacy at the α1 subtype.Citation79 The fact that benzodiazepine withdrawal is marked by an acetylcholine increase in the nucleus accumbens, which is also seen in other drug withdrawals, further begs clarification of the benzodiazepine–acetylcholine affiliation, and possibly of subunit-specific involvement, which has been overlooked.Citation78,Citation79
Although a recent primate study showed that α3 subtype might be reinforcing in those who are already dependent, α1 containing GABAA receptors contribute to most if not all of the reinforcing effects of benzodiazepines.
Mechanisms of benzodiazepine tolerance
Efficacy of benzodiazepine progressively reduces after long-term exposure; not only is a higher dosage of the drug required to experience the same therapeutic effects, but also discontinuation of prolonged treatment induces withdrawal.Citation80
A simple way to explain tolerance to any drug is down-regulation of the receptor as the aftermath of neuroplasticity. However, it is demonstrated that even after chronic administration of benzodiazepines, the number of benzodiazepine sites is not reduced, neither is the sensitivity of the benzodiazepine site.Citation81,Citation82
Benzodiazepine site downregulation does not seem to happen unless astronomical doses are given, in many studies over 100 mg/kg in rats, which translated into human doses that are far above the therapeutic range.Citation83,Citation84 Not to mention, only inconclusive and inconsistent results have been presented regarding possible changes in subtype composition and their mRNA expression.Citation85,Citation86 So, if its not downregulation, what is the underlying mechanism?
While tolerance to sedative and anticonvulsant effects seems to build quickly in both humans and animal models, a lack of tolerance regarding the anxiolytic and amnesic effects of long-term benzodiazepine use has been consistently demonstrated in clinical trials.Citation87–Citation89
Diazepam and alprazolam for the treatment of panic attacks, social phobia, and other anxiety-related disorders are effective even after chronic use.Citation90,Citation91 Could this mean α2 and α3 containing GABAA receptors, which mediate anxiety, have less significance in the tolerance building mechanism of benzodiazepines?
Other than putting the blame of addiction on α1 subtype, α5 subtype might be required for tolerance toward sedative effects of benzodiazepines. With the guidance of α1, α2, α3, and α5 subtype point mutated mice, repetitive dosing of diazepam showed that α5 containing GABAA receptors of the dentate gyrus lead the adaptive changes associated with sedative tolerance to benzodiazepines.Citation92 After chronic benzodiazepine administration, mice of the α5 point mutated species retained most of the motor-depressant effects of benzodiazepines, whereas α1, α2, and α3 point mutated mice started habituating from the sedative effects of benzodiazepines.Citation92
Now one might ask, how would you then explain tolerance of zolpidem since it has no α5 subtype efficacy? Well, in the same study, α1(H101R) mice showed downregulation of α5 containing GABAA receptors in the dentate gyrus of the hippocampus, but not in α2 and α3 point mutated mice.Citation92 Therefore, a hypothesis that the involvement of α5 as well as α1 subtypes is required to induce tolerance in benzodiazepine use was put forward.
Although long-term benzodiazepine treatment did not reduce the number of benzodiazepine sites or alter the binding affinity as assessed by 3H-flumazenil,Citation93 it did downregulate adenosine receptors in the striatum by almost half in mice of the same investigation. It has been consistently shown that benzodiazepine use is associated with downregulation of adenosine A1 and A2 receptors in animals.Citation94,Citation95 Although the mechanism of how benzodiazepines can influence adenosine receptors is unclear, a possible explanation for the downregulation of adenosine is for the attenuation of benzodiazepine-induced sedation.
It has been proven that benzodiazepines can indirectly increase adenosine after acute administration, through inhibition of adenosine reuptake. The adenosine reuptake inhibitor dipyridamole and the adenosine deaminase EHNA were able to reverse the sedative effects of benzodiazepines, as measured by excitatory currents within the hippocampus.Citation96 This mechanism was recently further validated when the sedative effects of benzodiazepines, barbiturates, and propofol all appeared to be mediated by the adenosine system.Citation97 Downregulation of adenosine receptors as discussed could in part explain the tolerance to the sedative effects of benzodiazepines.
