Abstract
Purpose
Previous studies have independently provided evidence for the effects of HIV infection, hypothalamic–pituitary–adrenal (HPA) axis dysfunction and early life trauma on neurocognitive impairment (NCI). This study examined the interaction between single-nucleotide polymorphisms (SNPs) of two HPA axis genes, corticotrophin-releasing hormone receptor 1 (CRHR1; rs110402, rs242924, rs7209436, and rs4792888) and corticotrophin-releasing hormone-binding protein (CRHBP; rs32897, rs10062367, and rs1053989), childhood trauma, and HIV-associated NCI.
Patients and methods
The sample comprised 128 HIV-positive Xhosa females of whom 88 (69%) had a history of childhood trauma. NCI was assessed using a battery of 17 measures sensitive to the effects of HIV, and the history of childhood trauma was assessed using the validated retrospective Childhood Trauma Questionnaire-Short Form. Generalized linear regression models were used to compare allelic distribution by trauma status and global NCI. The association between genotype, childhood trauma, and cognitive scores was also evaluated using generalized linear regression models, assuming additive models for the SNPs, and ANOVA.
Results
Of the seven polymorphisms assessed, only the rs10062367 variant of CRHBP was significantly associated with global NCI (P=0.034), independent of childhood trauma. This polymorphism was not significantly associated with z-scores on any specific cognitive domain. The interaction of childhood trauma and variants of CRHR1 was associated with poorer learning (rs110402) and/or recall (rs110402 and rs4792888).
Conclusion
These findings suggest that CRHBP rs10062367 A allele is a possible risk variant for NCI in HIV, independent of childhood trauma. Furthermore, results show that the interaction of childhood trauma with variants of CRHR1, rs110402 and rs4792888, confer added vulnerability to NCI in HIV-infected individuals in cognitive domains that are known to be impacted by HIV. While these findings need independent replication in larger samples, it adds CRHBP and CRHR1 to the list of known genes linked to HIV- and childhood trauma-associated neurocognitive phenotypes.
Introduction
In South Africa, the number of people living with HIV is estimated to be about 7.06 million (which is 12.6% of the total population) and includes more than one-fifth of all women aged 15–49 years.Citation1 South African women are disproportionately affected by HIV, and many also have trajectories characterized by trauma over the life course.Citation2–Citation5 Trauma has been associated with impaired neurocognitive functioning, as well as the development of various psychopathologies, including major depressive disorder (MDD) and posttraumatic stress disorder.Citation5,Citation6 The effects of early life trauma may exacerbate the neurocognitive deficits caused by HIV infection.
HIV infection is associated with neurological and neurocognitive complications, collectively referred to as HIV-associated neurocognitive disorders (HAND). HAND describes a spectrum of disorders ranging from asymptomatic neurocognitive impairment (ANI), an intermediate form termed mild neurocognitive disorder (MND), to a severe form which constitutes HIV-associated dementia (HAD).Citation7 The precise pathophysiology of neurocognitive impairment (NCI) in HIV-positive patients is not entirely clear, although it appears that only certain subsets of neurons and subpopulations of patients appear to be affected by HIV-induced central nervous system (CNS) injury, and both host and viral factors are important.Citation8
The development of HAND negatively impacts social and occupational functioning, quality of life, and medication adherence.Citation9,Citation10 However, since the introduction of highly active antiretroviral therapy (HAART), the pattern of HAND appears to have radically changed. In particular, there has been a significant reduction in HAD with an increased prevalence of ANI and MND.Citation7 Although less devastating than HAD, milder forms of HAND adversely impact important outcomes, including medication adherence and performance of cognitively demanding activities of daily living.Citation11,Citation12 It is still unclear why NCI persists in the post combined antiretroviral therapy (cART) era. Possible reasons include cardiovascular disease risk factors,Citation13 immune system activation and inflammation,Citation14–Citation16 genetic factors,Citation8,Citation17 antiretroviral therapy neurotoxicity,Citation18 substance abuse,Citation19 advancing age,Citation20 HIV reservoirs within the CNS,Citation21 and chronic dysfunction of the hypothalamic–pituitary–adrenal (HPA) axis.Citation22
The HPA axis is the primary regulator of environmental stress in humans. Stress triggers the release of corticotrophin- releasing hormone (CRH) which activates the HPA axis by binding to the corticotrophin-releasing hormone receptor 1 (CRHR1) in the anterior pituitary. The resultant neuroendocrine cascade culminates in the production of cortisol, which mediates the restoration of homeostasis.Citation23 Aberrant HPA axis regulation has been associated with hippocampal atrophy, cognitive deficits, and a number of psychiatric disorders.Citation24–Citation26 HPA axis dysregulation occurs during the course of HIV infection due to a chronic increase in basal endogenous cortisol levels.Citation22,Citation27–Citation29 Other possible mechanisms by which HIV may cause HPA axis dysfunction include chronic stress,Citation30,Citation31 direct CNS invasion and damage, secondary effects of cytokines, and side effects of antiretroviral therapy.Citation32,Citation33
Chronic dysregulation of the HPA axis has also been noted in several stress-related psychiatric disorders, especially in individuals traumatized in childhood.Citation34–Citation37 Therefore, early life trauma might, via its effect on the HPA axis, exacerbate NCI in HIV-positive patients. However, as genetic factors also play a significant role in the development of psychopathology following trauma exposure,Citation38 examining genetic influences on this susceptibility may aid in identifying individuals most vulnerable to HAND and facilitate more targeted therapeutic and preventive interventions.
