Abstract
Evidence has suggested that dysregulation of the dopaminergic system may play a significant role in the pathogenesis of attention-deficit/hyperactivity disorder (ADHD) in children. Manganese, a neurotoxicant, has been reported to exert its neurotoxicity by affecting the dopaminergic system. However, the association between peripheral manganese levels and ADHD has not been comprehensively reviewed. This study aimed to investigate the association between peripheral manganese levels and ADHD in children. An electronic search was performed on databases including PubMed, ProQuest, ClinicalKey, Cochrane Library, ClinicalTrials.gov, Embase, Web of Science, and ScienceDirect with last search on March 25th, 2018. As per the inclusion criteria, human observational studies investigating peripheral manganese levels in children with ADHD and controls were included. The meta-analysis was performed using a random-effects model, and possible confounders were examined by subgroup analysis. In total, four articles with 175 ADHD children and 999 controls were recruited. The manganese levels were significantly higher in ADHD children than in controls (p=0.033), when studies investigating blood levels and those investigating hair levels were included. However, when only studies investigating blood levels were included, there was no significant difference between ADHD children and controls (p=0.076). Our results support higher peripheral manganese levels in children diagnosed with ADHD than those in controls. Further primary studies are needed to clarify this association.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is defined as “a persistent pattern of inattention and/or hyperactivity-impulsivity that interferes with functioning or development”.Citation1 ADHD can be categorized into three subtypes: predominantly inattentive, predominantly hyperactive-impulsive, and combined.Citation2 The prevalence of ADHD is estimated to be 5.29% in children worldwide.Citation3 Inattention refers to wandering off task, lacking persistence, having difficulty sustaining focus, and being disorganized and is not due to defiance or lack of comprehension. Impulsivity refers to excessive motor activity when it is not appropriate, or excessive fidgeting, tapping, or talkativeness.Citation1 Further, it may cause impairments in social communication and functional limitations of effective communication, social participation, or academic achievement.Citation1
Although the exact pathogenesis of ADHD remains unknown, it has been found that people with ADHD have smaller brain volumes of specific structures, such as the prefrontal cortex and basal ganglia,Citation2 or have higher exposure to environmental pollution, such as leadCitation4 or polybrominated diphenyl ethers.Citation5 One of the most widely postulated pathogeneses of ADHD is the “dopamine hypothesis”,Citation2 attributing ADHD to a dysregulation of the dopaminergic system. A review article has shown that ADHD patients have decreased availability of dopamine receptor isoforms and increased dopamine transporter (DAT) binding compared with controls.Citation6 The most widely used medication for ADHD, methylphenidate with its derivatives, is postulated to have its effects by blocking DAT and promoting dopamine release.Citation6
In previous studies, manganese (elemental symbol: Mn), an environmental heavy metal element, has been found to cause neurologic toxicity to humans.Citation7,Citation8 It can be present in contaminated water, pesticides, batteries, and glass, among others, and then be ingested by humans.Citation9–Citation11 High concentration of manganese has been found in the basal ganglia of manganese-intoxicated rats.Citation12 After exposure to manganese, decreased long-term dopamine efflux in ratsCitation13 and attenuated dopaminergic neurotransmission in humansCitation14 have also been reported. In epidemiological studies, higher levels of manganese in the hair and blood samples from children with ADHD have been found.Citation15,Citation16 Another study has shown decreased intelligence quotient (IQ) in children with increased hair concentration of manganese.Citation17 There have been no studies investigating the postmortem levels of in vivo manganese in ADHD patients found in the literature, by using the same search strategy as the current meta-analysis.