It came forth that not only adenosine is involved in the tolerance toward benzodiazepines. NDMA and AMPA receptor upregulation was observed in the cerebral cortex of mice after abrupt ending of chronic diazepam administration.Citation98,Citation99 More recent inspections of this mechanism showed comparable results especially in the rat hippocampus.Citation100,Citation101
It appears that NMDA upregulation happens acutely after benzodiazepine administration, because NMDA antagonists dizocilpine (MK-801) and CPP were able to prevent tolerance to sedative and withdrawal effects after benzodiazepine administration.Citation102,Citation103 While the NMDA antagonist can suppress withdrawal symptoms in acute benzodiazepine abstinence, the AMPA antagonist prevented withdrawal during the long-term phase in mice.Citation104,Citation105 The upregulation and changes of glutamatergic system are most likely a compensatory mechanism.
Numerous investigations have observed uncoupling between allosteric linkage of GABA and the benzodiazepine site. Uncoupling is a mechanism wherein the benzodiazepine site loses its allosteric modulatory effects over GABAergic activities.Citation18,Citation89 It explains the reduced efficacy of benzodiazepines after chronic use and is further verified in numerous in vivo demonstrations.Citation89 Benzodiazepine site uncoupling is associated with negative modulation of GABAergic transmission and is likely a result of compensation to suppress repeated benzodiazepine-induced GABAergic enhancement.Citation106
In rats continuously exposed to either full agonist, partial agonist, or the antagonist flumazenil, the benzodiazepine efficacy correlates to the degree of uncoupling. Full agonists resulted in the highest percentage of benzodiazepine site uncoupling, especially in benzodiazepine sites that regulated anticonvulsant actions.Citation107 This not only explains the rapid tolerance building toward anticonvulsant effects but also indicates that partial agonists may be able to produce less tolerance.
Interestingly, a single flumazenil dose can reverse the anticonvulsant tolerance after chronic benzodiazepine exposure.Citation108,Citation109 The mechanisms of how flumazenil completely reverses the allosteric uncoupling of benzodiazepine and GABA are unclear. Flumazenil appears to be capable of yielding opposite downstream mechanisms of benzodiazepine agonists, and actually upregulate benzodiazepine binding and GABAergic chloride uptake.Citation110 Similar results have been observed in clinics, where flumazenil was able to reverse tolerance of daily clonazepam in users with partial seizures, who have been taking the medication for over a year.Citation111,Citation112
Benzodiazepine uncoupling recovers after 2 days of abstinence in rats, and only happens after repeated administration.Citation113 This correlates with previously mentioned human clinical data in terms of the quick adaptations to benzodiazepine’s anticonvulsant tolerance. Because about one third of all postsynaptic GABAA receptors are continuously activated, uncoupling might impact continual GABA transmission, which could also contribute to withdrawal.Citation114
In chick cortical neurons, benzodiazepine treatment leads to mild reductions of GABAA receptors on membrane surface; these missing receptors are discovered intracellularly and contribute around 7% of all GABAA receptors.Citation115 Tehrani et al also demonstrated that these intracellular GABAA receptors located on clathrin-bound vesicles are uncoupled as benzodiazepine sites with reduced affinity and allosteric modulatory control over the receptor.Citation116–Citation118
Long-term benzodiazepine treatment caused a significant 83% increase in the number of GABAA receptors on clathrin-coated vesicles versus control. Although benzodiazepines are still able to bind at these sites, they produce no modulatory effect. The internalization also meant a 12% reduction of GABAA receptors located on synaptic membranes, as assessed by 3H-flunitrazepam after a 7-day benzodiazepine treatment.Citation118
This internalization of GABAA receptors at the synaptic membrane possibly contributes to the tolerance of benzodiazepines. Although benzodiazepine agonists are able to bind to intracellular GABA receptors, they produce little or no allosteric modulation. A recent examination of benzodiazepine allosteric uncoupling has shown that benzodiazepine site internalization is part of the uncoupling mechanism.Citation114 This finding is likely the observation of benzodiazepine sites located on clathrin vesicles.
