Abstract
Different literature reviews of gambling disorder (GD) neurobiology have been focused on human studies, others have focused on rodents, and others combined human and rodent studies. The main question of this review was: which are the main neurotransmitters systems and brain structures relevant for GD based on recent rodent studies? This work aims to review the experimental findings regarding the rodent´s neurobiology of GD. A search in the Pub Med database was set (October 2012–October 2017) and 162 references were obtained. After screening, 121 references were excluded, and only 41 references remained from the initial output. More, other 25 references were added to complement (introduction section, neuroanatomical descriptions) the principal part of the work. At the end, a total of 66 references remained for the review. The main conclusions are: 1) according to studies that used noninvasive methods for drug administration, some of the neurotransmitters and receptors involved in behaviors related to GD are: muscarinic, N-methyl-D-aspartate (NMDA), cannabinoid receptor 1 (CB1), cannabinoid receptor 2 (CB2), dopamine 2 receptor (D2), dopamine 3 receptor (D3), and dopamine 4 receptor (D4); 2) moreover, there are other neurotransmitters and receptors involved in GD based on studies that use invasive methods of drug administration (eg, brain microinjection); example of these are: serotonin 1A receptor (5-HT1A), noradrenaline receptors, gamma-aminobutyric acid receptor A (GABAA), and gamma-aminobutyric acid receptor B (GABAB); 3) different brain structures are relevant to behaviors linked to GD, like: amygdala (including basolateral amygdala (BLA)), anterior cingulate cortex (ACC), hippocampus, infralimbic area, insular cortex (anterior and rostral agranular), nucleus accumbens (NAc), olfactory tubercle (island of Calleja), orbitofrontal cortex (OFC), medial prefrontal cortex (mPFC), prefrontal cortex (PFC) – subcortical network, striatum (ventral) and the subthalamic nucleus (STN); and 4) the search for GD treatments should consider this diversity of receptor/neurotransmitter systems and brain areas.
Introduction
Literature reviews regarding gambling disorder (GD) neurobiology have been specialized on human,Citation1,Citation2 rodents,Citation3,Citation4 or combination of both.Citation5,Citation6 The main question of this review was: which are the main neurotransmitters systems and brain structures relevant for GD based on recent rodent studies? Let me define first gambling and its epidemiological traits, before going in its neurobiological aspects.
Gambling conduct can be described as to put in peril anything significant, and to confide on the assumption of obtaining a gain in return.Citation7 GD is characterized by gaming behaviors that seriously disrupt the finances, social relations, and professional advancement of a fellow.Citation8 The lifetime prevalence of GD has been estimated at 0.4% to 4.2%.Citation9 Moreover, GD is presently included in the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM)-5, in a novel category, within the division of addictions (behavioral addictions).Citation10
There have been some literature reviews of the neurobiology of GD focused on human clinical studies.Citation1,Citation2,Citation11–Citation14 Specifically, the Lemieux and al´Absi´s review proposed that psychological and neurobiological aspects of the stress play a significant role in the starting, prolongation, and relapse of the addictions (including GD). Moreover, the mechanisms include interactions between biological mediators of the stress and the reward system; also, interactions between mediators of the stress and other systems related to addiction (endogenous opioids, the sympathetic-adrenal-medullary system, and endocannabinoids).Citation1
Another review work by Grant et al posed that GD is linked with alteration across different cognitive domains related to impulsivity and compulsivity;Citation2 moreover, it pointed that, based on imaging reports, GD relates to anatomical and functional anomalies of nexus involved in the reward processing and top-down monitoring.Citation2 In addition, it pointed that probably, diverse neural systems are involved in the pathophysiology (related to serotonin (5-HT), glutamate, dopamine (DA), opioids, and norepinephrine).Citation2
Then again, the Goulet-Kennedy et al´s review points that prefrontal cortex (PFC) and the striatum are the main conductors of decision processes, based on clinical studies.Citation11 Furthermore, that literature review states that the traits of decision making´s neural networks can be characterized by means of imaging technology; also, they consider that non-invasive neural stimulation in the PFC, and its network (striatum and others) have elucidated the neurobiological basis of decision-making processes;Citation11 Decision making is involved in different aspects of our daily life,Citation11 including all the spectrums of gambling behavior. Hence, a better understanding of the decision process´ neurobiology could be useful for a better quality of life of patients.Citation11
The publications review of the Banz et al group emphasizes in the capacity of neurobiological data to help in the promotion of improved norms and strategies for treatment and prevention.Citation12 Furthermore, another review by Levy and Glimcher concludes that imaging investigations in humans suggest the existence of a brain network that codifies the values of rewards by means of a standard neural scale.Citation13 Based on the authors, the brain area linked with this standard neural scale is a zone of the ventromedial prefrontal cortex (vmPFC)/orbitofrontal cortex (OFC). The authors propose that a better comprehension of brain mechanism for estimating and deciding might provide basic discernments of abnormal choice conducts like those of gambling.Citation13
