Abstract
Background
In recent years, there has been substantial research evaluating the relationship between arachidonate 5-lipoxygenase-activating protein (ALOX5AP) polymorphisms and ischemic stroke (IS). The objective of this study was to systematically review and analyze the existing evidence.
Methods
A comprehensive search of major electronic databases for studies published between 1990 and 2018 was carried out. Data were synthesized as OR and 95% CI using fixed-effects and random-effects models.
Results
A total of 30 studies were available for analysis. The aggregate sample size across all studies was 32,782 (16,294 cases and 16,488 controls). We found no association of the ALOX5AP rs10507391 (OR=1.03 for A allele vs T allele; 95% CI: 0.93–1.14; P=0.557), rs4769874 (OR=1.13 for A allele vs G allele; 95% CI: 1.00–1.28; P=0.050), rs9551963 (OR=1.03 for A allele vs C allele; 95% CI: 0.96–1.11; P=0.372), rs17222814 (OR=1.09 for A allele vs G allele; 95% CI: 0.96–1.24; P=0.195), rs17222919 (OR=0.89 for G allele vs T allele; 95% CI: 0.75–1.06; P=0.175), and rs4073259 (OR=1.20 for A allele vs G allele; 95% CI: 1.00–1.45; P=0.056) polymorphisms with IS risk. Haplotype analysis also did not yield significant findings for the HapA (rs17222814G–rs10507391T–rs4769874G–rs9551963A; OR=1.20; 95% CI: 0.91–1.56; P=0.192) and HapB (rs17216473A–rs10507391A–rs9315050A–rs17222842G; OR=1.11; 95% CI: 0.90–1.38; P=0.339) haplotypes.
Conclusion
Current evidence does not support an association of rs10507391, rs4769874, rs9551963, rs17222814, rs17222919, rs4073259, and HapA and HapB with IS risk.
Introduction
Stroke is the leading cause of mortality and serious long-term disability in China and worldwide, with ~15 million people throughout the world suffering a stroke every year.Citation1 Ischemic stroke (IS) accounts for ~85%–90% of all cases and is characterized by the sudden loss of blood circulation to an area of the brain. Strong evidence from twin, family, and animal model studies has consistently suggested a genetic influence on IS risk and prognosis, and in recent years, there has been substantial research evaluating specific genetic risk factors.Citation2
Leukotrienes are short-lived lipid mediators derived from the nuclear membrane of cells that are produced and excreted in response to various immune stimuli. The leukotriene pathway starts with oxidation of arachidonic acid to leukotriene A4 (LTA4) by lipoxygenase-activating protein (ALOX5AP).Citation3 The resultant LTA4 can then be converted to leukotriene B4 (LTB4) by leukotriene A4 hydrolase or it can be conjugated with reduced glutathione by the leukotriene C4.Citation3 As a crucial regulator of biosynthesis of proinflammatory leukotriene lipid mediators, ALOX5AP plays an important role in the development of atherosclerosis. Pharmacological targeting of ALOX5AP significantly reduced atherosclerosis burden and vascular as well as adipose tissue T-cell inflammation.Citation4 In addition, knockout of the ALOX5AP gene was associated with ceased leukotriene production and amelioration of stroke damage in a mouse model of middle cerebral artery occlusion.Citation5 The findings from clinical trials were consistent with those from animal experiments. Hakonarson et al found that the ALOX5AP inhibitor significantly decreased the concentration of the C-reactive protein, a contributor to the risk of myocardial infarction and IS.Citation6 Moreover, ALOX5AP mRNA and protein increased significantly in polymorphonuclear cells from OSA patients vs controls and were associated with carotid luminal diameter and intima–media thickness.Citation7
The human ALOX5AP is located on chromosome 13q12-13 and consists of five exons and four introns. Genetic variants in ALOX5AP gene have been studied in stroke cases, but case–control studies performed in different ethnic populations obtain contradictory results, indicating that the existing evidence regarding the association between ALOX5AP polymorphisms and IS risk needs to be systematically reviewed and analyzed. To reconcile inconsistencies across individual studies, we performed a quantitative meta-analysis of the effects of ALOX5AP polymorphisms on IS.