Therefore, as a neurotoxin affecting dopaminergic neurotransmission, manganese may play a role in the pathogenesis of ADHD in those exposed to the metal. Among the four datasets in the literature studying “blood” manganese levels, twoCitation16,Citation18 showed a significantly positive association between blood manganese level and ADHD, while the other twoCitation18,Citation19 revealed insignificant results. Three of the four datasets used case–control design, while the other dataset used cross-sectional design. Another case–control study showed a trend of positive association between “hair” manganese level and ADHD without statistical significance.Citation20 Difference in study design (case–control vs cross-sectional), source of manganese level (ie, blood vs hair), and population (described in the “Discussion” section) may contribute to the heterogeneity of the study results. Although there are two previous meta-analysesCitation17,Citation21 mentioning the possible relationship between manganese exposure and ADHD in children, they did not directly calculate the association. Therefore, the aim of the current study is to summarize the literature for the possible association between peripheral manganese levels and ADHD in children via a thorough meta-analysis.
Methods
We followed the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelinesCitation22 (). This preliminary meta-analysis followed an a priori defined but unpublished protocol. The Institutional Review Board of the Tri-Service General Hospital (TSGHIRB: B-105-12) approved the study.
Eligibility criteria
Inclusion criteria were the following: (a) observational studies, including a cohort or cross-sectional study design comparing peripheral levels of manganese in children with ADHD and controls; and (b) trials in humans. We excluded preclinical studies, review articles, meeting abstracts, and non-human studies; furthermore, to increase the specificity of the effect of manganese on ADHD, we excluded those studies that collected manganese sample sources further away from the diagnosis of ADHD (eg, collecting manganese when ADHD children were just delivered).
Search strategy and study selection
Two well-trained authors (JH Shih and PT Tseng) performed a systematic literature search from inception until August 30th, 2017 and updated search at March 25th, 2018. The following keywords were used in our search on PubMed, ProQuest, ClinicalKey, Cochrane Library, ScienceDirect, and ClinicalTrials.gov platforms: “(Manganese OR Mn) AND (ADHD OR attention-deficit/hyperactivity disorder)”. A similar search was conducted in Embase and Web of Science databases. The reference lists of included articles and recent reviews were also hand-searched to identify additional articles.Citation17,Citation21,Citation23–Citation25
Two authors (JH Shih and PT Tseng) independently screened the titles and abstracts of all retrieved results for potential eligibility. Both authors reviewed the full text of potentially eligible papers, and a final list of included studies was achieved. Any inconsistencies were resolved through discussion with a third reviewer (MK Wu).
Data extraction
The primary outcomes were the difference in manganese levels in children with ADHD compared to control groups (calculated as Hedges’ g statistic and corresponding 95% CIs and p-values). We did not choose difference in mean as the effect sizes (ESs) of our primary outcome due to presumed different units used in each study. The control groups were defined as those without ADHD. In addition, due to potential confounding effect of specific medication (eg, methylphenidate) on manganese,Citation18 we did not include the data of manganese level in children with ADHD who had been clearly described as receiving such medication. If data were available, we prefer to choose control groups as healthy (asymptomatic) individuals. When data were not available from the included studies, we contacted the primary authors to request the original data. We contacted the authors via email on two occasions if required (a second email was sent a week later if no response was received following an initial email). If there were no relevant data in the paper regarding manganese levels, wherever possible, we attempted to use another compatible statistical parameter (eg, p-value and sample sizes) to estimate the ESs according to the protocol in the Comprehensive Meta-Analysis manuals and guidance on the Comprehensive Meta-Analysis website (https://www.meta-analysis.com/downloads/Meta-analysis%20Converting%20among%20effect%20sizes.pdf) to covert and pool the ESs into Hedges’ g.
The variables of interest included manganese levels, mean age, gender distribution (in the form of % females), mean body mass index, cognitive performance (in the form of mean IQ), parental tobacco smoking and alcohol consumption, and the type of assay used for detection of manganese levels.
Methodological quality appraisal
We used the modified Newcastle–Ottawa Scale (NOS) to evaluate the quality of the included studies. In brief, the modified NOS was based on a version previously used in a meta-analysis study published in British Journal of Psychiatry in 2013, and score ranges from 0 to 6 ().Citation26
Meta-analysis procedure
Given the anticipated heterogeneity in the basic population of study, we conducted the meta-analyses with a random-effects model rather than a fixed-effects one.Citation27 As aforementioned, the primary ES was estimated as Hedges’ g with 95% CI to compare manganese levels in children with ADHD and controls. Subgroup analyses were conducted to determine whether results differed as we grouped them according to the sample sources (eg, blood, hair). We performed subgroup analyses whenever data from at least two independent data-sets were available. In addition, to overcome the limitation of the meta-analysis that we could not adjust the potential confounding effect by clinical variables, we collected the adjusted OR provided by the recruited articles to calculate the pooled adjusted OR.
