Abstract
Introduction
Low level of vitamin D is a potential risk factor for developing schizophrenia. Through interaction with its receptor (VDR) and the related enzymes (CYP27B1, CYP24A1), vitamin D modulates neurodevelopment, neuroprotection, and immunomodulation. Its deficiency leads to aberrant neurodevelopment in schizophrenic patients.
Methods
In this case–control study, relative expression of VDR, CYP27B1, and CYP24A1 in schizophrenic patients was compared with healthy individuals. Total RNA was extracted from whole blood of 50 patients with schizophrenia and 50 healthy controls. Real-time PCR was used to determine relative gene expression levels of VDR, CYP27B1, and CYP24A1.
Results
Significant upregulations were observed in VDR (P=0.004, 95% CI=0.77, 0.86), CYP27B1 (P=0.002, 95% CI=1.22, 4.98), and CYP24A1 (P≤0.0001, 95% CI=−2.721, 1.061) expressions in peripheral blood of schizophrenic patients compared with controls. Moreover, the gender-based analysis revealed upregulation of all genes in all the categories of male and female except for VDR gene in male group (P=0.234, 95% CI=−0.79, 3.35) and CYP27B1 gene in the female group (P=0.09, 95% CI=−0.21, 6.55). The age-based analysis demonstrated overexpression of VDR and CYP27B1 genes in all categories. Finally, there were significant correlations between expression levels of all genes (P<0.0001), while no correlation was found between age and expression of genes.
Conclusion
We hypothesized that the observed upregulation of the mentioned genes in schizophrenia patients might be the result of a compensatory mechanism to protect the affected individuals against adverse consequences of this disorder. Such imbalance in vitamin D processing pathway might also be implicated in the pathogenesis of schizophrenia. However, future studies should be designed to confirm the results of the current study.
Keywords:
Introduction
Schizophrenia is a debilitating psychiatric disorder with various etiologies affecting nearly 1% of the global population.Citation1 It consists of three major disabling categories: positive, negative, and cognitive symptoms.Citation2 Both environmental and genetic factors are involved in the etiology of this disorder.Citation2 Meanwhile, environmental factor might also provoke the disease onset in genetically predisposed persons. Among environmental factors, vitamin D is of particular interest. This vitamin plays a significant role in neurodevelopment as well as immunomodulation.Citation3 In the central nervous system (CNS), vitamin D contributes to both the synthesis of neurotransmitters and neuroprotection against damages and inflammation.Citation3 It is also capable of protecting the brain against reactive oxygen species (ROS), through upregulation of some known antioxidant molecules such as glutathione (GSH).Citation4 It has long been considered that prenatal exposure to low vitamin D may increase susceptibility to psychiatric disorders later in life. This finding is postulated by several epidemiological studies.Citation5 The active form of vitamin D is produced through two hydroxylation steps in liver and kidney. In the kidney, CYP27B1, which is a member of CYP/CYP450 superfamily is responsible for 1α hydroxylation on 25-hydroxy vitamin D3 (25OHD3) to create the active form 1α25-dihydroxyvitaminD3 (1α25OH2D3). It is worth mentioning that 1α25OH2D3 can regulate its production via CYP24A1 (another member of CYP/CYP450 enzymes). The latter enzyme deactivates 1α25OH2D3 through 24 oxidation pathway leading to the formation of calcitroic acid and lactone.Citation6 1α25OH2D3 exerts its effects on different tissues by genomic and nongenomic pathways. Genomic pathway initiates when 1α25OH2D3 binds to its receptor known as VDR.Citation7 Thereby, it modulates the expression of several genes involved in diverse physiological functions such as calcium hemostasis, growth control, differentiation, cognition, and immune response.Citation8 VDR is expressed in a wide variety of nerve cells and different regions of the nervous system. Moreover, hydroxylation process is not confined to kidney and liver, but it has been discovered in numerous other tissues.Citation8,Citation9 Overall, the importance of vitamin D and its metabolizing enzymes in the development of the nervous system are undeniable. Considering these and lack of studies related to the expression of genes involved in metabolism of vitamin D in schizophrenia, in this case–control study, we aimed to investigate the relative expression of VDR, CYP24A1, and CYP27B1 in peripheral blood of schizophrenic patients and healthy controls.