Methods
Search strategy
We searched Embase, Medline, PubMed, and China Knowledge Resource Integrated in July 2018 for studies investigating the relationship between ALOX5AP polymorphisms and IS risk. Searches were not limited by date restrictions and language. Search terms included: (“ALOX5AP polymorphism”, or “ALOX5AP variant”, or “ALOX5AP genotype”), AND (“cerebral infarction” OR “ischemic stroke”). The “AND” operator was used to combine these terms in varying combinations. Once articles had been collected, bibliographies were then hand-searched for additional references. The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.Citation8
Inclusion and exclusion criteria
Two investigators independently assessed titles and abstracts for relevance and full reports for inclusion. Discrepancies were resolved by discussion. Studies were included in our meta-analysis if they met the following criteria: 1) reported on case–control studies in adult humans; 2) published in peer-reviewed journals; and 3) reported genotypic and/or allelic frequencies. The exclusion criteria were as follows: 1) family-based studies; 2) case-only studies; 3) no information on genotypic and/or allelic frequencies; and 4) editorials, narrative reviews or other manuscripts not reporting primary data. If the paper, or author correspondence, suggested overlapping studies, we included only the most comprehensive study for meta-analysis.
Data extraction and quality assessment
Data were tabled in a standardized Excel sheet, and each group comparison was checked by two investigators to confirm accuracy of inclusion. The following information was abstracted from each study: journal, first author, year of publication, participant characteristics, geographical location, dominant ancestry of sample, diagnostic method for IS, numbers of patients and controls, DNA extraction and genotyping methods, allele frequency, and Hardy–Weinberg equilibrium (HWE) in controls. Authors were not contacted to request missing/additional data. For assessing the methodological quality of the primary studies, a quantitative Newcastle–Ottawa Scale (NOS) score was calculated for each study, which was based on the selection of the study groups, the comparability of the groups, and the ascertainment of the exposure.Citation9 Each study was graded as either low (scores 0–5) or high quality (scores 6–9).
Statistical analyses
All statistical analyses were performed using Stata 12.0 software (Stata, College Station, TX, USA). Since the majority of studies reported allele frequency instead of genotype data, the relationship between ALOX5AP polymorphisms and IS risk was assessed under allele contrast. All associations were presented as OR with the corresponding 95% CI. The significance of the pooled OR was determined using a Z-test. Heterogeneity was assessed by Cochrane’s Q test of heterogeneity and the I2 statistic. The I2 values of 25%, 50%, and 75% corresponded to low, medium, and high between-study heterogeneity. Summary ORs were calculated using random-effects models (DerSimonian and Laird) if there was significant between-study heterogeneity.Citation10 Random-effects modeling assumes a genuine diversity in the results of various studies and takes into account both within-study and between-study variances. Stratified analyses by sample ancestry were performed in order to assess the potential moderating effect of ancestry. HWE deviation was assessed by using the chi-squared goodness-of-fit test. Sensitivity analysis was conducted by omitting studies with HWE deviation. Funnel plots, Begg’s and Egger’s tests were used to assess publication bias. The standard error of log (OR) of each study was plotted against its log (OR) in funnel plots. The funnel plot symmetry was evaluated by Begg’s and Egger’s tests, which used the correlation between the ranks of effect sizes and the ranks of their variances. A P-value of less than 0.05 was considered statistically significant, except for Cochrane’s Q test, in which a P-value less than 0.10 was applied. No corrections for multiple comparisons were performed.
Results
Study characteristics
A total of 302 studies were identified by the search strategy. After duplicates were removed, 176 studies remained. On examination of the full text, 30 papers were eligible for inclusion in the review.Citation11–Citation40 Study selection is shown in . These 30 studies were reported between 2005 and 2018 and included 32,782 participants. Sample sizes ranged from 200 to 3,727 participants per study and represented Caucasian and Asian populations. Imaging (magnetic resonance imaging/computerized tomography) examination of the brain to support the clinical diagnosis was conducted in all studies. The distribution of genotypes in the controls of all studies was in agreement with HWE except for the studies by Cheng et al, Zhao et al, Xu et al, and Diakite et al.Citation21,Citation26,Citation29,Citation37 displays the study characteristics for the identified studies.