Genetic polymorphisms that could affect the function of genes known to regulate the HPA axis are considered likely determinants of the link between stress responsivity and risk for psychopathology.Citation39 One way in which the HPA axis is regulated is by the CRH-binding protein (CRHBP), which binds CRH with high affinity making it unavailable for binding with the CRH type 1 receptor (CRHR1).Citation40 CRHR1 and CRHBP gene variants have been associated with a number of clinical phenotypes, including MDDCitation41 and behavioral inhibition.Citation42 Bradley et al also showed that two CRHR1 single-nucleotide polymorphisms (SNPs; rs110402 and rs7209436) interacted with childhood maltreatment to predict depressive symptoms in adulthood.Citation43 In each case, maltreatment was associated with increased depression in carriers of a common allele (G for rs110402 and C for rs7209436), while carriers of the rare allele (A for rs110402 and T for rs7209436) showed no increase in depressive symptoms compared to controls. Similarly, variations in three CRHBP SNPs (rs6453267, rs7728378, and rs10474485) may predispose to suicidal behavior in individuals who have experienced childhood trauma.Citation44 Polymorphisms of genes in the CRH system have been associated with glucocorticoid resistance and reduced negative feedback of the HPA axis,Citation45 as well as cortisol reactivity.Citation46
In summary, genetic factors, childhood trauma, and HPA axis dysfunction might all be pathognomonic mechanisms and potentially interact in the development of psychopathology in individuals with HIV. To the best of our knowledge, no previous study has investigated the combination of these factors as possible determinants in the development of HAND. Spies et al previously reported on the independent and additive effects of HIV and early trauma in South African women.Citation47 Therefore, using data from this same cohort, the aim of the current study was to assess the relationship between polymorphic variants in CRHR1 and CRHBP, childhood trauma, and the development of HAND in South African women diagnosed with HIV.
Patients and methods
Study design
This study involved analysis of data from participants recruited as part of the Biological Endophenotypes of HIV and Childhood Trauma (BEHCT) study conducted at Stellenbosch University, Cape Town. The aim of the BEHCT study was to assess the genetic, cognitive, and neuroimaging outcomes in HIV-positive women. This study utilizes an adult female cohort (18–65 years), recruited between 2008 and 2013, with samples genotyped in 2017. The study design, data collection methods, and the inclusion/exclusion criteria for the BEHCT study have been described previously.Citation27 The main procedures applicable to the current study are briefly outlined below.
Study sample
Out of 140 eligible women, 12 (9%) were excluded from this study as genotyping data for these participants were not available. Therefore, a total of 128 HIV-positive, Black African Xhosa women who had full genetic and neurocognitive data were included in the present study. This particular demographic was chosen to reduce genetic heterogeneity. Eligibility criteria included willingness and ability to provide written informed consent, ability to read and write in either English or isiXhosa at fifth-grade level, aged between 18 and 65 years, and medically well enough to undergo neuropsychological testing. Exclusion criteria comprised a current or past history of schizophrenia, bipolar disorder, or other psychotic disorders; current substance or alcohol abuse or dependence; significant previous head injury; demonstrated frank dementia on the HIV dementia scale; current seizure disorders of any cause; history of CNS infections or neoplasms; hepatitis B-positive status; and current use within the last month of any psychotropic medication.