Also, an imaging meta-analyses review by Meng et al,Citation14 reports that GD fellows display a significantly higher activation (compared to healthy controls) in brain areas like right lentiform nucleus and left middle occipital gyrus. Moreover, the South Oaks Gambling Screen scores were linked with overactivity in the right lentiform nucleus and in the bilateral parahippocampus; but the scores were negatively linked to right middle frontal gyrus. Altogether, this suggests dysfunction within the frontostriatal cortical pathway in fellows with GD.Citation14
In addition, other reviews focus on both human and rodent.Citation5,Citation6,Citation15,Citation16 The Norbury and Husain publications review points that a high level of sensation seeking is a factor related to gambling and substance addiction.Citation5 Moreover, these authors support the existence of a relationship between sensation seeking and dopaminergic transmission, especially in the D2 receptors. Specifically, fellows with marked sensation seeking display also elevated DA tonic levels and an over-responsive midbrain dopaminergic responses to signals of future reward.Citation5 Moreover, Norbury and Husain propose that even for stimuli of similar strength, reactive responses could vary in terms of approach-avoidance displayed by the subject; the authors propose that these variations stem from differences in the efficiency of DA transmission at the level of the striatum.Citation5
Additionally, another review by Quintero concludes that pathological and nonpathological gamblers can differ in terms of brain´s anatomy, brain´s physiology, electroencephalography (EEG) profile, executive and cognitive efficiency.Citation6 For instance, fellows with GD can denote alterations in the insula, OFC, and frontal lobe;Citation6 moreover, fellows with GD compared to nonpathological gambler show differences in frontoparietal activation pattern (if winning or losing a game) and insular activity (altered cognitive interpretation of near-miss results and trial success) related to gaming.Citation6 With respect to anatomical differences between gamblers and non-gamblers, the first ones show more gray-matter volume compared to normal subjects, based on magnetic resonance imaging technology; furthermore, gamblers have a smaller size of right thalamus, right hippocampus, and left putamen compared to normal subjects.Citation6 Regarding research on rodent, this review states that the correctness of gambling decision is affected by the action of DA receptors and brain areas like insular cortex (rostral agranular zone), infralimbic, and prelimbic.Citation6
Another review by Potenza stresses that diverse neurotransmitters like glutamate, noradrenaline, DA, 5-HT, opioid, and brain structures like insula, ventral striatum, and vmPFC (among other areas) are linked to gambling and GD.Citation15 Furthermore, a literature review by van den Bos et al did focus on three almost ignored aspects of GD: developmental sex differences in GD, adolescence as a sensitive period for developing GD, and paths for upgrading ecological validity of investigative tools.Citation16
Finally, another set of reviews has been specialized on rodents.Citation3,Citation4,Citation17,Citation18 Particularly, a literature review by Alguacil and González-Martin stressed on the “umbrella category” of reward deficiency syndrome; this syndrome includes diverse neuropsychiatric and addiction disorders (including gambling).Citation3 More, these disorders share dysfunctional reward sensitivity, inadequate impulsivity, and/or compulsive conduct. That review considers that further investigation about the reward deficiency syndrome could ease the design of new drugs that are efficient for that cluster of disorders.Citation3
A publications review by Winstanley and Clark emphasizes that the adequate laboratory models for GD should screen fundamental cognitive procedures and have adequate translatability to different species;Citation4 moreover, models with these characteristics have a potential capacity to contribute to decision/neuroscience and the investigation of addictive behaviors.Citation4 Another review by Anselme stresses that deprivation and randomness, either psychological or physiological, increase the motivation for searching valuable stimuli;Citation17 moreover, this increase in motivation relates to the organism´s hardness for forecasting relevant environment´s stimulus and incidents.Citation17
FInally, a review by Cocker and Winstanley states that cognitive biases are important in the evolution of GD;Citation18 furthermore, these biases can be recreated in rodent models. In effect, some evidences suggest that biases can be linked to dopaminergic activity, especially in the D4 receptor; the authors suggest the exploration of the D4 receptor as an alternative for treating GD.Citation18
The present review aims to integrate recent research findings about the rodent neurobiology of GD and related behaviors. It is expected that this integration could ease the elaboration of most complete pharmacological and/or behavioral approaches for treating GD in human populations.
Materials and methods
Inclusion and exclusion criteria
The publications were selected based on the next inclusion criteria: a) rodent studies (mice or rat), b) experimental or quasi-experimental design, c) publications that include description about the relationship between the nervous system (brain and/or neurotransmitter) and gambling behavior or GD, d) publications that detail the number of animals, e) the sex of the animals could be male, female or not specified in the publication, f) publications written in English (at least title and abstract), and g) publications released within a recent five years temporal range: October 2012–October 2017. As a reference, some reviews were added, but principally for the introduction and discussion parts.
With respect to the exclusion criteria of the publications, these included the next: a) the non-compliance of the inclusion criteria, and b) it should not be an abstract, nor a publication of a scientific meeting, nor a publication included in non-scientific literature.