Materials and methods
Study design
Clinical confirmation of schizophrenia was done according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) by a certified psychiatrist. Controls were closely matched for age and gender with cases. The inclusion criteria for patients were defined as follows: definite diagnosis of the disorder based on DSM-V.Citation10,Citation11 Individuals recruited in control group were assessed through a structured psychiatric interview for ruling out the presence of any psychiatric disorder. Individuals who had the drug abuse, traumatic events, and severe medical conditions such as epilepsy and history of related disorders in first-degree relatives were excluded from the study. All the patients received Clozapine™ as treatment.Citation12
Blood sampling
In this study, 5 mL of peripheral blood was obtained from both groups. Written informed consent was obtained from all study participants. All procedures were performed with verification from the Ethics Committee of Shahid Beheshti University of Medical Sciences.
Laboratory analysis
Total RNA was isolated by Hybrid-RTM blood RNA extraction kit (cat no 305-101, GeneAll, Biotechnology Co. Ltd, Seoul, Korea). RNA integrity and purity were assessed by NanoDrop equipment. Low-quality RNA samples were excluded from the study. Purified RNA was stored at −80°C. In the succeeding step, the High-Capacity cDNA Reverse Transcription Kit (PN: 4,375,575, Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA) was used for the synthesis of cDNA. All the procedures were performed according to the manufacturer’s protocol. In order to perform TaqMan quantitative real-time PCR, the specific primers and probes were designed using Allele ID7 for x64 windows software (Premier Biosoft, Palo Alto, CA, USA). The specificity and validity of primers were determined by NCBI primer blast. The sequence of primers and probes are shown in . Finally, real-time PCR was carried out on a Corbett Rotor Gene 6000 system (Corbett Life Science, Sydney, Australia) with the TaqMan®, Universal PCR Master Mix (PN: 4,304,449, Applied Biosystems). In order to control the quality of method, we included negative control. Moreover, HPRT1 was used as a reference gene in order to normalize relative expression of target genes. Each sample was analyzed in triplicate. Relative expression of genes in patients compared with healthy subjects was estimated based on calculation of Ln [Efficiency^(CT reference gene−CT target gene)] values.
Statistical analysis
The independent sample t-test was applied to examine the differences between two groups. The one-way ANOVA test was used to compare means between control and patient groups. Pearson’s correlation coefficient was calculated to assess correlations between variables. The Kolmogorov–Smirnov test was used to investigate the normality. The level of significant P-value was set at ≤0.05. Data analysis was performed using SPSS version 18 (Chicago, IL, USA).
Result
Clinical and demographic information
The demographic features of the study groups are summarized in . No significant difference was found in age of cases and controls (P=0.0581) or in sex ratio (P=0.18). All the individuals were compared in three ways: A) total participants together (regardless of age and sex), B) sex-based analysis (male or female), and C) age-based analysis (<30, 30–40, and >40 years). Therefore, the group of schizophrenia patients was compared with controls, as well as each of the sex and age subgroup.
Table 1 Demographic features of schizophrenia patients and healthy controls
Relative expression level of the VDR
We observed upregulation of VDR expression in the peripheral blood of schizophrenic patients compared with healthy controls (P=0.004, 95% CI=0.77, 0.86). In the gender-based analysis of VDR expression, we detected a statistically significant change in expression levels of VDR in female affected by schizophrenia (P<0.0001, 95% CI=3.17, 6.15). Furthermore, schizophrenic females aged between 30 and 40 years and both male and female patients over the age of 40 years had a significant increase in expression of VDR (P=0.001, 95% CI=4.25, 7.09; P=0.047, 95% CI=0.03, 6.36; and P=0.03, 95% CI=1.15, 6.83, respectively) ().