Demographic, clinical, and psychological characteristics
Age, gender, marital status, ethnicity, years of education, and employment status were captured by questionnaires, while virologic markers of HIV infection (CD4 lymphocyte counts) were obtained from blood samples. Current and lifetime psychiatric disorders were evaluated using the MINI International Neuropsychiatric Interview-Plus (MINI-Plus).Citation48 Depressive and trauma symptomatology were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D) and the Davidson Trauma Scale, respectively.Citation49,Citation50
Childhood trauma and neurocognitive assessment
Histories of abuse and neglect were assessed using the Childhood Trauma Questionnaire-Short Form (CTQ-SF), a 28-item self-report inventory that provides valid screening for histories of abuse and neglect.Citation51 The tool assesses five types of maltreatment including emotional, physical, and sexual abuse and emotional and physical neglect. Each of these five subscales consists of five items, each of which is scored from 5 to 25. Therefore, the total score ranges from 25 to 125 and higher scores reflect higher levels of childhood trauma. In particular, 25–31 = no trauma, 41–51 = low-to moderate, 56–68 = moderate-to-severe, and 73–125 = severe-to-extreme. Similar to Spies et al,Citation47 we used a score ≥41 to identify trauma-exposed individuals, as this represents the lowest level score indicative of abuse or neglect (ie, low to moderate) for each subscale.
NCI was assessed using a battery of 17 tests that are sensitive to the effects of HIV infection. This battery was originally developed by the HIV Neurobehavioral Research Center at the University of California, San Diego, and has been culturally adapted for the South African context, as described by Spies et al.Citation52 Childhood trauma and neurocognitive assessments were performed by trained researchers and were conducted in either English or isiXhosa.
Genotyping
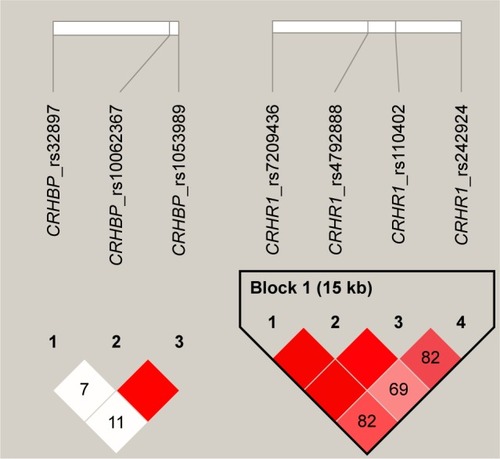
DNA was extracted from whole blood using phenol-chloroform extraction. Genotyping was conducted by LGC Genomics (United Kingdom). Validated SNPs with a minor allele frequency of >0.2 in at least one African population genotyped for the Hapmap project (www.hapmap.org), and which have been previously associated with some aspects of neuro-psychopathology, were selected for investigation.Citation43,Citation44,Citation53 We genotyped four CRHR1 SNPs (CRHR1; rs110402 (C to T) [n=127], rs242924 (C to A) [n=127], rs7209436 (C to T) [n=122], and rs4792888 (G to A) [n=122]) and three CRHBP SNPs (CRHBP; rs32897 (G to A) [n=122], rs1053989 (C to A) [n=126], and rs10062367 (G to A) [n=124]). Pair-wise linkage disequilibrium (LD) for the SNPs () was estimated using the solid spline method in Haploview, version 4.Citation54 All genetic variants were in Hardy–Weinberg equilibrium (P>0.05).
Data analysis
Demographic and clinical characteristics of study participants were summarized as counts and percentages for dichotomous traits, and means and SD or medians and 25th–75th percentiles (interquartile range) for quantitative traits.
Age- and education-corrected z-scores were calculated from all raw neuropsychological data and grouped into domains (motor function, verbal fluency, attention/working memory, speed, learning, recall, and executive functions). This was done by running a regression analysis on all raw neuropsychological data using test scores as the outcome and age and education as predictors. The residual was then saved, and a studentized residual was calculated. Within each domain, the studentized residual for each test was summed to obtain a domain z-score. Global NCI scores represent the mean of individual cognitive domain scores.