Inquiry strategy
A screening of publications in the Pub Med database was carried out based on the five recent years (October/01/2012–October/20/2017). The search terms included: “Gambling” AND “Brain”, “Gambling” AND “Neurobiology”, “Gambling Disorder” AND “Brain”, and “Gambling Disorder” AND “Neurobiology”. The next filters were added for the searching process: text availability (Abstract), species (other animals), Languages (English). Initially, 162 references were obtained in the Pub med search. A total of 121 references was eliminated by different factors (literature review or meta-analysis type, non-English language, human specie, duplicates, and others), resulting in a total of 41 references for subsequent analysis. In addition, another 25 complementary references were detected through references scanning or web searching, and added to the manuscript. As a reference, these 25 references related mainly to the background in the field (included in the introduction section) and to neuroanatomical references. The complete (full) forms of these 66 references were obtained on the web or solicited directly to the authors; later these references were evaluated for the preparation of the review.
More information is described in (Flow diagram of publication selection process). As a guide for the reader, the first part of the manuscript (results section) includes experimental works that manipulate neurotransmitters and receptors in a non-invasive way (for instance: subcutaneous administration (sc) or intraperitoneal administration (ip)). The second part describes brain structures, and also neurotransmitters/receptors that were evaluated in an invasive way (for instance, brain microinjection).
Figure 1 Flow diagram of publication selection process. The diagram presents the plan used for publications choice, starting from initial Pub Med database search, up to the final articles incorporated in the publication.
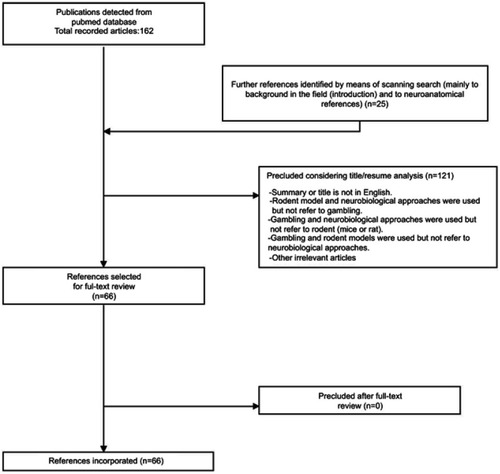
For the elaboration of this literature review, it was followed by the ethical principles and guidelines of the Helsinki Declaration.
Results
There are two tables summarizing the results: entitled “Summary of main studies included in the review – Neurotransmitters”, and entitled “Summary of main studies included in the review – Brain structures”. These tables detail different aspects of the studies revised like: neurotransmitters, messengers and receptors systems studied, the brain area, drug name, drug effects, drug route of administration, mental process and/or conduct analyzed, behavioral test (paradigm) used, specie (rat or mice), author and year of publication, and relevance of the study.
Table 1 Summary of main studies included in the review – Neurotransmitters
Table 2 Summary of main studies included in the review – Brain structures
Neurotransmitter
Acetylcholine receptor (cholinergic system)
The antagonism of muscarinic receptors (scopolamine, sc) but not of nicotinic receptors (mecamylamine hydrochloride, sc) impaired decision making in rat gambling tasks. Hence, muscarinic receptors can specifically disrupt decision making under conditions of risk and uncertainty (like those found in gambling).Citation19
NMDA antagonists
The blockade of NMDA receptors (but not AMPA (alpha-amino-3-hydroxy-5-methylisoxazolepropionate) receptors) with the antagonist MK-801 hydrogen maleate (non-competitive antagonist; sc) decreased sensitivity to delayed and uncertain reinforcement in rats, based on the delayed/probabilistic reinforcement, and on the sensitivity to reinforcer amount tests (operant conditioning chambers).Citation20 Moreover, the antagonism with ketamine hydrochloride (uncompetitive antagonist, ip) decreased sensitivity to reinforcer amount without altering delay/probability discounting in the same tests.Citation20 These findings suggest that NMDA receptors differentially mediate impulsivity, with MK-801 hydrogen maleate reducing impulsive choice, but augmenting risky decisions. It is relevant to consider this contrast for treating individuals displaying different psychiatric disorders characterized by impulsivity or risky decisions. In this case, a subject displaying marked impulsive choices would be treated better by means of a drug similar to MK-801 hydrogen maleate; nevertheless, the same medication might be inadequate for someone with GD.Citation20
CB1 and CB2
The blockade of CB1 (antagonist AM 4113, ip) or CB2 receptors (antagonist/inverse agonist AM 630, ip), or the inhibition of fatty-acid amide hydrolase (URB 597, ip) did not influence the rat gambling task performance.Citation21 However, the agonism of CB1 and CB2 receptors (WIN 55, 212-2, ip) improved choice strategy, and increased choice latency in the suboptimal group; but only increased perseverative behavior when punished, in the optimal group.Citation21 This could be interpreted as the stimulation of cannabinoid receptors could induce different gambling choice conducts based on the type of subjects; specifically, in healthy subjects (optimal group) induce inadequate conducts, but in dysfunctional subjects (suboptimal group) induced adequate conducts.Citation21
As a reference, it has been pointed out that the endocannabinoid system is associated with the reinforcing effects of drugs of abuse.Citation22 CB2 receptors have been linked to central functions, including a role in addictive processes.Citation23,Citation24 Moreover, CB1 receptors are located presynaptically, inhibit synaptic transmission, and allow synaptic modulation.Citation25 Furthermore, CB2 receptors are located in different zones of the nervous system: periphery,Citation26,Citation27 striatum, hippocampus, thalamus,Citation28 and ventral tegmental area (VTA).Citation29
DA receptors
An investigation did report that either D4 receptors agonism (PD 168077, ip) or D4 receptors antagonism (L-745,870, ip) had a minimal effect on latency measures and decision making, during the rodent gambling task.Citation30 Additionally, neither the D3 receptor agonism (PD 128907, ip) nor the D3 receptor antagonism (SB 277011-A, ip) influenced decision making.Citation30 Also, the antagonism of D2 receptor (L-741,626, sc) did not affect decision making.Citation30 In general, D2, D3, and D4 ligands did not influence significantly the choice behaviors of the rodent gambling task.