Sensitivity test, heterogeneity, publication bias, and meta-regression
We performed sensitivity test with “one-study removal” test to evaluate if the results of the meta-analysis were obtained from any outliers within the recruited studies or not.Citation28 Heterogeneity was assessed by the Cochran Q test and its corresponding p-value.Citation29 The I2 statistic was interpreted as the proportion of heterogeneity a study estimates that is due to heterogeneity.Citation30 We performed meta-regression analyses with unrestricted maximum likelihood random effects when data on each potential moderator were provided by at least five different studies. We assessed publication bias via the inspection of funnel plotsCitation31 and with the Egger’s regression test.Citation32 When evidence of publication bias was found, the Duval and Tweedie’s trim-and-fill procedure, which is a validated model to estimate an ES, was employed.Citation33 The current meta-analysis was conducted using the Comprehensive Meta-Analysis software, version 3 (BioStat, Frederick, MD, USA). The threshold for statistical significance was set at a two-tailed p-value <0.05.
Results
Study selection
summarizes study selection process. In brief, a total of 22 studies entered the full-text review stage. Eighteen articles were excluded for various reasons (). A list of excluded articles is presented in . In total, four articles met the inclusion criteria, the details of which are summarized in .Citation16,Citation18–Citation20
Figure 1 The flowchart of meta-analysis.
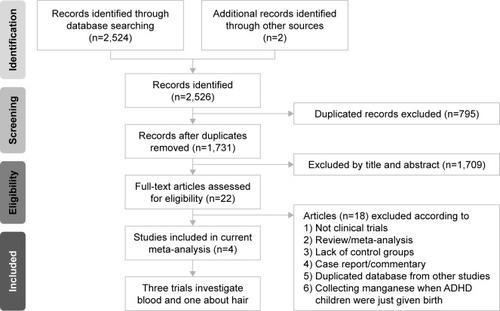
Table 1 Characteristics of the recruited studies
Table 2 Comparison of previous and current meta-analyses
Among the included studies, four provided comparison of the peripheral manganese levels in children with ADHD and controls (children with ADHD: n=175, mean age=8.8, mean female proportion=16.0%; controls without ADHD: n=999, mean age=9.1, mean female proportion=45.9%).Citation16,Citation18–Citation20 Two of them provided the data of adjusted OR for the association between manganese levels and ADHD.Citation16,Citation20
Characteristics and methodological quality of included studies
Among the recruited studies, the diagnosis of ADHD was made according to widely acceptable criteria, including the Diagnostic and Statistical Manual of Mental Disorders (DSM)Citation16,Citation18,Citation20 or Diagnostic Interview Schedule for Children Version IV.Citation19 In addition, the sample sources ranged from peripheral bloodCitation16,Citation18,Citation19 to hair sample.Citation20 The assays used to detect manganese levels included inductively coupled plasma mass spectrometry and graphite furnace atomic absorption spectrometry (). Regarding the methodological quality of included studies, the average NOS score was 15.5 with a SD of 1.0 ().