Table 1 Summary of included studies
Data synthesis
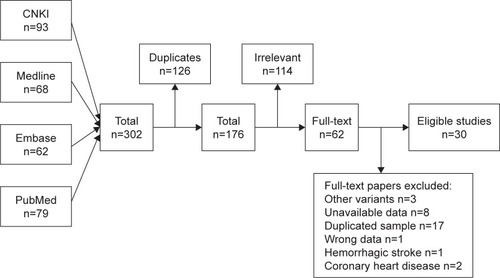
Twenty studies provided summaries of data on the ALOX5AP rs10507391 polymorphism.Citation13–Citation18,Citation20,Citation22,Citation23,Citation25,Citation26,Citation28,Citation29,Citation31,Citation32,Citation34–Citation38 Random-effects meta-analysis indicated no association of the polymorphism with IS in the overall population (OR=1.03 for A allele vs T allele; 95% CI: 0.93–1.14; P=0.557) (; ). There was significant between-study heterogeneity (I2=81.6%, P<0.001). In the subgroup analysis, according to ethnicity, no association between the ALOX5AP rs10507391 polymorphism and IS risk was found in Asian (OR=1.10 for A allele vs T allele; 95% CI: 0.98–1.23; P=0.109) and Caucasian (OR=0.89 for A allele vs T allele; 95% CI: 0.76–1.02; P=0.101) populations (; ).
Figure 2 Forest plots for the association of ALOX5AP polymorphisms with IS risk.
Abbreviation: IS, ischemic stroke.
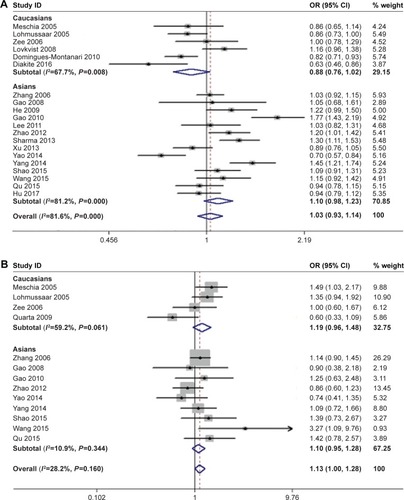
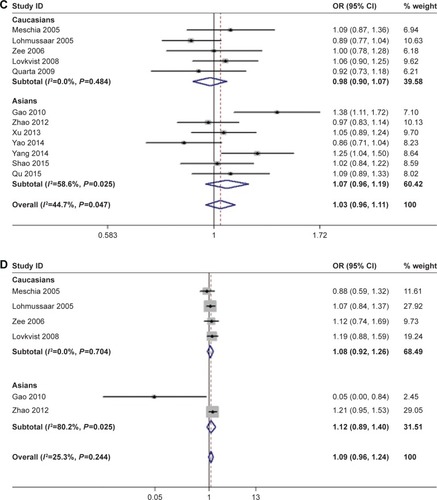
Table 2 Meta-analysis of association between ALOX5AP polymorphisms and IS risk
Thirteen studies contributed to the pooled analysis of the ALOX5AP rs4769874 polymorphism (A allele vs G allele).Citation13–Citation17,Citation19,Citation23,Citation26,Citation31,Citation32,Citation34–Citation36 Fixed-effect meta-analysis gave an estimated OR of 1.13 (95% CI: 1.00–1.28; P=0.050), indicating no significant association with IS (; ). There was no significant between-study heterogeneity (I2=10.9%, P=0.160). Neither Asian (OR=1.10, 95% CI 0.95–1.28, P=0.210) nor Caucasian (OR=1.19, 95% CI 0.96–1.48, P=0.106) studies found an association with IS (; ).
Twelve studies examined the ALOX5AP rs9551963 polymorphism.Citation13–Citation15,Citation18,Citation19,Citation23,Citation26,Citation29,Citation31,Citation32,Citation34,Citation36 In the primary pooled analysis (; ), we identified no significant association between the polymorphism and IS risk (OR=1.03 for A allele vs C allele; 95% CI: 0.96–1.11; P=0.372). There was significant between-study heterogeneity (I2=44.7%, P=0.047). In the subgroup analysis based on ethnicity, studies with patients of Asian descent or Caucasian descent yielded similar findings (Asian population: OR=1.07, 95% CI 0.96–1.19, P=0.230; Caucasian population: OR=0.98, 95% CI 0.90–1.07, P=0.656), indicating no association with IS (; ).