As reference, the D4 receptors can be found in the next areas within the nervous system: cerebral cortex, amygdala, hypothalamus, pituitary gland, visual system (retina),Citation31 and the basal ganglia.Citation32–Citation36 Furthermore, D3 receptors are localized in the islands of Calleja, mamnillary bodies, nucleus accumbens (NAc) shell, frontoparietal cortex, the substantia nigra/VTA, basolateral amygdala (BLA), and lateral habenula.Citation37–Citation40 In general, some authors agree that the specific localizations of D3 and D4 dopamine receptors in the nervous system support their roles in cognition and emotion.Citation41,Citation42
Another investigation reported that pramipexole (an agonist of D2 and D3 receptors, sc) induced GD tendencies based on a probability discounting task in rats.Citation43 Specifically, pramipexole augmented unfavorable decisions, disrupted the discounting of probabilistic losses, augmented risk-taking behaviors, distorted the representation of rewards, and impaired the ability to discern favorable from unfavorable contingencies.Citation43 Moreover, the results of complementary studies (voltammetry recordings and High Performance Liquid Chromatography (HPLC)) focused in the NAc suggested that pramipexole behavioral effects were separated from the dynamic changes related to mesolimbic DA release.Citation43
As a reference, the HPLC also measured besides dopamine level, the level of serotonin and norepinephrine. Moreover, the pump speed (Shimadzu LC-6A liquid chromatograph, Columbia, Maryland, United States of America) was 1.5 mL/min. The reverse-phase column utilized was a Rexchrom (Regis Technologies, Morton Grove, Illinois, United States of America) S50100-ODS C18 column with a length of 25 cm and an internal diameter of 4.6 mm. The compounds were measured at +0.7 V using a Shimadzu L-ECD-6A electrochemical detector.
Brain structures
Amygdala
Rat studies did show that amygdala low serotonergic metabolism, or its sustained activity related to poor decision making in the rat gambling task.Citation44 Moreover, the lesions of the rat BLA related to reduced risk seeking for losses, but intact risk aversion for gains, based on the loss – chasing task, and the betting task;Citation45 these data supported the hypothesis that the amygdala plays a more prominent role in choice biases related to losses. The Tremblay´s data suggested that risk seeking for losses being explained by changes in the amygdalar activity, because the amygdala roles in representing the negative affect, and the aversive emotional reaction to loss. Also, these findings discouraged the explanation of risk seeking for losses, because the aberrant estimations of probability or loss magnitude.Citation45 This result suggested that communication between these areas is vital for the appropriate assessment of reward value to influence choice.Citation45
In addition, another rat investigation explored the disconnection between BLA pathways and the OFC, and found a retarded acquisition in the gambling task.Citation46 Based on Zeeb opinion, this disconnection prevented modifications in the value of a specific reward for contributing appropriately to cost-benefit decision making.Citation46 Also, it seems that pathways from the OFC to BLA are important in the decision process, and for the adequate assessment of reward value to influence choice.Citation46
As a reference, a rat study reported that this specie has decision-making processes that are influenced by a previous reference point;Citation47 this study used a modified T maze paradigm. Specifically, the modification did consist of adding “pockets” at both sides (right and left) of the T´s stem; because, these pockets in the stem stored pellets, rats could set reference values for each arm of the maze, before selecting.
The ¨previous expectation¨ had been previously reported in human research.Citation47 It is known that decision-making processes can be disrupted in GD. If the decision making can be influenced by the ¨previous expectation”, then this expectation should be studied, and its neurobiology for easing the treatment of GD. It is still necessary to find out which brain structure(s) is(are) involved in a ¨previous expectation”.