Meta-analysis of overall differences in manganese levels in children with ADHD and controls without ADHD
Overall
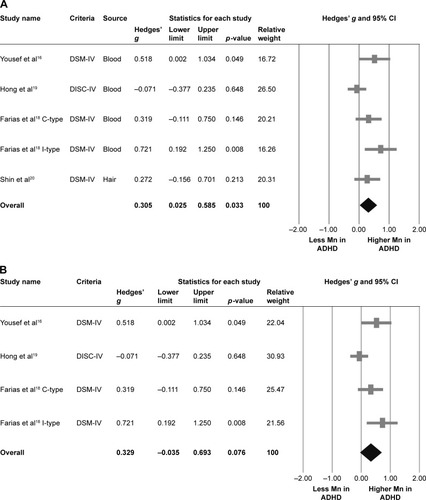
Four articles provided five datasets in this part of meta-analysis.Citation16,Citation18–Citation20 The meta-analysis suggested that overall manganese levels, either in blood or in hair, in children diagnosed with ADHD were significantly higher than those in controls (k=5, Hedges’ g=0.305, 95% CI=0.025–0.585, p=0.033) () without significant evidence of heterogeneity (Q value=8.419, df=4, I2=52.486%, p=0.077, tau=0.229), but significant publication bias via inspection of funnel plot and Egger’s regression was found (t=10.525, df=3, p=0.002). The significant results of the meta-analysis changed to insignificant after refilling two potential missing data to the left side of means according to Duval and Tweedie’s trim-and-fill test (Hedges’ g=0.145, 95% CI=−0.140 to 0.430).
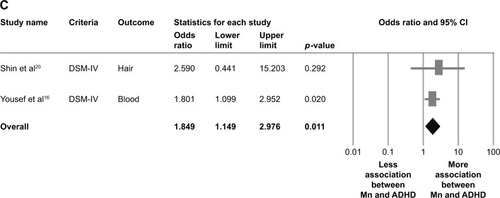
Figure 2 Meta-analysis of (A) overall manganese levels in ADHD children and controls, (B) blood manganese levels in ADHD children and controls, and (C) pooled adjusted OR for the association between manganese levels and ADHD.
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; C-type, combined type; DISC-IV, Diagnostic Interview Schedule for Children Version IV; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; I-type, inattention type.
Sensitivity test
The significant results of the meta-analysis changed to insignificant after removing any one of the recruited studies, which might be due to smaller sample sizes after removal of one of the recruited studies. However, the significant results of the meta-analysis changed to more predominantly significant after removing the data from Hong et al (Hedges’ g=0.425, 95% CI=0.191–0.660, p<0.001).Citation19
Meta-regression
The meta-regression could be performed only on association between ESs and mean age due to lack of data. To be specific, there was no significant association between ESs and mean age (p=0.656).
Meta-analysis of differences in manganese levels in children with ADHD and controls without ADHD in blood samples
Blood manganese
Three articles provided four datasets in this part of meta-analysis.Citation16,Citation18,Citation19 The meta-analysis suggested that the blood manganese levels in children diagnosed with ADHD and controls without ADHD were not significantly different, but a trend of higher blood manganese levels in ADHD children compared to controls was observed (k=4, Hedges’ g=0.329, 95% CI=−0.035 to 0.693, p=0.076) () with significant evidence of heterogeneity (Q value=8.394, df=3, I2=64.260%, p=0.039, tau=0.295) and significant publication bias via inspection of funnel plot and Egger’s regression (t=9.335, df=2, p=0.011). However, the results of the meta-analysis did not change after refilling according to Duval and Tweedie’s trim-and-fill test.
Sensitivity test
The insignificant results of the meta-analysis changed to significant after removing the data from Hong et al (Hedges’ g=0.491, 95% CI=0.210–0.771, p=0.001).
Meta-regression
All the meta-regression procedures were not performed because there were less than five datasets.
Meta-analysis of differences in manganese levels in children with ADHD and controls without ADHD in hair samples
Hair manganese
Only one article provided one dataset in this part of meta-analysis.Citation20 Therefore, the meta-analysis was not done due to lack of enough datasets. However, in the research results according to the description in the article by Shin et al,Citation20 the authors indicated that the proportion of abnormal manganese levels, either higher or lower, in children with ADHD was significantly higher than those in controls (p=0.039).
Meta-analysis of overall adjusted OR for the association between manganese levels and diagnosis of ADHD
Two articles provided two datasets in this part of meta-analysis.Citation16,Citation20 The meta-analysis of the adjusted ORs indicated significant association between manganese levels and the diagnosis of ADHD (k=2, adjusted OR=1.849, 95% CI=1.149–2.976, p=0.011) () without significant evidence of heterogeneity (Q value=0.150, df=1, I2<0.001%, p=0.698, tau<0.001).