Considerable evidence has been compiled recently underlying a complete and complex molecular mechanism of phosphorylation and posttranslational modifications of GABAA as a response to benzodiazepines that possibly contribute to the observed uncoupling and tolerance building mechanism.Citation119 These complex molecular mechanisms of posttranslational modifications of the GABAA receptor resulting from palmitoylation, ubiquitination, and especially phosphorylation, are believed to dictate the role in regulating the recycling of GABAA receptors through different protein kinases and ultimately impact inhibitory currents.Citation18,Citation84,Citation119
cAMP-dependent protein kinase A (PKA) has been identified to lead the changes and alterations of GABAA receptor functioning of CA1 pyramidal cells within the hippocampus after long-term flurazepam administration.Citation120 Further evidence accumulated over the importance of PKA in the formation of benzodiazepine tolerance showed that mutations to a single PKA phosphorylation site prevented uncoupling, even after chronic diazepam treatment.Citation121
As compared to wild-type mice, a wide range of transcripts that are thought to contribute to the neuroplastic mechanisms of tolerance remained unchanged in α1(H101R) point mutated mice after diazepam administration.Citation122 Transcripts changes such as brain-derived neurotrophic factor and calcium/calmodulin-dependent kinase II play important roles in synaptic plasticity; α1(H101R) mice did not produce transcript changes after diazepam treatment.Citation122 This is direct evidence regarding the involvement of α1 containing GABAA receptors in the role of benzodiazepine tolerance. It agrees with earlier studies wherein α1 subtype inactive benzodiazepines imidazenil and TPA023 consistently failed to show anticonvulsant tolerance in chronic dosing in mice and monkeys.Citation123–Citation129
Interestingly, there seems to be almost no downregulation of GABAA receptors despite long-term heavy administration of imidazenil as compared to diazepam.Citation83 Future studies could compare the uncoupling and posttranslational modification differences between traditional benzodiazepine agonists such as diazepam and α1 subtype ineffective compounds like TPA023 and imidazenil.
Conclusion
Although partial agonism at α1 containing GABAA receptors appears to reduce abuse potential, it appears insufficient. Bretazenil and etizolam which showed reduced abuse potential in animal models do not keep their promise.Citation129,Citation130 When the bretazenil dose increased from 2 to 4 mg in humans, users reported liking the drug. While etizolam has seen popular recreational use, it is sold online as a research chemical capable of inducing euphoria.Citation131 As a result, if clinical effects can be achieved without the involvement of α1 subtype, it should be avoided in future drug design of benzodiazepines and similar compounds.
As already discussed, both α2 and α3 containing GABAA receptors and their involvement in anxiety suppressing effects of benzodiazepines are critical. This is because most current models have characterized α2 containing GABAA receptors as the sole mediator of anxiety in benzodiazepines.Citation35–Citation39 As highlighted in the model presented in this review, the search for a selective anxiolytic should not be constrained to α2 selective.Citation29–Citation41
Benzodiazepine tolerance is complicated and appears to result from a combination of various factors. Complex mechanisms involving uncoupling, intracellular trafficking, post-translational modifications of GABAA receptors all appear to contribute toward benzodiazepine tolerance.Citation89,Citation119 Various studies have also suggested the involvement of other neurotransmitters, especially adenosine and glutamate, during the tolerance and withdrawal effects of benzodiazepines.Citation92–Citation105
The efficacy of benzodiazepines may also play a role in tolerance as full agonists produce more tolerance than partial agonists.Citation107
In the light of current evidence, α1 dormant, α2, α3, and α5 subtype partial agonists not only possess low abuse potential, but are also low or devoid of tolerance building. There is evidence that α1 containing GABAA receptors directly contribute to the downstream effects of tolerance, because α1(H101R) mice have been shown to maintain expressions in neuroplasticity-coding transcripts after diazepam administration.Citation120 This perfectly agrees with data from animal studies regarding the lack of tolerance in α1 subtype inactive compounds such as TPA023B and imidazenil.Citation121–Citation126 Future drug discovery involving tranquilizers should look for partial agonists of α2, α3, and α5 containing GABAA receptors; Valium without its side effects is potentially achievable.
Disclosure
The authors report no conflicts of interest in this work.