Six studies provided data for the ALOX5AP rs17222814 polymorphism.Citation13–Citation15,Citation18,Citation23,Citation26 Fixed-effect meta-analysis gave an estimated OR of 1.09 (95% CI: 0.96–1.24; P=0.195), indicating no association with IS in the overall population (; ). There was no significant between-study heterogeneity (I2=25.3%, P=0.244). In the subgroup analysis by ethnicity, neither Asian nor Caucasian studies yielded significant findings ().
Four Asian studies in three publications investigated the role of the ALOX5AP rs17222919 polymorphism.Citation24,Citation27,Citation33 Random-effects meta-analysis indicated no association of the SNP with IS (OR=0.89 for G allele vs T allele; 95% CI: 0.75–1.06; P=0.175) (). Similarly, four Asian studies providing summaries of data on the ALOX5AP rs4073259 polymorphism did not find any association with IS risk (OR=1.20 for A allele vs G allele; 95% CI: 1.00–1.45; P=0.056) ().Citation21,Citation30,Citation40
In addition to individual polymorphisms, we performed combined analysis for haplotypes. HapA was defined by rs17222814, rs10507391, rs4769874, and rs9551963 polymorphisms, with alleles G, T, G, and A. No association between HapA and IS risk was found (OR=1.20, 95% CI: 0.91–1.56, P=0.192) (). The pooled effect estimate for HapB (rs17216473A–rs10507391A–rs9315050A–rs17222842G) was similar, indicating no significant association with IS (OR=1.11, 95% CI: 0.90–1.38, P=0.339) ().
Publication bias and sensitivity analysis
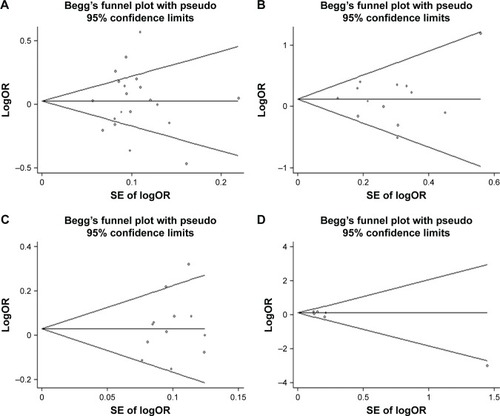
Funnel plots did not suggest publication bias (). The results of Begg’s and Egger’s tests for small-study effect biases were not significant, indicating no evidence of publication bias (). For the ALOX5AP rs10507391 and rs9551963 polymorphisms, sensitivity analyses were performed to assess the stability of the results by omission of studies, which were not in HWE. The significance of pooled ORs was not influenced.
Figure 3 Funnel plots to detect publication bias.
Abbreviations: IS, ischemic stroke; SE, standard error.
Discussion
IS is the leading cause of morbidity and mortality in industrialized countries. Given the limited therapeutic options for IS patients, there remains great interest in identifying novel therapeutic target that may prevent stroke or improve stroke recovery.
The ALOX5AP gene encodes a major regulator for 5-lipoxygenase that catalyzes the oxidation of arachidonic acid to LTA4, which is released by inflammatory cells at injured sites and thus plays an important role in atherosclerosis and other vascular damage.Citation41 In the literature, there are apparently conflicting associations reported between ALOX5AP polymorphisms and IS risk. The conflicting results were possibly because of small effects of ALOX5AP polymorphisms on IS risk or the relatively low statistical power of published studies. To summarize the available evidence, we combined studies involving a total of 32,782 participants. Six single polymorphisms and two haplotypes were analyzed. Our analysis did not support an association of the ALOX5AP rs10507391, rs4769874, rs9551963, rs17222814, rs17222919, and rs4073259 polymorphisms with IS risk in Asian and Caucasian populations. Haplotype analysis also did not yield significant findings for the HapA (rs17222814G– rs10507391T–rs4769874G–rs9551963A) and HapB (rs17216473A–rs10507391A–rs9315050A–rs17222842G) haplotypes. To the best of our knowledge, this is the largest and most comprehensive meta-analysis on the association between ALOX5AP polymorphisms and IS risk.