Cingulate cortex
Researches regarding the inactivation of the anterior cingulate cortex (ACC) by means of GABAA and GABAB agonism reported opposed results. Specifically, one study stated that inactivation of the ACC by means of a mixture of the GABAA agonist (muscimol; infusion in the brain) and the GABAB agonist (baclofen; infusion in the brain) impaired rodent´s ability to differentiate winning from losing outcomes in a rat slot machine task.Citation48 However, the other study that inactivated ACC by means of GABAB (baclofen hydrochloride, brain microinfusion) and GABAA (muscimol hydrobromide, brain microinfusion) receptors agonism reported no effect on decision making based on a rat gambling task.Citation49
Moreover, another study explored the effect of D4 agonism (PD168077; infusion in the brain) in the ACC, and it found a disruption of the rat´s ability to differentiate winning from losing outcomes in the slot machine task.Citation48 Also, it found an augmentation of the reward expectancy, but only on archetypal “near-miss” trials (ie, when the first two of three stimuli in the array were concordant with a rewarding outcome, and only the last stimulus critically signaled a non-win);Citation48 Cocker considered that the ACC is fundamental for analyzing the adequate response when competing stimulus and outcome associations are activated; also, this author considered that the D4 receptor antagonists might be an effective treatment for GD.Citation48
Hippocampus
High levels of DA, 5-HT, and noradrenaline in the hippocampus predicted the emergence of more exploratory and risky behaviors in a strain of healthy inbred mice, based on a gambling task.Citation50 In this study, they focused on postmortem brain analysis, rather than administration of drugs, or experimental treatments on living brains.
Insular cortex
Some studies evaluated different areas of the insular cortex like: overall insular cortex, anterior insular cortex, or agranular insular cortex (rostral or caudal). The insular cortex did seem relevant to the rapid (30 mins) disruptive action of corticosteroid hormones (C174 Corticosterone HBC-complex, sc) on decisions (reward based) of the rat´s Iowa gambling task.Citation51 This corticosteroid action was related to stress experience. As a reference, the disruption on decision was accompanied by significant changes in the insular cortex (based on c-fos immuno-histochemistry).
Another study showed that inactivation of insular cortex by a mixture of GABAA (muscimol; brain microinjection) and GABAB (baclofen hydrochloride; brain microinjection) receptors agonists induced risky behaviors linked to altered decisions, based on a rat gambling test (a radial arm maze).Citation52
Other studies investigated the anterior insular cortex relevance in gambling-related behaviors, and found mixed results. Specifically, the first investigation found a decreased in risk preference, based on two rats gambling tasks (the amount gambling task and the delay gambling task); the treatment was a mixture of GABAA (muscimol; brain microinjection) and GABAB (baclofen; brain microinjection) receptor agonists.Citation53 Moreover, the second investigation blocked the D2 receptors of the anterior insular cortex (eticlopride hydrochloride, brain microinjection) and found augmentation of risk preference, after winning in a previous risky choice; also, the blockade of the 5-HT1A receptors (WAY100635; brain microinjection) of the anterior insular cortex increased risk preference, after losing in a previous risky choice of a gambling task.Citation54 As reference, the antagonism of dopamine 1 receptor (D1) (SCH23390 hydrochloride) or the antagonism of serotonin 2A receptor (5-HT2A) (M100907, brain microinjection) in the anterior insular cortex did not alter risk preference in the rat gambling task.Citation54
Regarding the agranular insular cortex, its inactivation by a mixture of GABAA (muscimol, brain microinjection) and GABAB receptors (baclofen, brain microinjection) agonism decrease risk preference based on two different rat gambling tasks; besides, in risk-free control situations, the agranular insular cortex inactivation did not impair decision making.Citation53 Furthermore, the inactivation of the caudal agranular insular cortex by either lesion (ibotenic acid; brain microinjection) or by a mixture of GABAA (muscimol; brain microinjection) and GABAB (baclofen; brain microinjection) receptors agonists did not disrupt decision-making behavior under risk in a rat gambling task.Citation55
Lateral ventricles
Brain anatomical abnormalities like the enlargement of the lateral ventricles did not alter decision making in the rat gambling task.Citation56 As a reference, the enlargement of ventricles was induced by a maternal (before and during pregnancy) diet deficient in vitamin D; subsequently, the whole litters were placed in a standard diet and evaluated.
Limbic area
Different experimental manipulations of the infralimbic area generated poor decision making in the rat gambling task; for instance: a higher serotonergic metabolism, a rapid action of corticosteroids hormones (30 mins), and inactivation by gamma-aminobutyric acid (GABA) receptors agonism. Specifically, the relationships between a higher serotonergic metabolism in the infralimbic area, and poor decision making in the rat gambling task was inferred based on the postmortem brain analysis after gambling behavioral tests.Citation44 Moreover, the rapid and disruptive action of corticosteroid hormones of stress (C174 Corticosterone HBC-complex; sc) on decision making of rats was performed by means of noninvasive brain manipulations.Citation51 Furthermore, the disruption of decision making after agonism of GABAA (muscimol hydrobromide; brain microinjection) and GABAB (baclofen hydrochloride; brain microinjection) receptors was performed by means of direct brain injections; specifically, this inactivation allowed an augmented preference for disadvantageous options and reduced choice for optimal options.Citation49
However, other conditions like manipulations of the D2 receptors did not alter neither choice preference nor optimal performance in the rat gambling task; specifically, the infralimbic cortex was treated by means of administering the D2 receptor antagonist (eticlopride hydrochloride; brain microinjection).Citation49
On the other hand, another set of studies targeted the prelimbic cortex by the agonism of GABAergic receptors and the antagonism of D2 receptors obtaining opposed results.Citation49 Specifically, the inactivation of prelimbic cortex by means of the agonism of GABAA (muscimol hydrobromide; brain microinjection) and GABAB (baclofen hydrochloride; brain microinjection) receptors disrupted decision making in the rat gambling task.Citation49 Moreover, this inactivation allowed an augmented choice for disadvantageous options, and a reduced choice for optimal options.Citation49 Under other conditions, the disruption of the prelimbic cortex by means of treatment with the D2 receptor antagonist (eticlopride hydrochloride; brain microinjection) did not alter choice preference neither optimal performance in the rat gambling task.Citation49
Neocortex
Nervous system anatomical abnormalities like a tiny cerebral cortex did not modify decision making in the rat gambling task.Citation56 As a reference, the reduction of the cerebral cortex was produced by a maternal diet (before and during pregnancy) deficient in vitamin D; afterward, the offsprings were placed in a standard diet and evaluated.