References
- OlfsonMKingMSchoenbaumMBenzodiazepine use in the United StatesJAMA Psychiatry201572213614225517224
- DantzerRBehavioral effects of benzodiazepines: a reviewNeurosci Biobehav Rev1977127186
- ShaderRGreenblattDBalterMAppropriate use and regulatory control of benzodiazepinesJ Clin Pharmacol19913197817841687147
- SternbachLThe benzodiazepine storyJ Med Chem19792211734039
- OlsenRWamsleyJLeeRLomaxPBenzodiazepine/barbiturate/GABA receptor-chloride ionophore complex in a genetic model for generalized epilepsyAdv Neurol1986443653783010677
- HarrisIAddiction to barbiturates and the barbiturate abstinence syndromeAnn Intern Med195033110812115426096
- WolfBGrohmannRBiberDBrennePRütherEBenzodiazepine abuse and dependence in psychiatric inpatientsPharmacopsychiatry19892225460
- BustoUSellersENaranjoCCappellHSanchez-CraigMSimpkinsJPatterns of benzodiazepine abuse and dependenceAddiction19868118794
- PerraultGMorelESangerDZivkovicBLack of tolerance and physical dependence upon repeated treatment with the novel hypnotic zolpidemJ Pharmacol Exp Ther199226312983031403792
- Victorri-VigneauCDaillyEVeyracGJollietPEvidence of zolpidem abuse and dependence: results of the French Centre for Evaluation and Information on Pharmacodependence (CEIP) network surveyBr J Clin Pharmacol200764219820917324242
- KlepnerCLippaABensonDSanoMBeerBResolution of two biochemically and pharmacologically distinct benzodiazepine receptorsPharmacol Biochem Behav1979114457462523502
- AtackJRegional differences in the inhibition of mouse in vivo [3H] Ro 15-1788 binding reflect selectivity for α1 versus α2 and α3 subunit-containing GABAA receptorsNeuropsychopharmacology199920325526210063485
- SieghartWGABAA receptors: ligand-gated Cl− ion channels modulated by multiple drug-binding sitesTrends Pharmacol Sci199213124464501338138
- StudyRBarkerJDiazepam and (–)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neuronsProc Natl Acad Sci U S A19817811718071846273918
- SieghartWSperkGSubunit composition, distribution and function of GABA-A receptor subtypesCurr Top Med Chem20022879581612171572
- RudolphUCrestaniFBenkeDBenzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypesNature1999401675579680010548105
- FritschyJMohlerHGABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunitsJ Comp Neurol199535911541948557845
- VinkersCOlivierBMechanisms underlying tolerance after long-term benzodiazepine use: a future for subtype-selective receptor modulators?Adv Pharmacol Sci20122012119
- WaffordKGABAA receptor subtypes: any clues to the mechanism of benzodiazepine dependence?Curr Opin Pharmacol200551475215661625
- HindmarchITrickLRidoutFA double-blind, placebo- and positive-internal-controlled (alprazolam) investigation of the cognitive and psychomotor profile of pregabalin in healthy volunteersPsychopharmacology2005183213314316205916
- McKernanRRosahlTReynoldsDSedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtypeNat Neurosci20003658759210816315
- KnablJWitschiRHöslKReversal of pathological pain through specific spinal GABAA receptor subtypesNature2008451717633033418202657
- AutaJKadriuBGiustiPCostaEGuidottiAAnticonvulsant, anxiolytic, and non-sedating actions of imidazenil and other imidazo-benzodiazepine carboxamide derivativesPharmacol Biochem Behav201095438338920227434
- AtackJTPA023 [7-(1,1-Dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine], an agonist selective for α2- and α3-containing GABAA receptors, is a nonsedating anxiolytic in rodents and primatesJ Pharmacol Exp Ther2005316141042216183706
- HuangMRadadiaKMaconeBAuerbachSDattaSEffects of eszopi-clone and zolpidem on sleep–wake behavior, anxiety-like behavior and contextual memory in ratsBehav Brain Res20102101546620153782
- AtackJGABAA receptor subtype-selective efficacy: TPA023, an α2/α3 selective non-sedating anxiolytic and α5IA, an α5 selective cognition enhancerCNS Drug Rev20081412535
- ChengVMartinJErinMα5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidateJ Neurosci200626143713372016597725
- RoehrsTMerlottiLZorickFRothTSedative, memory, and performance effects of hypnoticsPsychopharmacology199411621301347862941
- LöwKCrestaniFKeistRMolecular and neuronal substrate for the selective attenuation of anxietyScience2000290548913113411021797
- DiasREvidence for a significant role of α3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepinesJ Neurosci20052546106821068816291941