Our results are in agreement with a previous meta-analysis published in 2009. Zintzaras et al analyzed the relationship between ALOX5AP polymorphisms and risk of stroke with a total of 5,194 cases and 4,566 controls, finding no association of rs17222814, rs10507391, rs4769874, rs9551963, HapA, and HapB with stroke.Citation42 Although the two meta-analyses obtained similar findings, there were still some differences. First, both hemorrhagic and IS patients were included in the Zintzaras et al’s study, whereas we only took into account IS patients. Second, Zintzaras et al’s meta-analysis overtook some Asian studiesCitation16,Citation17,Citation20 because they excluded the non-English papers. Third, Zintzaras et al’s analysis arose from 12 studies, considerably less in number (5,194 cases and 4,566 controls) than the overall 30 in our study (16,294 cases and 16,488 controls). Besides Zintzaras et al’s analysis, the Wang et al’sCitation43 meta-analysis involving 10 studies and the Chen et al’sCitation44 meta-analysis combining seven studies investigated rs10507391.Citation43,Citation44 The positive association between rs10507391 and IS risk reported by these two small size meta-analyses was not confirmed by our study applying a large sample.
It should be mentioned that much effort has been performed to conduct appropriately the meta-analysis and avoid any possible source of bias. To avoid the local literature bias, we included both English and non-English articles. We properly assessed deviations from HWE in the control population and performed sensitivity analysis excluding studies where the sample violated HWE to ensure stability of the pooled ORs. In addition, we evaluated small-study effects and publication bias through Begg’s and Egger’s tests; there was no evidence of systematic missingness of scientific results from the literature. Finally, subgroup analysis by ethnicity confirmed the lack of association between the ALOX5AP polymorphisms and IS risk not only in the overall populations but also in each ethnic group.
Previous studies demonstrated the presence of strong linkage disequilibrium in the ALOX5AP gene.Citation14,Citation15,Citation32 It is believed that haplotype analysis can provide more information than single-locus analysis because it allows us to explore potential interactions among alleles. In this meta-analysis, we evaluated the relationship of the HapA (rs17222814G– rs10507391T–rs4769874G–rs9551963A) and HapB (rs17216473A–rs10507391A–rs9315050A–rs17222842G) haplotypes with IS risk. Although no associations of these haplotypes with IS were identified, we could not exclude that other haplotypes may play a role in the development of IS. The study by Zhao et al reported a statistically significant association between the haplotype GCGA constructed by rs17222814, rs10507391, rs4769874, and rs9551963 and IS risk.Citation26 In addition, Yao et al found that the haplotype TGC constructed using rs10507391, rs4769874, and rs9551963 was associated with the increased risk of IS (OR=1.60, 95% CI: 1.28–1.98, P<0.001), while the haplotype AGA was protective (OR=0.66, 95% CI: 0.53–0.81) in a case–control study, involving a total of 420 cases and 488 controls.Citation31 Considering the large sample sizes applied by these studies, further studies are warranted to confirm their findings.
Our study has some limitations that should be considered. First, the relationship between the ALOX5AP polymorphisms and the levels of leukotriene lipid mediators in IS patients was not evaluated due to lack of published data. Increased levels of products of the 5-lipoxygenase (5-LO)/5-LO-activating protein (FLAP) pathway including LTB4 had been reported in IS patients.Citation45 The evaluation of potential interactions between ALOX5AP polymorphisms and leukotriene lipid mediators in IS patients could provide valuable insights into the development of IS. Second, because there was a paucity of data, we did not assess gender differences in the effects of ALOX5AP polymorphisms on IS risk. It is widely known that gender has a complex and interactive effect on IS risk and there are gender differences in the prevalence of IS.Citation46 More research is needed to evaluate gender differences in the association of ALOX5AP polymorphisms with IS development.
In conclusion, our meta-analysis of 30 studies involving 16,294 IS patients and 16,488 controls indicates no significant association of the ALOX5AP polymorphisms rs10507391, rs4769874, rs9551963, rs17222814, rs17222919, and rs4073259 with IS risk. In addition, the HapA and HapB haplotypes also did not show any association with IS.
Acknowledgments
This work was supported, in part, by the National Natural Science Foundation of China (81660776 and 81360535), the Natural Science Foundation of Guangxi Zhuang Autonomous Region (2016JJA140115), and the Science Research and Technology Development Project of Guangxi Zhuang Autonomous Region (1598012-55).
Disclosure
The authors report no conflicts of interest in this work.