Table 2 Expression levels of genes in schizophrenia patients compared with healthy controls
Relative expression level of the CYP27B1
CYP27B1 total expression level was significantly higher in the peripheral blood of schizophrenic patients compared with controls (P=0.002, 95% CI=1.22, 4.98). Gender-based assessments revealed upregulation in both male and female groups. However, this increase was merely significant in the male group (P=0.012, 95% CI=0.7, 5.41). In addition, we observed CYP27B1 upregulation in schizophrenic male patients aged over 40 years compared with the corresponding control group (P=0.01, 95% CI=1.32, 8.7) ().
Relative expression level of the CYP24A1
CYP24A1 expression level was increased in the peripheral blood of schizophrenic patients compared with controls (P<0.001, 95% CI=−2.721, −1.061). Such upregulation was also significant in male and female subgroups (P=0.003, 95% CI=−2.819, −0.577 and P=0.042, 95% CI=−4.42, −0.93, respectively). However, in the subgroup of patients aged over 40 years, both male and female patients demonstrated downregulation of CYP24A1 expression (P=0.002, 95% CI=−0.679, −3.064 and P=0.044, 95% CI=−0.421, −2.721, respectively) ().
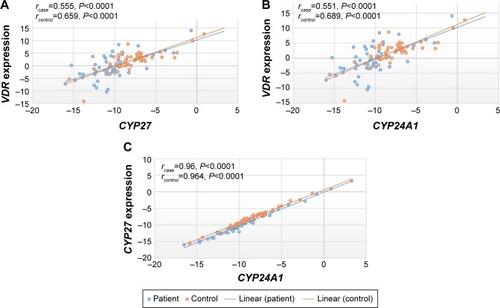
Correlation between the expression levels of VDR/CYP27B1, VDR/CYP24A1, and CYP27B1/CYP24A1 in schizophrenic patients and healthy controls
Correlations between expression levels of VDR/CYP27B1, VDR/CYP24A1, and CYP27B1/CYP24A1 are demonstrated in . All correlations were statistically significant (P<0.0001).
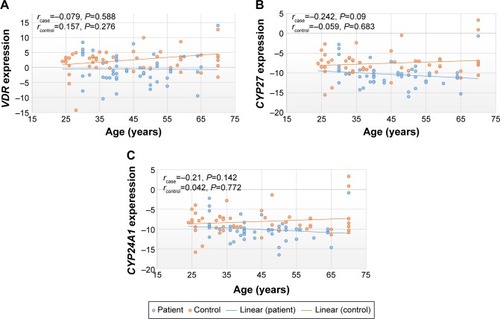
Correlation between expressions of vitamin D-related genes and age of study participants
Increase in the age of schizophrenia patients did not affect the expression level of the vitamin D-related genes. As in , there were not any statistically significant correlation between expression of genes and age either in patients or healthy subjects (P>0.05).
Discussion
In this study, we compared the expression of vitamin D-related genes in schizophrenic patients and healthy controls using quantitative real-time PCR. Our data revealed significant upregulation of CYP27B1, VDR, and CYP24A1 in schizophrenia patients compared with controls (P=0.002, P=0.004, and P<0.0001, respectively). In order to examine the clinical relevance of our results, we also assessed the expression of these genes in brain samples using the public available data of the SZDB database (http://www.szdb.org/). The dataset GSE12649 showed upregulation of VDR (fold change = 1.54, P=0.047 with 204255_s_at probe). However, expressions of the other genes were not different between cases and controls using the same dataset.