- AtackJHutsonPCollinsonNAnxiogenic properties of an inverse agonist selective for α3 subunit-containing GABAA receptorsBr J Clin Pharmacol20051443357366
- NavarroJBurónEMartín-LópezMAnxiolytic-like activity of SB-205384 in the elevated plus-maze test in micePsicothema200618110010417296016
- CrestaniFMöhlerHRudolphUAnxiolytic-like action of diazepam: mediated by GABAA receptors containing the α2-subunitTrends Pharmacol Sci2001228403
- ReynoldsDMcKernanRDawsonGAnxiolytic-like action of diazepam: which GABAA receptor subtype is involved?Trends Pharmacol Sci2001228402
- RudolphUCrestaniFMöhlerHGABAA receptor subtypes: dissecting their pharmacological functionsTrends Pharmacol Sci200122418819411282419
- TanKRudolphULüscherCHooked on benzodiazepines: GABAA receptor subtypes and addictionTrends Neurosci201134418819721353710
- RudolphUKnoflachFBeyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypesNat Rev Drug Discov201110968569721799515
- SamardzicJSvob StracDBenzodiazepines and anxiety disorders: from laboratory to clinicNew Developments in Anxiety DisordersCroatia, RijekaInTech2016
- MirzaNMunroGThe role of GABA(A) receptor subtypes as analgesic targetsDrug News Perspect201023635136020697602
- McEownKTreitDα2 GABAA receptor sub-units in the ventral hippocampus and α5 GABAA receptor sub-units in the dorsal hippocampus mediate anxiety and fear memoryNeuroscience201325216917723962649
- MorrisHDawsonGReynoldsDAtackJStephensDBoth α2 and α3 GABAA receptor subtypes mediate the anxiolytic properties of benzodiazepine site ligands in the conditioned emotional response paradigmEur J Neurosci20062392495250416706856
- BaileyDTetzlaffJCookJHeXHelmstetterFEffects of hippocampal injections of a novel ligand selective for the α5β2γ2 subunits of the GABA/benzodiazepine receptor on Pavlovian conditioningNeurobiol Learn Mem200278111012071663
- CrestaniFKeistRFritschyJTrace fear conditioning involves hippocampal α5 GABAA receptorsProc Natl Acad Sci U S A200299138980898512084936
- DawsonGMaubachKACollinsonNAn inverse agonist selective for α5 subunit-containing GABAA receptors enhances cognitionJ Pharmacol Exp Ther200531631335134516326923
- RudolphUCrestaniFBenkeDBenzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypesNature1999401675579680010548105
- KadriuBGuidottiACostaEAutaJImidazenil, a non-sedating anticonvulsant benzodiazepine, is more potent than diazepam in protecting against DFP-induced seizures and neuronal damageToxicology2009256316417419111886
- CrestaniFLöwKKeistRMandelliMMöhlerHRudolphUMolecular targets for the myorelaxant action of diazepamMol Pharmacol200159344244511179437
- MilićMDivljakovićJRallapalliSvan LinnMTimićTCookJSavićMThe role of α1 and α5 subunit-containing GABAA receptors in motor impairment induced by benzodiazepines in ratsBehav Pharmacol201223219119722327019
- LicataSPlattDCookJVan LinnMRowlettJContribution of α1 subunit-containing γ-aminobutyric acid A (GABAA) receptors to motor-impairing effects of benzodiazepines in squirrel monkeysPsychopharmacology2008203353954619031072
- AtackJWaffordKStreetLMRK-409 (MK-0343), a GABAA receptor subtype-selective partial agonist, is a non-sedating anxiolytic in preclinical species but causes sedation in humansJ Psychopharmacol201025331432820147571
- AtorNAtackJHargreavesRBurnsHDawsonGReducing abuse liability of GABAA/benzodiazepine ligands via selective partial agonist efficacy at α1 and α2/3 subtypesJ Pharmacol Exp Ther2009332141619789360
- RowlettJPlattDLelasSAtackJDawsonGDifferent GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primatesProc Natl Acad Sci U S A2005102391592015644443
- FischerBAtackJPlattDReynoldsDDawsonGRowlettJContribution of GABAA receptors containing α3 subunits to the therapeutic-related and side effects of benzodiazepine-type drugs in monkeysPsychopharmacology2010215231131921190016
- AtackJHalletDJTyeSPreclinical and clinical pharmacology of TPA023B, a GABAA receptor α2/α3 subtype-selective partial agonistJ Psychopharmacol201025332934420156926
- PiantadosiSFrenchBPoeMSex-dependent anti-stress effect of an α5 subunit containing GABAA receptor positive allosteric modulatorFront Pharmacol2016744627920723
- Boeuf-CazouOBongueBAnsiauDMarquiéJLapeyre-MestreMImpact of long-term benzodiazepine use on cognitive functioning in young adults: the VISAT cohortEur J Clin Pharmacol201167101045105221494764