References
- VenketasubramanianNYoonBWPandianJNavarroJCStroke epidemiology in south, east, and south-east Asia: a reviewJ Stroke201719328629429037005
- MalikRDichgansMChallenges and opportunities in stroke geneticsCardiovasc Res201811491226124029554300
- MashimaROkuyamaTThe role of lipoxygenases in pathophysiology; new insights and future perspectivesRedox Biol2015629731026298204
- BäckMSultanAOvchinnikovaOHanssonGK5-Lipoxygenase-activating protein: a potential link between innate and adaptive immunity in atherosclerosis and adipose tissue inflammationCirc Res2007100794694917379835
- StrömJOStridTHammarströmSDisruption of the alox5ap gene ameliorates focal ischemic stroke: possible consequence of impaired leukotriene biosynthesisBMC Neurosci20121314623194405
- HakonarsonHThorvaldssonSHelgadottirAEffects of a 5-lipoxygenase-activating protein inhibitor on biomarkers associated with risk of myocardial infarction: a randomized trialJAMA2005293182245225615886380
- Stanke-LabesqueFPépinJLde JouvencelTLeukotriene B4 pathway activation and atherosclerosis in obstructive sleep apneaJ Lipid Res20125391944195122761257
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementBMJ2009339jul21 1b253533619622551
- StangACritical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analysesEur J Epidemiol201025960360520652370
- DerSimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- HelgadottirAManolescuAThorleifssonGThe gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and strokeNat Genet200436323323914770184
- HelgadottirAGretarsdottirSSt ClairDAssociation between the gene encoding 5-lipoxygenase-activating protein and stroke replicated in a Scottish populationAm J Hum Genet200576350550915640973
- MeschiaJFBrottTGBrownRDPhosphodiesterase 4D and 5-lipoxygenase activating protein in ischemic strokeAnn Neurol200558335136116130105
- LõhmussaarEGschwendtnerAMuellerJCALOX5AP gene and the PDE4D gene in a central European population of stroke patientsStroke200536473173615731479
- ZeeRYChengSHegenerHHErlichHARidkerPMGenetic variants of arachidonate 5-lipoxygenase-activating protein, and risk of incident myocardial infarction and ischemic stroke: a nested case-control approachStroke20063782007201116778124
- ZhangWLYangXMShiJSunKHuiRTPolymorphism of SG13S114T/A in the ALOX5AP gene and the risk for stroke in a large Chinese cohortYi Chuan Xue Bao200633867868416939001
- GaoZSQcDTo study relationship between polymorphism of SG13S114T/A and SG13S89G/A in the ALOX5AP gene and thrombotic strokeNingxia Med J2008305388390
- LövkvistHSmithJGLuthmanHIschaemic stroke in hypertensive patients is associated with variations in the PDE4D genome regionEur J Hum Genet20081691117112518398440
- QuartaGStanzioneREvangelistaAPhosphodiesterase 4D and 5-lipoxygenase activating protein genes and risk of ischemic stroke in SardiniansEur J Hum Genet200917111448145319417766
- HeYLZhuMJinXPZhouYLRelationship of polymorphism of SG13S114A/T in ALOX5AP gene with atherosclerotic cerebral infarctionZhejiang Da Xue Xue Bao Yi Xue Ban200938663063320014490
- ChengHJinQWDingXSAssociation of ALOX5AP and PDE4D genes with the risk of lacunar infarction in Nanjing city of Jiangsu provinceActa Univ Med Nanjing20103013945
- Domingues-MontanariSFernández-CadenasIdel Rio-EspinolaAAssociation of a genetic variant in the ALOX5AP with higher risk of ischemic stroke: a case-control, meta-analysis and functional studyCerebrovasc Dis201029652853720357438
- GaoZTianGPRelationship between ALOX5AP gene single nucleotide polymorphism and stroke of Han population in Shenyang CityJ Apop Nerve Dis2010271110221026
- KimDHAhnWYKimDKA Promoter polymorphism (rs17222919, -1316T/G) of ALOX5AP is associated with intracerebral hemorrhage in Korean populationProstaglandins Leukot Essent Fatty Acids2011853–411512021816595