In schizophrenia, males are more affected than females. Several lines of evidence suggest that estrogen in female can have a protective role against neuropsychiatric diseases. Both estrogen and vitamin D activate TPH2 gene responsible for serotonin production.Citation13–Citation15 Therefore, we hypothesized that the change in expression ratio of VDR and CYP27B1 is more probable in male than female or it is plausible that male display alteration in vitamin D gene expression with more intensity. As a result, we carried out gender-based analysis. However, in contrast to our speculation, female patients revealed a significant increase in VDR expression (P<0.0001). Furthermore, in CYP27B1 expression analysis, both male and female patients had considerable upregulation compared with healthy controls. However, only CYP27B1 upregulation in male category was statistically significant (P=0.012). In the age-based analysis, based on estrogen hypothesis that was mentioned earlier, we expected upregulation in VDR and CYP27B1 gene expression in affected female over 40 years old because these individuals have lower amounts of estrogen. For VDR gene, these results were not compatible with the suggested hypothesis. For instance, both female and male over 40 years old showed increase in the VDR expression (P=0.03 and P=0.04, respectively), while female aged between 30 and 40 years also represented this increase (P=0.001). CYP27B1 overexpression was only significant for schizophrenic male patients aged over 40 years (P=0.01). CYP24A1 gene revealed downregulation in both male and female patients aged over 40 years (P=0.002 and P=0.004, respectively). To our knowledge, this was the first study evaluating the expression levels of VDR and related enzymes in schizophrenia. So far, most of the studies that have assessed vitamin D-related gene in schizophrenia have mainly focused on polymorphisms of these genesCitation16,Citation17 or assessed serum level of vitamin D in schizophrenic patients and reported lower serum level of vitamin D in these patients compared with controls.Citation18 In this study, we also assessed the correlation between the expression of vitamin D-related genes and the age of study participants. Expressions of the assessed genes were not correlated with age either in schizophrenia patients or in controls, which is in line with the findings reported in multiple sclerosis patients.Citation19 On the contrary, all pairwise correlations between expression levels of genes were significant. In our study, schizophrenia patients had been treated with an equal dose of clozapine. Clozapine is an atypical drug, and its mechanism of action is not comprehensively known. It acts on histaminergic, muscarinic, adrenergic, and serotonin receptors. Gene expression studies using microarray in both rat and human postmortem brain showed that clozapine regulates several genes acting in various biological pathways. Genes that are modulated by this drug are suggested as candidate genes in schizophrenia. However, vitamin D signaling pathway was not among those possibly regulated pathways by clozapine.Citation20,Citation21 This may indicate that changes in vitamin D in schizophrenia can be the consequence of this disorder not the cause of it. Moreover, clozapine does not obviate the action of DHCR7 enzyme and does not alter the level of vitamin D precursor (7DHC) either in rat or in human.Citation22 Overall, it is unlikely that change in VDR, CYP24A1, and CYP27B1 expression observed in schizophrenia patients in this study is the result of clozapine action on gene regulation. Our findings suggest that increased expression levels of VDR and CYP27B1 may be involved in the pathophysiology of schizophrenia through various pathways. There are growing evidences linking vitamin D to neuroplasticity, neuroprotection, and neurotransmission. Low level of this vitamin is considered as a potential risk factor for developing psychiatric and neurological disorders.Citation9 Vitamin D deficiency can cause long-term dysregulation in genes that are involved in mitochondrial function, cytoskeletal maintenance, and neurotransmission.Citation5 Previous reports indicate the presence of vitamin D metabolite 25(OH)D in the cerebrospinal fluid (CSF) and the correlation between CSF and serum 25(OH)D levels.Citation23 Besides, vitamin D and its carrier protein infiltrate to the CNS.Citation24 So, we suggest that the expression of metabolizing enzymes in the blood might affect vitamin D levels in the brain. However, future studies are needed to verify this hypothesis.