- MoldofskyHLueFMouslyCRoth-SchechterBReynoldsWThe effect of zolpidem in patients with fibromyalgia: a dose ranging, double blind, placebo controlled, modified crossover studyJ Rheumatol19962335295338832997
- NickollsSMaceHFishRA comparison of the α2/3/5 selective positive allosteric modulators L-838,417 and TPA023 in preclinical models of inflammatory and neuropathic painAdv Pharmacol Sci20112011112
- HeikkinenAMöykkynenTKorpiELong-lasting modulation of glutamatergic transmission in VTA dopamine neurons after a single dose of benzodiazepine agonistsNeuropsychopharmacology200934229029818563060
- CrestaniFMartinJMöhlerHRudolphUMechanism of action of the hypnotic zolpidem in vivoBr J Clin Pharmacol2000131712511254
- StephensDPistovcakovaJWorthingLAtackJDawsonGRole of GABAA α5-containing receptors in ethanol reward: the effects of targeted gene deletion, and a selective inverse agonistEur J Pharmacol20055261–324025016253225
- TanKBrownMLabouèbeGNeural bases for addictive properties of benzodiazepinesNature2010463728276977420148031
- EnginEBakhurinKSmithKNeural Basis of Benzodiazepine Reward: Requirement for α2 Containing GABAA Receptors in the Nucleus AccumbensNeuropsychopharmacology201239818051815
- ShindayNSawyerEFischerBReinforcing effects of compounds lacking intrinsic efficacy at α1 subunit-containing GABAA receptor subtypes in midazolam- but not cocaine-experienced rhesus monkeysNeuropsychopharmacology20123861006101423303046
- LaliveARudolphULüscherCTanKIs there a way to curb benzodiazepine addiction?Swiss Med Wkly2011141w1327722012428
- BustoUKaplanHZawertailoLSellersEPharmacologic effects and abuse liability of bretazenil, diazepam, and alprazolam in humansClin Pharmacol Ther19945544514638162672
- VashchinkinaEPanhelainenAVekovischevaOGABA site agonist gaboxadol induces addiction-predicting persistent changes in ventral tegmental area dopamine neurons but is not rewarding in mice or baboonsJ Neurosci201232155310532022496576
- HarveySFosterKMcKayPThe GABAA receptor α1 subtype in the ventral pallidum regulates alcohol-seeking behaviorsJ Neurosci20022293765377511978852
- KaminskiBVan LinnMCookJYinWWeertsEEffects of the benzodiazepine GABAA α1-preferring ligand, 3-propoxy-β-carboline hydrochloride (3-PBC), on alcohol seeking and self-administration in baboonsPsychopharmacology2012227112713623271191
- EilerWJuneHBlockade of GABAA receptors within the extended amygdala attenuates D2 regulation of alcohol-motivated behaviors in the ventral tegmental area of alcohol-preferring (P) ratsNeuropharmacology20075281570157917451754
- CooperSJBenzodiazepine–opiate antagonist interactions and reward processes: implications for drug dependencyNeuropharmacology19832245355386134251
- SolhiHMostafazadehBRezaKVHRezaGAShooshtarizadehABenefit effect of naloxone in benzodiazepines intoxication: findings of a preliminary studyHum Exp Toxicol201030753554020573655
- KangWStevenWMarleneWOverexpression of proenkephalin in the amygdala potentiates the anxiolytic effects of benzodiazepinesNeuropsychopharmacology2000221778810633493
- ConnollyJBoulterJHeinemannSα4-β2 and other nicotinic acetylcholine receptor subtypes as targets of psychoactive and addictive drugsBr J Clin Pharmacol19921053657666
- SherTChenYSharplesTJPhysiological roles of neuronal nicotinic receptors subtypes: new insights on the nicotinic modulation of neurotransmitter release, synaptic transmission and plasticityCurr Top Med Chem200343283297
- BuissonBBertrandDNicotine addiction: the possible role of functional upregulationTrends Pharmacol Sci200223313013611879680
- WilliamsMAdinoffBThe role of acetylcholine in cocaine addictionNeuropsychopharmacology20073381779179717928814
- RadaPHoebelBAcetylcholine in the accumbens is decreased by diazepam and increased by benzodiazepine withdrawal: a possible mechanism for dependencyEur J Pharmacol20055081–313113815680263
- DazziLMotzoCMairaGSannaASerraMBiggioGEnhancement of acetylcholine release by flumazenil in the hippocampus of rats chronically treated with diazepam but not with imidazenil or abecarnilPsychopharmacology199512121801858545523
- PeturssonHLaderMBenzodiazepine dependenceAddiction1981762133145
- TietzERosenbergHChiuTAutoradiographic localization of benzodiazepine receptor downregulationJ Pharmacol Exp Ther198623612842923001290