Discussion
To the best of our knowledge, this is the first meta-analysis to investigate the relationship between peripheral manganese levels and ADHD in children. The results of the current meta-analysis showed significantly higher overall (ie, in blood or hair) peripheral manganese levels in children diagnosed with ADHD than those in controls, with no significant heterogeneity. Among the four included studies with five datasets in total, our results are consistent with two datasets,Citation16,Citation18 but not with the other three.Citation18–Citation20 However, among the three studies mentioned,Citation18–Citation20 twoCitation18,Citation20 also showed a trend of positive association (p=0.146 and 0.213, respectively), though not significant, which may be due to small number of cases. Sensitivity test showed that the results became insignificant after removing any one of the included studies, which may also be due to small number of cases. Subgroup analysis showed a trend of higher “blood” manganese levels in ADHD children than in controls, though not significant (p=0.076). However, the results changed to significant after removing the data from Hong et al.Citation19 The change from insignificance to significance may have been due to the difference in the study designs. Among the four studies included in the current meta-analysis, the study by Hong et alCitation19 used cross-sectional design, while the other three studiesCitation16,Citation18,Citation20 used case–control design. Moreover, the study by Hong et alCitation19 used Diagnostic Interview Schedule for Children Version IV, while the other studies used DSM criteria.Citation16,Citation18,Citation20
Although the meta-analysis was not done in the “hair” subgroup due to the availability of only one dataset, the result of this article showed that the hair manganese levels were significantly higher in ADHD children than in controls. To overcome the potential confounding effects, we performed a meta-analysis of overall adjusted ORs, which revealed a significantly positive association (adjusted OR=1.849, p=0.011) between manganese levels and ADHD in children, with no significant heterogeneity.
A comparison of the two previous meta-analysesCitation17,Citation21 mentioning the association between manganese and ADHD and the current study is summarized in . In the two studies, the one by Rodríguez-Barranco et alCitation17 calculated only the association between manganese exposure and IQ, and the other by Scassellati et alCitation21 did not perform a meta-analysis for manganese exposure.
Since “dopamine hypothesis”Citation2 is currently the most widely postulated etiologic scheme for ADHD, disrupted dopamine neurotransmission caused by manganese exposure found in several previous studiesCitation13,Citation14 has prompted us to perform this study. Our study results also support the positive association between peripheral manganese levels and ADHD. Although there have been no studies investigating the effect of removing excessive manganese from children with ADHD, the results of our current study suggest that this treatment approach may be promising.
Because manganese levels in children (biological monitoring) are affected by environmental manganese levels, such as in soil, water, and food, it would be difficult to thoroughly investigate. Therefore, we chose blood and hair levels as an indicator of internal dose.