The Shapiro–Wilk W test was used to test for normality. Differences in normally distributed demographic and clinical variables (age, global NCI scores, NCI domain scores for verbal fluency, attention/working memory, speed, learning, recall, and executive functions) according to trauma status were determined using Student’s t-test, while differences in non-parametric variables (CTQ scores, CES-D scores, viral load, CD4 counts, and NCI domain scores for motor function) were determined using Welch’s unequal variances t-test. Pearson’s chi-squared test was used to assess the relationship between categorical variables.
The contribution of each SNP to global NCI scores was evaluated using generalized linear regression models. When the results were significant, additional generalized linear regression models were used to evaluate the contribution of the SNP to individual cognitive domain scores. Generalized linear models, assuming additive models for the SNPs, were also used to evaluate if there was any association between genotype and CTQ scores, as well as to investigate the relationships between genotype, childhood trauma, and cognitive scores. Finally, analysis of variance (ANOVA) was used to determine whether including the interaction between genotype × childhood trauma explained more of the variance in cognitive domain scores than these two predictors acting individually. Antiretroviral (ARV) treatment and CES-D scores were included as covariates in the regression models.
Analyses were carried out using R statistical software version 3.2.2 (The R Foundation for Statistical Computing, Vienna, Austria) with the R package SNPassoc.Citation55 Statistical significance was set at P<0.05.
Ethical considerations
The study was approved by the Health Research Ethics Committee (HREC # N07/07/153A) of Stellenbosch University (Cape Town, South Africa). All participants gave written informed consent for participation according to locally and internationally accepted ethical guidelines. Participation in the present study was entirely voluntary and participants were informed of their right to withdraw their participation at any point in the study. Women who required further care were referred to their local community health care facility. Participants were reimbursed for their transport costs to attend study visits.
Results
shows demographic and clinical variables for the overall sample and by trauma status. Of the 128 participants, 88 (69%) had a history of childhood trauma. Those participants with childhood trauma had higher self-reported depression on the CES-D. This converged with interviewer-based assessment of psychopathology using the MINI-Plus, with a significantly greater proportion in the childhood trauma-exposed group meeting diagnostic criteria for recurrent MDD.
Table 1 Demographic and clinical characteristics of study participants (n=128) showing differences between those with/without childhood trauma exposure
Less than half the sample (41.4%) were on ARVs. ARV status had no significant impact on cognitive domain scores, except for the recall domain ().
Table 2 Differences in mean global NCI scores (z-scores) by ART status
Regression analysis revealed that individuals carrying at least one CRHBP rs10062367 G allele had significantly higher global NCI z-scores compared to subjects homozygous for the A allele (). On further analysis, this polymorphism was not significantly associated with z-scores on specific cognitive domains.
Table 3 Differences in neurocognitive impairment (z-scores) across genotypes for CRHBP rs10062367
There were no significant differences in childhood trauma CTQ-SF scores across genotypes. In addition, childhood trauma status was not significantly associated with global NCI (P=0.441) or specific domain scores (motor function: P=0.089, verbal fluency: P=0.894, attention/working memory: P=0.458, processing speed: P=0.430, learning: P=0.785, recall: P=0.238, executive function: P=0.996). However, cognitive domain z-scores were influenced by the interaction between childhood trauma and polymorphisms in CRHR1. In particular, the presence of at least one T allele in CRHR1 rs110402 interacted with childhood trauma to worsen learning and recall. In contrast, the presence of at least one C allele in CRHR1 rs7209436 interacted with childhood trauma to improve learning and recall. Finally, the presence of at least one A allele in rs4792888 interacted with childhood trauma to worsen recall ().
Table 4 Predictive value of CRHR1 variants × childhood trauma interactions on cognitive domain scores
Discussion
We evaluated the interaction between childhood trauma, variants of two HPA-axis genes, CRHBP and CRHR1, and NCI in a sample of Black African Xhosa women living with HIV. Of the seven polymorphisms assessed, only the rs10062367 variant of CRHBP was significantly associated with NCI, independent of childhood trauma. Compared to carriers of the rs10062367 G allele, A homozygotes had significantly lower global NCI scores. However, this polymorphism was not significantly associated with z-scores on specific cognitive domains. Host genetic factors have been found to predispose HIV-infected individuals to NCI.Citation56,Citation57 To the best of our knowledge, CRHBP rs10062367 has not been previously studied in the context of HAND. Therefore, our findings, while requiring further independent replication in a larger sample, may be the first to suggest a possible link between rs10062367 and NCI in HIV-infected individuals.