- GallagerDLakoskiJGonsalvesSRauchSChronic benzodiazepine treatment decreases postsynaptic GABA sensitivityNature1984308595474776322004
- ZhaoTChiuTRosenbergHReduced expression of gamma-aminobutyric acid type A/benzodiazepine receptor gamma 2 and alpha 5 subunit mRNAs in brain regions of flurazepam-treated ratsMol Pharmacol19944546576638183244
- PoisbeauPWilliamsSModyISilent GABAA synapses during flurazepam withdrawal are region-specific in the hippocampal formationJ Neurosci19971710346734759133372
- PesoldCCarunchoHImpagnatielloFTolerance to diazepam and changes in GABAA receptor subunit expression in rat neocortical areasNeuroscience19977924774879200730
- ArnotMDaviesMMartinIBatesonAGABAA receptor gene expression in rat cortex: differential effects of two chronic diazepam treatment regimesJ Neurosci Res200164661762511398186
- KalesAScharfMKalesJRebound insomnia: a new clinical syndromeScience1978201436010391041684426
- BrowneTPenryJBenzodiazepines in the treatment of epilepsy: a reviewEpilepsia19731432773104204764
- BatesonABasic pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawalCurr Pharm Des20028152111812247
- SchweizerEMaintenance drug treatment of panic disorderArch Gen Psychiatry199350151608422222
- CowleyDBenzodiazepine sensitivity in panic disorder: effects of chronic alprazolam treatmentNeuropsychopharmacology19951221471577779243
- RijnsoeverVCRequirement of α5-GABAA receptors for the development of tolerance to the sedative action of diazepam in miceJ Neurosci200424306785679015282283
- HawkinsMPravicaMRadulovackiMChronic administration of diazepam downregulates adenosine receptors in the rat brainPharmacol Biochem Behav19883023033082845443
- HawkinsMPanWStefanovichPRadulovackiMDesensitization of adenosine A2 receptors in the striatum of the rat following chronic treatment with diazepamNeuropharmacology19882711113111402849727
- KaplanGCotreauMGreenblattDEffects of benzodiazepine administration on A1 adenosine receptor binding in-vivo and ex-vivoJ Pharm Pharmacol19924487007031359103
- NarimatsuEAokiMInvolvement of the adenosine neuromodulatory system in the benzodiazepine-induced depression of excitatory synaptic transmissions in rat hippocampal neurons in vitroNeurosci Res1999331576410096472
- NarimatsuENiiyaTKawamataMNamikiAThe mechanisms of depression by benzodiazepines, barbiturates and propofol of excitatory synaptic transmissions mediated by adenosine neuromodulationMasui2006556684691 Japanese16780077
- TsudaMChibaYSuzukiTMisawaMUpregulation of NMDA receptor subunit proteins in the cerebral cortex during diazepam withdrawalEur J Pharmacol19983412–3R1R29543260
- TsudaMShimizuNYajimaYSuzukiTMisawaMHypersusceptibility to DMCM-induced seizures during diazepam withdrawal in mice: evidence for upregulation of NMDA receptorsNaunyn Schmiedebergs Arch Pharmacol199835733093159550303
- BonavitaCFerreroACeresetoMVelardezMRubioMWikinskiSAdaptive changes in the rat hippocampal glutamatergic neurotrans-mission are observed during long-term treatment with lorazepamPsychopharmacology2003166216316712545333
- SongJShenGGreenfieldLTietzEBenzodiazepine withdrawal-induced glutamatergic plasticity involves up-regulation of GluR1-containing α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in hippocampal CA1 neuronsJ Pharmacol Exp Ther2007322256958117510319
- FileSFernandesCDizocilpine prevents the development of tolerance to the sedative effects of diazepam in ratsPharmacol Biochem Behav19944748238268029250
- KoffJPritchardGGreenblattDMillerLThe NMDA receptor competitive antagonist CPP modulates benzodiazepine tolerance and discontinuationPharmacology19975552172279399331
- FernandesCFileSDizocilpine does not prevent the development of tolerance to the anxiolytic effects of diazepam in ratsBrain Res199981524314349878864
- SteppuhnKTurskiLDiazepam dependence prevented by glutamate antagonistsProc Natl Acad Sci U S A19939014688968938341715
- JacobTMichelsGSilayevaLHaydonJSuccolFMossSBenzodiazepine treatment induces subtype-specific changes in GABAA receptor trafficking and decreases synaptic inhibitionProc Natl Acad Sci U S A201210945185951860023091016
- HernandezTHeningerCWilsonMGallagerDRelationship of agonist efficacy to changes in GABA sensitivity and anticonvulsant tolerance following chronic benzodiazepine ligand exposureEur J Pharmacol198917031451552515976