The current study has several limitations. First, although our main results showed higher manganese levels in children diagnosed with ADHD than those in controls, significant publication bias and heterogeneity were found. Further, the results changed to insignificant after refilling two potential missing data by using Duval and Tweedie’s trim-and-fill test (Hedges’ g=0.145, 95% CI=−0.140 to 0.430). The publication bias may be overcome when more primary researches are published. The heterogeneity might be due to the difference in study designs. Among the four included studies, the study by Hong et alCitation19 used cross-sectional design, while the other three studiesCitation16,Citation18,Citation20 used matched case–control design. Another source of heterogeneity may have come from the fact that Farias et alCitation18 had two datasets of different diagnostic criteria, which were ADHD-combined type (C-type) and ADHD-inattentive type (I-type), respectively. Further, the four included studies investigated different populations: in terms of countries, for example, their subjects were from United Arab Emirates,Citation16 Brazil,Citation18 and Republic of Korea.Citation19,Citation20 Second, due to the small number of eligible studies, we had to combine the results from blood and hair samples, which could be a source of bias. Third, there were only two datasets provided with adjusted ORs, so the potential confounding factors may not have been addressed well enough. Fourth, due to difficulties in experimental designing and ethical concerns, few studiesCitation34,Citation35 provided central nervous system (CNS) concentrations of manganese, and none of them studied the relations between CNS manganese concentrations and ADHD. Although CNS concentrations are likely to provide more accurate information for manganese neurotoxicity, only blood or hair concentrations were available for this meta-analysis. Fifth, although eligible articles studied manganese levels in blood and hair, there have been no well-validated biomarkers for manganese exposure in children.Citation36 Moreover, hair samples are susceptible to exogenous contamination.Citation36 Sixth, although we provided evidences of higher manganese levels in ADHD children than in controls, we could not provide further detailed information about “to what extent” the manganese levels were higher than controls. Because the peripheral samples of manganese and method of detection in each included study were different from the others, we could not directly count and pool the difference in mean manganese levels between ADHD children and controls in each study. Seventh, although two of the studies provided information about ADHD subtype,Citation16,Citation18 there was still limited information about the correlation between peripheral manganese levels and ADHD subtype. Therefore, we could not derive further evidence about the correlation between peripheral manganese levels and ADHD subtype. Finally, because there had been limited articles included in the current meta-analysis, the subjects distribution was as restricted as only from three countries, including United Arab Emirates,Citation16 Brazil,Citation18 and Republic of Korea.Citation19,Citation20 Therefore, when clinicians apply our results into clinical practice, they should pay more attention about the applicability due to limited number of countries included and regional bias.
Conclusion
The results of this meta-analysis support higher peripheral manganese levels in children diagnosed with ADHD than those in controls. Further studies are warranted to replicate these findings, especially in other countries and in different ADHD subtypes, and to quantify the degree of the association for it to be useful in clinical settings.
Supplementary materials
Figure S1 Whole flowchart of the meta-analysis.
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; N/A, not available.
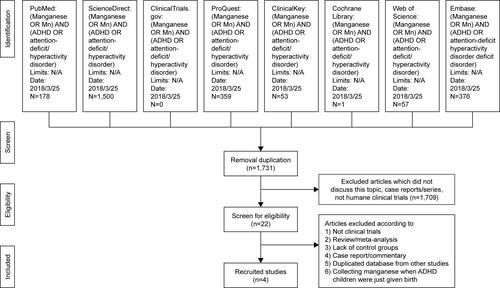
Table S1 Results of Newcastle–Ottawa Scale scores of recruited studies
Table S2 Excluded studies and reasons
References
- ShinDWKimEJLimSWShinYCOhKSKimEJAssociation of hair manganese level with symptoms in attention-deficit/hyperactivity disorderPsychiatry Investig20151216672
- HongSBKimJWChoiBSBlood manganese levels in relation to comorbid behavioral and emotional problems in children with attention-deficit/hyperactivity disorderPsychiatry Res20142201–241842525064383