NAc
A study found a relationship between the activity of specific cue responsive NAc neurons, and the cue onset during a go/no go task. Despite a gambling task was not used, the go/no go task has relevance to impulsivity, that is a core trait of GD.Citation57 Specifically, electrophysiological recordings of neurons in the NAc during the go/no go tasks showed that individual cue-responsive neurons displayed either increases or decreases in activity at the cue onset; NAc cue responses correlated with action, regardless of cue type or accuracy.Citation57
Olfactory tubercle
An investigation found a correlation between the messenger ribonucleic acid (mRNA) levels of D3 receptors in the island of Calleja (r=–0.91), in the islands of Calleja major (r=0.62) and the performance of male rats in the rodent gambling task (this study only used males).Citation58 This finding was consistent with a human imaging study (positron emission tomography) that reported a link between D3 receptor binding index and the severity of disordered gambling.Citation59
OFC
A group of studies with different manipulations (neurotransmitters and receptors systems) reported diverse results: eg, increase in risk preference (inactivation by GABAergic agonism, or rapid corticosteroid action),Citation51,Citation53 decrease in risk preference (antagonism of 5-HT1A),Citation54 no effects on risk preference (antagonism of D1, D2, or 5-HT2A receptors),Citation54 and no effect on decision making (inactivation by GABAergic agonism or D2 receptor antagonism).Citation49 However, it is important to note that under risk-free control situations, the GABAergic agonism of OFC did not affect decision making; hence, the degree of risk of the task should be considered.Citation53 Specific details of all the previous reports are explained in the next paragraphs.
The OFC was inactivated by a mixture of GABAA (muscimol; brain microinjection) and GABAB (baclofen; brain microinjection) receptors agonists; this treatment augmented risk preference in the rats, based on two gambling tasks (the amount gambling task, and the delay gambling task).Citation53 However, under risk-free control situations, the inhibition of the OFC did not disrupt decision making. According to the authors, the OFC denoted relevance at the time of accepting or declining a risk.Citation53 Moreover, the lateral OFC did show relevance for the rapid (30 mins) disruptive action of corticosteroid hormone (C174 Corticosterone HBC-complex; sc) on decision making (reward based) in a rat Iowa gambling task.Citation51 This was inferred because the disruption on decision process was accompanied by significant changes in gene expression in the lateral OFC (increase in c-fos expression, based on c-fos immuno-histochemistry).Citation51
Nevertheless, the antagonism of 5-HT1A receptors (WAY100635; brain microinjection) in the rat´s OFC decreased risk preference on a modified gambling task.Citation54 Finally, other studies reported absence of effects of OFC manipulation; specifically, the antagonism of either D1 receptors (SCH 23390 hydrochloride; brain microinjection), D2 receptors (eticlopride hydrochloride; brain microinjection), or 5-HT2A receptors (M100907; brain microinjection) did not alter risk preference on a modified gambling task.Citation54 Furthermore, the inactivation of the OFC by means of GABAA (muscimol hydrobromidel; brain microinjection) and GABAB (baclofen hydrochloride; brain microinjection) receptors did not affect decision making in rats, based on a gambling task.Citation49 Moreover, D2 receptor antagonism (eticlopride hydrochloride; brain microinjection) did not affect decision making in the same paradigm.Citation49
mPFC
Some studies showed that direct manipulation (ibotenic acid lesion, GABAergic antagonism) or developmental manipulation (adolescence/juvenile social isolation) of the mPFC disrupted decision making.Citation60–Citation62 More details about the previous reports are explained in the next paragraphs.