The lack of an association between NCI and other variants of CRHBP suggest an independent effect of rs10062367 on global neurocognitive deficits, or the effect of another polymorphism in LD with rs10062367. Other plausible reasons for the lack of a significant association between other SNPs and NCI may be the limited number of CRHBP polymorphisms that we assessed and the relatively small sample of HIV-infected participants.
In the current study, the interaction of childhood trauma and CRHR1 variants was associated with poorer learning (rs110402) and/or recall (rs110402 and rs4792888). Early-life trauma has been associated with impaired neurocognitive functioning,Citation6,Citation47 and previous studies have demonstrated an association between childhood trauma and polymorphisms in CRHR1 and CRHBP in non-HIV samples.Citation43,Citation44 There may be a few reasons why trauma did not interact with CRHBP gene variants in contributing to NCI in HIV-infected individuals. Firstly, the rs10062367 CRHBP variant might confer vulnerability to NCI independent of environmental stress exposure, at least in this population. Various CRHBP polymorphisms have previously been associated with psychopathology including suicidality and anxiety,Citation44,Citation58–Citation61 with emerging evidence also indicating that variants of the CRHBP gene (eg, rs28365143) can robustly predict pharmacotherapy outcomes in depression (ie, symptom change, response, and remission).Citation61,Citation62 Nevertheless, results show that the interaction of childhood trauma with variants of CRHR1, specifically rs110402 and rs4792888, confer added vulnerability to neurocognitive decline in HIV-infected individuals.
A possible limitation of this study was that childhood trauma was assessed retrospectively and is, as such, prone to recall bias. Moreover, there are potential confounding variables which we did not control for, namely, 1) the possible trauma of contracting HIV and/or an effect of HIV infection overriding any effect(s) of childhood trauma, 2) any effect(s) of adult-onset trauma, 3) viral load and ARV regimen differences between the groups (there were, however, no significant differences in CD4 count between trauma exposed and unexposed groups), and 4) the higher level of self-reported depression in the childhood trauma group. Another limitation is that our sample included only Black African women living with HIV and we, therefore, cannot generalize our findings to HIV-uninfected individuals, other races or ethnic groups, or to HIV-infected males. Finally, this was a relatively small sample, and larger sample replication studies would be helpful to validate these findings.
Future studies should ideally include data from HIV-negative controls (unexposed to either child or adult trauma) and consist of larger sample sizes. There is also a need to investigate the functional effects these particular CRH1 and CRHBP SNPs. Moreover, to further interrogate the CRHBP rs10062367 genotype and stress response abnormalities, future studies could investigate HPA-axis neuroendocrine endophenotypes of NCI (eg, cortisol). Future studies should also take durationCitation63 and typeCitation53 of trauma into account as these trauma variables can impact NCI.
Conclusion
The study shows that HPA-axis genes are possible risk variants for NCI among HIV-infected women and may interact with childhood trauma to impact specific domains of cognitive function. The combination of these vulnerabilities might be early markers of NCI in HIV-infected individuals and might represent potential targets for therapeutic action. However, replication studies involving whole gene-based association analyses of SNPs in CRHBP, as well as a more detailed analysis of childhood trauma, are needed.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
We acknowledge the assistance from Michael McCaul (Center for Evidence-based Health Care, Stellenbosch University) and Muiruri Macharia (Department of Psychiatry, Stellenbosch University).
Disclosure
The authors report no conflicts of interest in this work.