- GonsalvesSGallagerDPersistent reversal of tolerance to anticonvulsant effects and GABAergic subsensitivity by a single exposure to benzodiazepine antagonist during chronic benzodiazepine administrationJ Pharmacol Exp Ther1988244179833121850
- KleinRMasciaMHarknessPHadinghamKWhitingPHarrisRRegulation of allosteric coupling and function of stably expressed gamma-aminobutyric acid (GABA)A receptors by chronic treatment with GABAA and benzodiazepine agonistsJ Pharmacol Exp Ther19952743148414927562525
- MillerLHellerJLumpkinMWeillCGreenblattDShaderRAugmentation of GABAA receptor function by chronic exposure to GABA-neutral and GABA-negative benzodiazepine ligands in cultured cortical neuronsBiochem Pharmacol1990406133713442169744
- LugoboniFLeoneRWhat is stopping us from using flumazenil?Addiction20121077135922509854
- SavicIFeasibility of reversing benzodiazepine tolerance with flumazenilLancet199133787341331371670787
- TietzEChiuTRosenbergHRegional GABA/benzodiazepine receptor/chloride channel coupling after acute and chronic benzodiazepine treatmentEur J Pharmacol1989167157652476326
- GutiérrezMFerreriMGravielleMGABA-induced uncoupling of GABA/benzodiazepine site interactions is mediated by increased GABAA receptor internalization and associated with a change in subunit compositionNeuroscience201425711912924215979
- TehraniMBarnesEAgonist-dependent internalization of gamma-aminobutyric acid A/benzodiazepine receptors in chick cortical neuronsJ Neurochem1991574130713121654391
- TehraniMBarnesEIdentification of GABAA/benzodiazepine receptors on clathrin-coated vesicles from rat brainJ Neurochem1993605175517618386222
- TehraniMBarnesESequestration of γ-aminobutyric acid A receptors on clathrin-coated vesicles during chronic benzodiazepine administration in vivoJ Pharmacol Exp Ther199728313843909336347
- TehraniMBaumgartnerBBarnesEClathrin-coated vesicles from bovine brain contain uncoupled GABAA receptorsBrain Res1997761–2195203
- GravielleMActivation-induced regulation of GABAA receptors: is there a link with the molecular basis of benzodiazepine tolerance?Pharmacol Res20161099210026733466
- LillySZengXTietzERole of protein kinase A in GABAA receptor dysfunction in CA1 pyramidal cells following chronic benzodiazepine treatmentJ Neurochem200385498899812716430
- AliNOlsenRChronic benzodiazepine treatment of cells expressing recombinant GABAA receptors uncouples allosteric binding: studies on possible mechanismsJ Neurochem200879511001108
- HuopaniemiLKeistRRandolphACertaURudolphUDiazepam-induced adaptive plasticity revealed by α1 GABAA receptor-specific expression profilingJ Neurochem20048851059106715009662
- AutaJImpagnatielloFKadriuBGuidottiACostaEImidazenil: a low efficacy agonist at α1- but high efficacy at α5-GABAA receptors fail to show anticonvulsant cross tolerance to diazepam or zolpidemNeuropharmacology200855214815318555494
- ZanottiAMariotRContarinoALipartitiMGiustiPLack of anticonvulsant tolerance and benzodiazepine receptor down regulation with imidazenil in ratsBr J Clin Pharmacol19961174647652
- GhianiCSerraMMotzoCChronic administration of an anti-convulsant dose of imidazenil fails to induce tolerance of GABAA receptor function in miceEur J Pharmacol199425432993028013567
- KadriuBGocelJLarsonJAbsence of tolerance to the anticonvulsant and neuroprotective effects of imidazenil against DFP-induced seizure and neuronal damageNeuropharmacology20116181463146921903116
- AutaJGuidottiACostaEImidazenil prevention of alprazolam-induced acquisition deficit in patas monkeys is devoid of toleranceProc Natl Acad Sci U S A20009752314231910696114
- VinkersCvan OorschotRNielsenEGABAA receptor α subunits differentially contribute to diazepam tolerance after chronic treatmentPLoS One201278e4305422912786
- RundfeldtCWlaźPHönackDLöscherWAnticonvulsant tolerance and withdrawal characteristics of benzodiazepine receptor ligands in different seizure models in mice. Comparison of diazepam, bretazenil and abecarnilJ Pharmacol Exp Ther199527526937027473156
- SannaEBusoneroFTalaniGLow tolerance and dependence liabilities of etizolam: molecular, functional, and pharmacological correlatesEur J Pharmacol20055191–2314216107249
- O’ConnellCSadlerCToliaVLyBSaitmanAFitzgeraldROverdose of etizolam: the abuse and rise of a benzodiazepine analogAnn Emerg Med201565446546625805032