First, the excitotoxic lesion of mPFC induced by ibotenic acid (brain microinjection) worsened decision making (fewer selection of advantageous or optimal choices) based on a rat gambling task;Citation60 however, this deficit in decision making was attenuated after treatment with D1 receptors antagonism (SCH23390; ip). However, the D2 receptors antagonism (haloperidol; sc) did not attenuate the deficit.Citation60
Furthermore, the antagonism of GABAA receptors (bicuculline methiodide; brain microinjection) in the mPFC disrupted decision making in the rat gambling task. Despite this study described this application for schizophrenia treatment, this finding is also useful for GD treatment, because it studied decision process during the rat gambling task.Citation61 Finally, social isolation from early adolescent to juvenile period (post-natal day 21 (P21) to post-natal day 42 (P42)) induced lasting cellular and synaptic changes in the pyramidal neurons of the adult mPFC.Citation62 Besides, isolation consequences counteract the DA enhancement induced by a DA agonism bolsterer (amphetamine sulfate; ip) or by a DA reuptake inhibitor (GBR12909 dihydrochloride; ip) in the five-choice serial reaction time task (challenging conditions).Citation62 Also, the social isolation decreased sensitivity to DA in the pyramidal neurons of the mPFC. Impulsivity was measured in the rat gambling task and other tests. Also, social isolation impaired impulsive action and decision making under novel or challenging circumstances based on the rat gambling task and other tests. However, impulsive choices were not affected by social isolation.Citation62
PFC – subcortical network and related structures
A rats’ study combined gambling tasks, post-mortem analysis (DA and 5-HT turnovers), and c-fos immuno-detection in the brain prefrontal – subcortical network.Citation44 Differences between good and bad decision making was found. Good decision making was characterized by a wider network (but once good choices had been made), and a disengagement of the key prefrontal areas (insular and infralimbic cortices) and the amygdala. On the other hand, poor decision making was related to a lower network recruitment and to a sustained amygdala activity.Citation44 Besides, poor decision making was linked to an imbalance of monoaminergic metabolism (ie: a higher infralimbic vs a lower amygdalar serotonergic metabolism), and to an aberrant low recruitment of brain areas linked to executive functions and affective valence during decision processes.Citation44
Striatum
Studies have looked at the relevance of the striatum in general (rats), and its specific zones like olfactory tubercle and ventral striatum (mice). In general, it was found that striatum activity was linked to wager sensitivity, motivated behavior, discrimination of rewards, stereotypical behavior, and compulsivity.Citation63–Citation65 Additional technical details are described in the next paragraphs.
Specifically, lower striatal D2 and D3 receptors densities correlated to high wager sensitivity, based on a novel task for decision making in rats, micro-possitron emission tomography, and autoradiography using [11C] raclopride.Citation63
In addition, a mice study reported that the olfactory tubercle (a sub-region of the ventral striatum) robustly encoded the onset and progression of motivated behaviors (organization of goal-directed behaviors), and discriminated the type and magnitude of a reward (process of reward information).Citation64 As reference, this mice investigation did use a novel water-motivated instrumental task, and “in vivo” electrophysiological recordings; despite this investigation did not perform explicit behavioral tests about gambling, it was proposed by the authors, that the findings were conceptually/theoretically related to GD.Citation64 Finally, another investigation performed on mice found that an augmented activity of the ventral striatum related to stereotypical behavior;Citation65 the mice were evaluated by means of a stereotypical behavior paradigm, and the brains analyzed by immunohistochemical staining of FosB and delta FosB.Citation65 The authors (Phillips et al) proposed that the stereotypy observed could be relevant to the compulsivity described in GD and other disorders (eating and drug seeking).Citation65
STN
There was scarce research regarding the involvement of the STN in gambling behavior. Specifically, a study reported that several sessions of bilateral deep brain stimulation (DBS) of the STN induced a subsequent increment of premature responding in the gambling task; this increment even persisted after finishing the stimulation.Citation66 As a reference, DBS of the STN had been also associated with impulsivity in the absence of Parkinsonism (under specific conditions).Citation66
Discussion
The main question of this review was: which are the main neurotransmitters systems and brain structures relevant for GD based on recent rodent studies? This question was answered in this section by contrasting the present review main points and those from previous literature reviews in the field (reviews cited in the Introduction). The present review found that NMDA receptor antagonism influence reinforcement sensitivity and impulsivity; also, that D2 and D3 receptors’ agonism induces GD tendencies. These points agree with the publications review by Grant et al;Citation2 it concluded that probably diverse neural systems participate in the pathophysiology of GD like those related to glutamate and DA among others messengers.Citation2
The present work considers that the BLA–OFC pathway is relevant for the assessment of reward among other functions in the rat gambling task. This partially agrees with the Levy and Glimcher’s review;Citation13 precisely, those authors proposed that the vmPFC/OFC is part of a brain network that codify the values of rewards by means of a standard neural scale (based on human neuroimaging).Citation13 It seems that the OFC is related to the assessment and codification of rewards in gambling activities.
Based on the present review, a higher level of DA, noradrenaline, and 5-HT in the hippocampus predicted exploratory and risky behaviors in gambling. Related to this, a review by Meng et al pointed out that bilateral overactivity of the parahippocampus among other structures, positively correlated with South Oaks Gambling Screen scores.Citation14 Taking these together, it seems that a higher metabolism and activity of the zone of the hippocampus and its surroundings (parahippocampus) relates to more risky gambling tendencies.
Furthermore, this work found that insular cortex activity relates to decision making in the rat gambling task; in the same sense, a previous literature review stated that the insular cortex (including rostral agranular zone) among difference structures influence the correctness of gambling decision.Citation6 Taking these together, it seems that insular cortex relates to decision making in gambling tasks.