References
- Statistics South Africa Midyear population statistics2017 Available from: http://www.statssa.gov.za/publications/P0302/P03022017.pdfAccessed May 31, 2018
- AnderssonNCockcroftASheaBGender-based violence and HIV: relevance for HIV prevention in hyperendemic countries of southern AfricaAIDS200822Suppl 4S73S8619033757
- JewkesRPenn-KekanaLLevinJRatsakaMSchrieberMPrevalence of emotional, physical and sexual abuse of women in three South African provincesS Afr Med J200191542142811455808
- KalichmanSCSimbayiLCSexual assault history and risks for sexually transmitted infections among women in an African township in Cape Town, South AfricaAIDS Care200416668168915370057
- SeedatSInterventions to improve psychological functioning and health outcomes of HIV-infected individuals with a history of trauma or PTSDCurr HIV/AIDS Rep20129434435023007792
- SpiesGAfifiTOArchibaldSLFennema-NotestineCSareenJSeedatSMental health outcomes in HIV and childhood maltreatment: a systematic reviewSyst Rev20121113022587946
- HeatonRKFranklinDREllisRJHIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictorsJ Neurovirol201117131621174240
- JayadevSGardenGAHost and viral factors influencing the pathogenesis of HIV-associated neurocognitive disordersJ Neuroimmune Pharmacol20094217518919373562
- TozziVBalestraPGalganiSNeurocognitive performance and quality of life in patients with HIV infectionAIDS Res Hum Retroviruses200319864365213678465
- GormanAAFoleyJMEttenhoferMLHinkinCHvan GorpWGFunctional consequences of HIV-associated neuropsychological impairmentNeuropsychol Rev200919218620319472057
- HinkinCHCastellonSADurvasulaRSMedication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexityNeurology200259121944195012499488
- AlbertSMMarderKDooneiefGNeuropsychologic impairment in early HIV infection. A risk factor for work disabilityArch Neurol19955255255307733849
- McCutchanJAMarquie-BeckJAFitzsimonsCARole of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorderNeurology201278748549222330412
- CliffordDBAncesBMHIV-associated neurocognitive disorderLancet Infect Dis2013131197698624156898
- AncutaPKamatAKunstmanKJMicrobial translocation is associated with increased monocyte activation and dementia in AIDS patientsPLoS One200836e251618575590
- EdénAPriceRWSpudichSFuchsDHagbergLGisslénMImmune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapyJ Infect Dis2007196121779178318190258
- BorjabadAVolskyDJCommon transcriptional signatures in brain tissue from patients with HIV-associated neurocognitive disorders, Alzheimer’s disease, and Multiple SclerosisJ Neuroimmune Pharmacol20127491492623065460
- RobertsonKLinerJMeekerRBAntiretroviral neurotoxicityJ Neurovirol201218538839922811264
- FerrisMJMactutusCFBoozeRMNeurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDSNeurosci Biobehav Rev200832588390918430470
- Rodriguez-PenneyATIudicelloJERiggsPKComorbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of lifeAIDS Patient Care STDS201327151623305257
- GrayLRRocheMFlynnJKWesselinghSLGorryPRChurchillMJIs the central nervous system a reservoir of HIV-1?Curr Opin HIV AIDS20149655255825203642
- VagoTClericiMNorbiatoGGlucocorticoids and the immune system in AIDSBailliere Clin Endocrinol Metab199484789802
- SapolskyRMRomeroLMMunckAUHow do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actionsEndocr Rev2000211558910696570
- ChaouloffFSerotonin, stress and corticoidsJ Psychopharmacol200014213915110890308
- YoungAHThe effects of HPA axis function on cognition and its implications for the pathophysiology of bipolar disorderHarv Rev Psychiatry201422633133325377604
- FrodlTO’KeaneVHow does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humansNeurobiol Dis201352243722426398
- VergesBChavanetPDesgresJAdrenal function in HIV infected patientsActa Endocrinol (Copenh)198912156336372555993
- BiglinoALimonePFornoBAltered adrenocorticotropin and cortisol response to corticotropin-releasing hormone in HIV-1 infectionEur J Endocrinol199513321731797655641
- LortholaryOChristeffNCasassusPHypothalamo-pituitary-adrenal function in human immunodeficiency virus-infected menJ Clin Endocrinol Metab19968127917968636305
- ReifSMugaveroMRaperJHighly stressed: stressful and traumatic experiences among individuals with HIV/AIDS in the Deep SouthAIDS Care201123215216221259127
- HandGAPhillipsKDDudgeonWDPerceived stress in HIV-infected individuals: physiological and psychological correlatesAIDS Care20061881011101717012093
- GeorgeMMBhangooAHuman immune deficiency virus (HIV) infection and the hypothalamic pituitary adrenal axisRev Endocr Metab Disord201314210511223728720