- LeeJDLeeTHHuangYCALOX5AP genetic variants and risk of atherothrombotic stroke in the Taiwanese populationJ Clin Neurosci201118121634163822051033
- ZhaoJWangXXuJAssociation of inflammatory response gene polymorphism with atherothrombotic stroke in Northern Han ChineseActa Biochim Biophys Sin201244121023103023076369
- WangYWangGNSunHChenCXiaoHZhangJSAssociation of ALOX5AP with ischemic stroke in eastern ChineseWorld J Emerg Med20123210811325215047
- SharmaVDadheechSKaulSJyothyAMunshiAAssociation of ALOX5AP1 SG13S114T/A variant with ischemic stroke, stroke subtypes and aspirin resistanceJ Neurol Sci20133311–210811323746795
- XuCQiangLLimeiCCorrelation between cerebral infarction and ALOX5AP gene expressionCell Biochem Biophys201367389990423546934
- ZhangRGuoXLiXArachidonate 5-lipoxygenase-activating protein (ALOX5AP) gene rs4073259 polymorphism not associated with ischemic stroke in the northeastern Chinese Han populationClin Neurol Neurosurg2014119646924635928
- YaoQZhangCZhangXSynergistic effect of ALOX5AP polymorphisms and cigarette smoking on the risk of atherosclerotic cerebral infarction in a Northern Han Chinese populationJ Clin Neurosci201421697597924411318
- YangDHeYLiMA novel risk haplotype of ALOX5AP gene is associated with ischemic stroke in Chinese Han populationJ Mol Neurosci201453349349924198186
- FanYChenHLiAA promoter polymorphism (rs17222919, -1316T/G) of ALOX5AP gene is associated with decreased risk of ischemic stroke in two independent Chinese populationsPLoS One2015103e012239325815512
- ShaoMYiXChiLLinJZhouQHuangRIschemic stroke risk in a southeastern Chinese population: insights from 5-lipoxygenase activating protein and phosphodiesterase 4D single-nucleotide polymorphismsJ Formos Med Assoc2015114542242924485247
- WangJNYangZNSuiQThe polymorphism of ALOX5AP and PDE4D gene and molecular epidemiology of the susceptibility of cerebral infarctionJ Bengbu Med Coll2015401013381340
- QuZSuFZhuYA tagging ALOX5AP polymorphism and risk of ischemic stroke in a northeastern Chinese Han populationInt J Clin Exp Med2015811213432135026885075
- DiakiteBHamziKHmimechWNadifiSGMRAVCGenetic polymorphisms of T-1131C APOA5 and ALOX5AP SG13S114 with the susceptibility of ischaemic stroke in MoroccoJ Genet201695230330927350673
- HuXWangJLiYThe β-fibrinogen gene 455G/A polymorphism associated with cardioembolic stroke in atrial fibrillation with low CHA2DS2-VaSc scoreSci Rep2017711751729235504
- LiuDLiuLSongZHuZLiuJHouDGenetic variations of oxidative stress related genes ALOX5, ALOX5AP and MPO modulate ischemic stroke susceptibility through main effects and epistatic interactions in a chinese populationCell Physiol Biochem20174341588160229041000
- ShiYXuLFengQAllele-specific methylation contributed by CpG-SNP is associated with regulation of ALOX5AP gene expression in ischemic strokeNeurol Sci2018391017171724
- PasterkampGvan der LaanSWHaitjemaSHuman validation of genes associated with a murine atherosclerotic phenotypeArterioscler Thromb Vasc Biol20163661240124627079880
- ZintzarasERodopoulouPSakellaridisNVariants of the arachidonate 5-lipoxygenase-activating protein (ALOX5AP) gene and risk of stroke: a HuGE gene-disease association review and meta-analysisAm J Epidemiol2009169552353219126581
- WangGLiuRZhangJThe arachidonate 5-lipoxygenase-activating protein (ALOX5AP) gene SG13S114 polymorphism and ischemic stroke in Chinese population: a meta-analysisGene2014533246146824148560
- ChenZZhengJLiuWThe SG13S114 polymorphism of the ALOX5AP gene is associated with ischemic stroke in Europeans: a meta-analysis of 8062 subjectsNeurol Sci201738457958728101761
- WangGWangYSunHVariants of the arachidonate 5-lipoxygenase-activating protein (ALOX5AP) gene and risk of ischemic stroke in Han Chinese of eastern ChinaJ Biomed Res201125531932723554707
- Roy-O’ReillyMMcculloughLDAge and sex are critical factors in ischemic stroke pathologyEndocrinology201815983120313130010821