- YousefSAdemAZoubeidiTKosanovicMMabroukAAEapenVAttention deficit hyperactivity disorder and environmental toxic metal exposure in the United Arab EmiratesJ Trop Pediatr201157645746021300623
- FariasACCunhaABenkoCRManganese in children with attention-deficit/hyperactivity disorder: relationship with methylphenidate exposureJ Child Adolesc Psychopharmacol201020211311820415606
- KonstantopoulosKVogazianosPThodiCNikopoulou-SmyrniPA normative study of the Children’s Color Trails Test (CCTT) in the Cypriot populationChild Neuropsychol201521675175824898762
- HuDWangYXChenWJAssociations of phthalates exposure with attention deficits hyperactivity disorder: a case-control study among Chinese childrenEnviron Pollut201722937538528614761
- KonradKGauggelSSchurekJCatecholamine functioning in children with traumatic brain injuries and children with attention-deficit/hyperactivity disorderBrain Res Cogn Brain Res200316342543312706222
- HanJYKwonHJHaMThe effects of prenatal exposure to alcohol and environmental tobacco smoke on risk for ADHD: a large population-based studyPsychiatry Res20152251–216416825481018
- Rodríguez-BarrancoMLacasañaMAguilar-GarduñoCAssociation of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysisSci Total Environ2013454–455562577
- ScassellatiCBonviciniCFaraoneSVGennarelliMBiomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analysesJ Am Acad Child Adolesc Psychiatry2012511010031019.e2023021477
- ZengXXuXBoezenHMHuoXChildren with health impairments by heavy metals in an e-waste recycling areaChemosphere201614840841526829309
- GrandjeanPLandriganPJNeurobehavioural effects of developmental toxicityLancet Neurol201413333033824556010
- LucchiniRPlacidiDCagnaGManganese and developmental neurotoxicityAdv Neurobiol201718133428889261
- HongSBImMHKimJWEnvironmental lead exposure and attention deficit/hyperactivity disorder symptom domains in a community sample of South Korean school-age childrenEnviron Health Perspect2015123327127625280233
- BouchardMLaforestFVandelacLBellingerDMerglerDHair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap waterEnviron Health Perspect20071151122127
- LucchiniRGZoniSGuazzettiSInverse association of intellectual function with very low blood lead but not with manganese exposure in Italian adolescentsEnviron Res2012118657122925625
- ChanTJGutierrezCOgunseitanOAMetallic burden of deciduous teeth and childhood behavioral deficitsInt J Environ Res Public Health20151266771678726084001
- EricsonJECrinellaFMClarke-StewartKAPrenatal manganese levels linked to childhood behavioral disinhibitionNeurotoxicol Teratol200729218118717079114
- RucklidgeJJohnstoneJHarrisonRBoggisAMicronutrients reduce stress and anxiety in adults with attention-deficit/hyperactivity disorder following a 7.1 earthquakePsychiatry Res2011189228128721802745
- BhangSYChoSCKimJWRelationship between blood manganese levels and children’s attention, cognition, behavior, and academic performance – a nationwide cross-sectional studyEnviron Res201312691623790803
- LiuWHuoXLiuDZengXZhangYXuXS100β in heavy metal-related child attention-deficit hyperactivity disorder in an informal e-waste recycling areaNeurotoxicology20144518519125451971
- OdeARylanderLGustafssonPManganese and selenium concentrations in umbilical cord serum and attention deficit hyperactivity disorder in childhoodEnviron Res201513737338125601741
Disclosure
The authors report no conflicts of interest in this work.
References
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders5th edArlington, VAAmerican Psychiatric Publishing2013
- KliegmanRBehrmanRENelsonWENelson Textbook of Pediatrics20th edAtlanta, GAElsevier2016
- PolanczykGde LimaMSHortaBLBiedermanJRohdeLAThe worldwide prevalence of ADHD: a systematic review and metaregression analysisAm J Psychiatry2007164694294817541055
- HuangSHuHSánchezBNChildhood blood lead levels and symptoms of attention deficit hyperactivity disorder (ADHD): a cross-sectional study of Mexican childrenEnviron Health Perspect2016124686887426645203
- LamJLanphearBPBellingerDDevelopmental PBDE exposure and IQ/ADHD in childhood: a systematic review and meta-analysisEnviron Health Perspect2017125808600128799918
- CorteseSThe neurobiology and genetics of attention-deficit/hyperactivity disorder (ADHD): what every clinician should knowEur J Paediatr Neurol201216542243322306277
- AschnerMGuilarteTRSchneiderJSZhengWManganese: recent advances in understanding its transport and neurotoxicityToxicol Appl Pharmacol2007221213114717466353