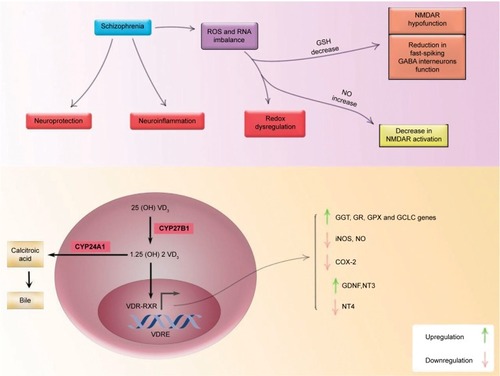
As mentioned earlier, schizophrenia is a disorder of heterogeneous nature. Several studies have concentrated on the dopaminergic hypothesis of schizophrenia. Nevertheless, in recent years, growing number of studies have focused on other aspects of this disorder such as neuroinflammation and redox dysregulation along with NMDA receptor (NMDAR) hypofunction. Redox dysregulation is observed in schizophrenia patients.Citation25 The imbalance in the production of ROS and reactive nitrogen species along with deficiency in enzymatic and nonenzymatic antioxidants can lead to oxidative and nitro-sative stress.Citation25 GSH is a cellular antioxidant. Its synthesis and metabolism are controlled by gammaglutamyl transpeptidase (GGT). It is now postulated that the active form of vitamin D (1,25OH2D3) upregulates activation and gene expression of GGT. Furthermore, 1,25OH2D3 increases activation of GSH reductase, GSH peroxidase, and glutamyl cysteine ligase catalytic subunit.Citation26–Citation29 Vitamin D serum level can elevate GSH expression in astrocytes. Additionally, diminished levels of GSH were observed in prefrontal cortex and CSF of schizophrenia patients and its lower levels associated with more severe negative symptoms.Citation2,Citation26–Citation28,Citation30 Interestingly, this antioxidant agent has the capability to regulate different neurotransmitter systems such as NMDARs and GABA-A receptor. GSH deficiency as a result of redox dysregulation can lead to NMDAR hypofunction and decreased myelination in schizophrenia patients, two conditions that can reduce fast-spiking GABA interneurons function causing negative, positive, and cognitive symptoms of schizophrenia.Citation25,Citation31 Based on the available evidence, low level of GSH can be a potential factor for NMDAR hypofunction, which is reported in schizophrenia. 1,25OH2D3 also acts as a neuroprotective agent through downregulation of inducible nitric oxide synthase (iNOS).Citation2,Citation30 This enzyme releases nitric oxide (NO) during the reaction. NO metabolites damage neurons as well as oligodendrocytes when they are produced in large amount. In postmortem studies, elevated levels of NO and NOS was identified in schizophrenic patients.Citation26,Citation32 There is also evidence indicating immune activation in schizophrenia. Inflammatory symptoms have been observed in postmortem studies in schizophrenia patients. As a result, inflammation might be a possible pathogenic mechanism in this disorder. COX2 (cyclooxygenase 2 is a rate-limiting enzyme in pros-taglandin biosynthesis) mediates inflammatory response, and it is induced by cytokines such as IL2, IL6, and IL10. By recruiting genomic pathway, 1,25OH2D3 suppresses transcription of COX2 gene, which can lead to the succeeding inhibition of proinflammatory cytokines secretion.Citation33,Citation34 On the contrary, 1,25OH2D3 works as a neurotrophic support through upregulation of GDNF (glial cell line-derived neurotrophic factor), NT3 (neurotrophin 3), and downregulation of NT4 (neurotrophin 4).Citation35 has summarized the role of vitamin D in four pathways involved in the etiology of schizophrenia. CYP27B1 converts vitamin D to its active form 1,25OH2D3, which is then bound to its receptor VDR. VDR heterodimerized with retinoid × receptor and modulates the expression of target genes. As mentioned above, it elevates GSH levels and upregulates mRNA expression of NGF, NT3, GDNF, and GGT, while it downregulates inflammation and mRNA expression of iNOS, COX2, and NT4 in astrocytes and microglia. CYP24A1 degrades this active form and terminates its effects.Citation30 In this study, we observed approximately two-fold increase in CYP24A1 expression level, which can be justified by positive feedback through VDR as a result of increase in CYP27B1. Moreover, we found that patients with schizophrenia had significant upregulation in VDR and CYP27B1 gene expression. Therefore, we hypothesized that these findings might be a consequence of schizophrenic disorder in order to apply the compensatory mechanism to promote antioxidant protection against oxidative and nitrosative stress as well as alleviating neuroinflammation and boosting neurotrophic support in patients affected by schizophrenia. Finally, the main limitation of this study is the lack of first-episode schizophrenia patients in order to evaluate the gene expression free of possible confounding effects of medication. Moreover, we did not have information about vitamin D status of study participants, and we did not have access to postmortem brain tissues to evaluate expression of genes in them.