- CostaANappiREPolattiFPomaAGrossmanABNappiGStimulating effect of HIV-1 coat protein gp120 on corticotropin-releasing hormone and arginine vasopressin in the rat hypothalamus: involvement of nitric oxideExp Neurol2000166237638411085902
- CarpenterLLCarvalhoJPTyrkaARDecreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatmentBiol Psychiatry200762101080108717662255
- SecklJRMeaneyMJGlucocorticoid “programming” and PTSD riskAnn N Y Acad Sci20061071135137816891583
- HeimCMletzkoTPurselleDMusselmanDLNemeroffCBThe dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood traumaBiol Psychiatry200863439840517825799
- de KloetCSVermettenEGeuzeEKavelaarsAHeijnenCJWestenbergHGAssessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a reviewJ Psychiatr Res200640655056716214171
- NugentNRAmstadterABKoenenKCGenetics of post-traumatic stress disorder: informing clinical conceptualizations and promoting future researchAm J Med Genet C Semin Med Genet2008148C212713218412098
- TyrkaARPriceLHGelernterJSchepkerCAndersonGMCarpenterLLInteraction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivityBiol Psychiatry200966768168519596121
- BehanDPKhongsalyOOwensMJChungHDNemeroffCBDe SouzaEBCorticotropin-releasing factor (CRF), CRF-binding protein (CRF-BP), and CRF/CRF-BP complex in Alzheimer’s disease and control postmortem human brainJ Neurochem1997685205320609109532
- LiuZZhuFWangGAssociation of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depressionNeurosci Lett2006404335836216815632
- SmollerJWYamakiLHFagernessJAThe corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorderBiol Psychiatry200557121485149215953484
- BradleyRGBinderEBEpsteinMPInfluence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor geneArch Gen Psychiatry200865219020018250257
- RoyAHodgkinsonCADelucaVGoldmanDEnochMATwo HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behaviorJ Psychiatr Res2012461727921978546
- PrattWBToftDOSteroid receptor interactions with heat shock protein and immunophilin chaperonesEndocr Rev19971833063609183567
- SheikhHIKryskiKRSmithHJHaydenEPSinghSMCorticotropin-releasing hormone system polymorphisms are associated with children’s cortisol reactivityNeuroscience201322911123131710
- SpiesGFennema-NotestineCArchibaldSLChernerMSeedatSNeurocognitive deficits in HIV-infected women and victims of childhood traumaAIDS Care20122491126113522672200
- SheehanDVLecrubierYSheehanKHThe Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10J Clin Psychiatry199859Suppl 202233
- RadloffLSThe CES-D Scale: A self-report depression scale for research in the general populationApplPsychol Meas197713385401
- DavidsonJRBookSWColketJTAssessment of a new self-rating scale for post-traumatic stress disorderPsychol Med19972711531609122295
- BernsteinDPSteinJANewcombMDDevelopment and validation of a brief screening version of the Childhood Trauma QuestionnaireChild Abuse Negl200327216919012615092
- SpiesGFennema-NotestineCChernerMSeedatSChanges in cognitive function in women with HIV infection and early life stressAIDS Care20062911423
- MajerMNaterUMLinJMCapuronLReevesWCAssociation of childhood trauma with cognitive function in healthy adults: a pilot studyBMC Neurol2010106120630071
- BarrettJCFryBMallerJDalyMJHaploview: analysis and visualization of LD and haplotype mapsBioinformatics200521226326515297300
- GonzálezJRArmengolLSoléXSNPassoc: an R package to perform whole genome association studiesBioinformatics200723565465517237056
- KallianpurARLevineAJHost genetic factors predisposing to HIV-associated neurocognitive disorderCurr HIV/AIDS Rep201411333635224996618
- LevineAJPanosSEHorvathSGenetic, transcriptomic, and epigenetic studies of HIV-associated neurocognitive disorderJ Acquir Immune Defic Syndr201465448150324583618
- de LucaVTharmalingamSKennedyJLAssociation study between the corticotropin-releasing hormone receptor 2 gene and suicidality in bipolar disorderEur Psychiatry200722528228717532191
- EnochMAShenPHDucciFCommon genetic origins for EEG, alcoholism and anxiety: the role of CRH-BPPLoS One2008310e362018974851
- Haass-KofflerCLHenryATMelkusGDefining the role of corticotropin releasing factor binding protein in alcohol consumptionTransl Psychiatry2016611e95327845775
- BinderEBOwensMJLiuWAssociation of polymorphisms in genes regulating the corticotropin-releasing factor system with antidepressant treatment responseArch Gen Psychiatry201067436937920368512
- O’ConnellCPGoldstein-PiekarskiANNemeroffCBAntidepressant outcomes predicted by genetic variation in corticotropin-releasing hormone binding proteinAm J Psychiatry2018175325126129241359
- NavaltaCPPolcariAWebsterDMBoghossianATeicherMHEffects of childhood sexual abuse on neuropsychological and cognitive function in college womenJ Neuropsychiatry Clin Neurosci2006181455316525070