- GuilarteTRChenMKMcGlothanJLNigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primatesExp Neurol2006202238139016925997
- GrantKGoldizenFCSlyPDHealth consequences of exposure to e-waste: a systematic reviewLancet Glob Health201316e350e36125104600
- HeacockMKellyCBAsanteKAE-waste and harm to vulnerable populations: a growing global problemEnviron Health Perspect2016124555055526418733
- KlaassenCDCasarettLJDoullJCasarett and Doull’s Toxicology: The Basic Science of Poisons8th edNew York, NYMcGraw-Hill Education/Medical2013
- MorelloMCaniniAMattioliPSub-cellular localization of manganese in the basal ganglia of normal and manganese-treated rats: an electron spectroscopy imaging and electron energy-loss spectroscopy studyNeurotoxicology2008291607217936361
- McDougallSAReichelCMFarleyCMPostnatal manganese exposure alters dopamine transporter function in adult rats: potential impact on nonassociative and associative processesNeuroscience2008154284886018485605
- ChenPChenZLiACatalytic metalloporphyrin protects against paraquat neurotoxicity in vivoBiomed Environ Sci200821323323818714822
- LiuWHuoXLiuDZengXZhangYXuXS100β in heavy metal-related child attention-deficit hyperactivity disorder in an informal e-waste recycling areaNeurotoxicology20144518519125451971
- YousefSAdemAZoubeidiTKosanovicMMabroukAAEapenVAttention deficit hyperactivity disorder and environmental toxic metal exposure in the United Arab EmiratesJ Trop Pediatr201157645746021300623
- Rodríguez-BarrancoMLacasañaMAguilar-GarduñoCAssociation of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysisSci Total Environ2013454–455562577
- FariasACCunhaABenkoCRManganese in children with attention-deficit/hyperactivity disorder: relationship with methylphenidate exposureJ Child Adolesc Psychopharmacol201020211311820415606
- HongSBKimJWChoiBSBlood manganese levels in relation to comorbid behavioral and emotional problems in children with attention-deficit/hyperactivity disorderPsychiatry Res20142201–241842525064383
- ShinDWKimEJLimSWShinYCOhKSKimEJAssociation of hair manganese level with symptoms in attention-deficit/hyperactivity disorderPsychiatry Investig20151216672
- ScassellatiCBonviciniCFaraoneSVGennarelliMBiomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analysesJ Am Acad Child Adolesc Psychiatry201251101003.e201019.e2023021477
- StroupDFBerlinJAMortonSCMeta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) groupJAMA2000283152008201210789670
- ZengXXuXBoezenHMHuoXChildren with health impairments by heavy metals in an e-waste recycling areaChemosphere201614840841526829309
- GrandjeanPLandriganPJNeurobehavioural effects of developmental toxicityLancet Neurol201413333033824556010
- LucchiniRPlacidiDCagnaGManganese and developmental neurotoxicityAdv Neurobiol201718133428889261
- AnglinRESamaanZWalterSDMcDonaldSDVitamin D deficiency and depression in adults: systematic review and meta-analysisBr J Psychiatry201320210010723377209
- BorensteinMHedgesLVHigginsJPRothsteinHRA basic introduction to fixed-effect and random-effects models for meta-analysisRes Synth Methods2010129711126061376
- TobiasAAssessing the influence of a single study in meta-analysisStata Tech Bull1999471517
- HigginsJPThompsonSGQuantifying heterogeneity in a meta-analysisStat Med200221111539155812111919
- BorensteinMHigginsJPHedgesLVRothsteinHRBasics of meta-analysis: I2 is not an absolute measure of heterogeneityRes Synth Methods20178151828058794
- HigginsJPGreenS10.4.3.1 Recommendations on testing for funnel plot asymmetryHigginsJPGreenSCochrane Handbook for Systematic Reviews of Interventions5.1.0 edCochrane Library2011 Available from: http://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htmAccessed July 02, 2018
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- DuvalSTweedieRTrim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysisBiometrics200056245546310877304
- FraněkTKotaškaKPrůšaRManganese and selenium concentrations in cerebrospinal fluid of seriously ill childrenJ Clin Lab Anal2017316e22122
- RomarísEMCervantesIILópezJMMarcénJFConcentration of calcium and magnesium and trace elements (zinc, copper, iron and manganese) in cerebrospinal fluid: a try of a pathophysiological classificationJ Trace Elem Med Biol201125Suppl 1S45S4921146970
- EastmanRRJursaTPBenedettiCLucchiniRGSmithDRHair as a biomarker of environmental manganese exposureEnviron Sci Technol20134731629163723259818