Conclusion
Schizophrenia is a severe mental condition with a diversified set of pathologies such as perturbed dopamine and glutamate pathways, aberrant redox regulation, and neuroinflammation, all of which create a common central pathway.Citation29 The active form of vitamin D upregulates or downregulates numerous factors acting in these pathways. Both VDR coding mRNA and its protein are present in the CNS. The mRNA expression of VDR gene has been identified both in neuronal and glial cells. Our findings suggest that increased mRNA expression of VDR and CYP27B1 may be involved in the modulation of mentioned pathways through the genomic mechanism in order to ameliorate symptoms of the disease. Further investigations are needed to support this claim and determine the exact regulatory mechanism of vitamin D in each pathway. It is also valuable to measure the expression levels of VDR and related enzymes simultaneously with that of other molecular agents involved in oxidative, nitrosative stress, immune activation, and neurotrophic support in schizophrenia patients.
Acknowledgments
The authors would like to thank the schizophrenic patients and the Schizophrenia Society of Iran for their kind contribution in conducting this study. This study was supported technically and financially by Shahid Beheshti University of Medical Sciences.
Supplementary material
Table S1 The sequences of probes and primers
Disclosure
The authors report no conflicts of interest in this work.
References
- EscuderoIJohnstoneMGenetics of schizophreniaCurr Psychiatry Rep2014161150225200985
- AmaralADCalhauCCoelhoRSchizophrenia: implications of vitamin D deficit on brain developmentIJCNMH2014116114
- CieslakKFeingoldJAntoniusDLow vitamin D levels predict clinical features of schizophreniaSchizophr Res20141592–354354525311777
- YükselRNAltunsoyNTikirBCorrelation between total vitamin D levels and psychotic psychopathology in patients with schizophrenia: therapeutic implications for add-on vitamin D augmentationTher Adv Psychopharmacol20144626827525489478
- EylesDAlmerasLBenechPDevelopmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brainJ Steroid Biochem Mol Biol20071033–553854517293106
- ProsserDEJonesGEnzymes involved in the activation and inactivation of vitamin DTrends Biochem Sci2004291266467315544953
- GrovesNJMcGrathJJBurneTHVitamin D as a neurosteroid affecting the developing and adult brainAnnu Rev Nutr20143411714125033060
- SchusterICytochromes P450 are essential players in the vitamin D signaling systemBiochim Biophys Acta20111814118619920619365
- DelucaGCKimballSMKolasinskiJRamagopalanSVEbersGCReview: the role of vitamin D in nervous system health and diseaseNeuropathol Appl Neurobiol201339545848423336971
- RahimiSSayadAMoslemiEGhafouri-FardSTaheriMBlood assessment of the expression levels of matrix metalloproteinase 9 (MMP9) and its natural inhibitor, TIMP1 genes in Iranian schizophrenic patientsMetab Brain Dis20173251537154228578515
- AzimiTGhafouri-FardSDavood OmraniMVaccinia related kinase 2 (VRK2) expression in neurological disorders: schizophrenia, epilepsy and multiple sclerosisMult Scler Relat Disord201819151929100046
- Nafisi-FarNGhafouri-FardSPanahASTSayadATaheriMA gender dimorphism in up-regulation of BACE1 gene expression in schizophreniaMetab Brain Dis201833393393729500546
- KulkarniJRiedelAde CastellaAREstrogen – a potential treatment for schizophreniaSchizophr Res200148113714411278160
- PatrickRPAmesBNVitamin D hormone regulates serotonin synthesis. Part 1: relevance for autismFASEB J20142862398241324558199
- PatrickRPAmesBNVitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behaviorFASEB J20152962207222225713056
- KuningasMMooijaartSPJollesJSlagboomPEWestendorpRGvan HeemstDVDR gene variants associate with cognitive function and depressive symptoms in old ageNeurobiol Aging200930346647317714831
- AhmadiSMirzaeiKHossein-NezhadAShariatiGVitamin D receptor FokI genotype may modify the susceptibility to schizophrenia and bipolar mood disorder by regulation of dopamine D1 receptor gene expressionMinerva Med2012103538339123042374
- NerhusMBergAOKvitlandLRLow vitamin D is associated with negative and depressive symptoms in psychotic disordersSchizophr Res20161781–3444927595553
- SmoldersJThewissenMTheunissenRVitamin D-related gene expression profiles in immune cells of patients with relapsing remitting multiple sclerosisJ Neuroimmunol20112351–2919721507492
- FatemiSHFolsomTDReutimanTJNovakJEngelRHComparative gene expression study of the chronic exposure to clozapine and haloperidol in rat frontal cortexSchizophr Res20121342–321121822154595
- LeeBJMarchionniLAndrewsCEAnalysis of differential gene expression mediated by clozapine in human postmortem brainsSchizophr Res2017185586628038920
- KoradeŽLiuWWarrenEBArmstrongKPorterNAKonradiCEffect of psychotropic drug treatment on sterol metabolismSchizophr Res2017187748128202290
- HolmøyTMoenSMGundersenTA25-hydroxyvitamin D in cerebrospinal fluid during relapse and remission of multiple sclerosisMult Scler200915111280128519808741
- SmoldersJSchuurmanKGvan StrienMEExpression of vitamin D receptor and metabolizing enzymes in multiple sclerosis-affected brain tissueJ Neuropathol Exp Neurol20137229110523334593
- DoKQCabungcalJHFrankASteulletPCuenodMRedox dysregulation, neurodevelopment, and schizophreniaCurr Opin Neurobiol200919222023019481443
- BerridgeMJVitamin D cell signalling in health and diseaseBiochem Biophys Res Commun20154601537125998734
- GarcionESindjiLLeblondelGBrachetPDarcyF1,25-dihy-droxyvitamin D3 regulates the synthesis of gamma-glutamyl transpep-tidase and glutathione levels in rat primary astrocytesJ Neurochem199973285986610428085
- KogaMSerritellaAVSawaASedlakTWImplications for reactive oxygen species in schizophrenia pathogenesisSchizophr Res20161761527126589391
- SteulletPCabungcalJMoninARedox dysregulation, neuroinflammation, and NMDA receptor hypofunction: a “central hub” in schizophrenia pathophysiology?Schizophr Res20161761415125000913
- GarcionEWion-BarbotNMontero-MeneiCNBergerFWionDNew clues about vitamin D functions in the nervous systemTrends Endocrinol Metab200213310010511893522
- SnyderMAGaoWJNMDA hypofunction as a convergence point for progression and symptoms of schizophreniaFront Cell Neurosci201373123543703
- BitanihirweBKWooTUOxidative stress in schizophrenia: an integrated approachNeurosci Biobehav Rev201135387889320974172
- MüllerNUlmschneiderMScheppachCCOX-2 inhibition as a treatment approach in schizophrenia: immunological considerations and clinical effects of celecoxib add-on therapyEur Arch Psychiatry Clin Neurosci20042541142214991374
- WangQHeYShenYVitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4J Biol Chem201428917116811169424619416
- KrivoyAHochmanESendtKVAssociation between serum levels of glutamate and neurotrophic factors and response to clozapine treatmentSchizophr Res201819222623128599751