Moreover, the present review found that infralimbic area relates to decision making in the rat gambling task; this agrees with another publications review that states that infralimbic area among other structures is involved in the correctness of gambling decision on rodent tasks.Citation6
The present review found that OFC activity is related to risk preference; relevant to this, another review states that alterations in the OFC among other structures are found in fellows with GD.Citation6 It seems that alteration of the OFC activity is relevant for GD. Additionally, this review found that mPFC is involved in decision making, and its disruption impairs decision making. Other reviews have proposed similar ideas; Goulet-Kennedy et al´s review pointed that PFC among other structures is fundamental for decision processes based on clinical studies.Citation11 Moreover, a review by Potenza states that vmPFC among other areas is relevant for GD.Citation15 In general, it seems that PFC (including medial and ventromedial area) is relevant in the dynamics of GD.
Besides, the present review points that PFC–subcortex network activity is linked to poor decision making if lower network action and sustained activity of the amygdala are present; moreover, PFC–subcortex network is associated with good decision if a wider network and disengagement of key prefrontal areas and the amygdale are present. These points agree with Grant et al´s review;Citation2 Grant et al work concludes that based on imaging reports, GD relates to anatomical and functional anomalies of nexus involved in reward processing and top-down monitoring.Citation2 Hence, both reviews agree that disruption of top-down circuits is a common element in problems linked to gambling.
Regarding the striatum, the present literature review states that the striatum´s density of dopamine receptors relates to wager sensitivity; also, the ventral striatum activity relates to stereotypy (like the GD compulsivity). Moreover, the olfactory tubercle relates to the onset and progression of motivated behaviors and reward´s discrimination. Similarly, other publication reviews like the one by Goulet-Kennedy et al pointed that the striatum is a conductor of decision processes (which are relevant to gambling behaviors) based on clinical studies.Citation11
Furthermore, the review by Norbury and Husain states that marked sensation seeking relates to GD, and to dopaminergic transmission;Citation5 specifically, fellows with marked sensation seeking display high tonic DA levels and over-responsive midbrain dopaminergic responses to signals of future reward.Citation5 Also, differences in subject reactions (variability in approach – avoidance reactions) to stimuli stems from differences in the efficiency of DA transmission at the level of striatum.Citation5 Another review by Potenza, stresses that the ventral striatum among other structures is linked to gambling and GD.Citation15 Integrating, the reviews state that striatum relates to wager sensitivity (based on DA receptor density), stereotypy (ventral zone), conduction of decision processes (including those of gambling behaviors), and variability in approach – avoidance to stimuli.
The different points contrasted above in the Discussion section, between the present and other reviews published, have been integrated for elaborating clinical indications. These indications are the next: a) glutamate and DA seem relevant in the pathophysiology of GD; however, other neurotransmitters should also be considered, b) the OFC is relevant for the assessment and codification of rewards in gambling activities, c) a higher metabolism and activity of the hippocampus and its surroundings (parahippocampus) relates to risky gambling tendencies, d) the insular cortex and the infralimbic area are relevant for gambling-related decisions, e) the alteration of OFC activity is relevant for GD, f) the PFC (including mPFC and vmPFC) is relevant for the dynamic of GD, g) the disruption of top (cortical)–down (subcortical) circuits can be relevant to gambling problems, and h) the striatum relates to wager sensitivity, stereotypy, decision processes, and approach/avoidance to stimuli related to gambling.
Conclusion
Based on the studies revised that used noninvasive methods for drug administration, some of the receptors involved in behaviors related to GD are: muscarinic, NMDA, CB1, CB2, D2, D3, and D4 receptors. Moreover, based on the studies revised that used invasive methods for drug administration, some of the neurotransmitters and receptors involved in GD are: 5-HT1A, noradrenaline receptors, GABAA, and GABAB. According to this work, the next brain structures are involved in behaviors related to GD: amygdala (including BLA), BLA–OFC pathways, ACC, hippocampus, infralimbic area, prelimbic cortex, insular cortex (including anterior and rostral agranular zones), NAc, olfactory tubercle (island of Calleja and the island of Calleja major), OFC, mPFC, PFC–subcortical network, striatum (including ventral zone and olfactory tubercle), and STN. The present review and others described in the field agree that DA and glutamate, among other neurotransmitters, are relevant to GD. The present review and others described in the field agree that the next brain areas are relevant for GD: OFC, hippocampus/parahippocampus, insular cortex, infralimbic area, PFC, PFC–subcortical network, and striatum. The search for GD treatments should consider and integrate this diversity of neurotransmitters, receptors, and brain areas.
Abbreviation list
AMPA, alpha-amino-3-hydroxy-5-methylisoxazolepropionate; ACC, anterior cingulate cortex; BLA, basolateral amygdala; CB1, cannabinoid receptor 1; CB2, cannabinoid receptor 2; DBS, deep brain stimulation; DSM-5, Diagnostic and Statistical Manual of Mental Disorders-5; DA, dopamine; D1, dopamine 1 receptor; D2, dopamine 2 receptor; D3, dopamine 3 receptor; D4, dopamine 4 receptor; EEG, electroencephalography; GD, gambling disorder; GABA, gamma-aminobutyric acid; GABAA, gamma-aminobutyric acid receptor A; GABAB, gamma-aminobutyric acid receptor B; HPLC, High Performance Liquid Chromatography; ip, intraperitoneal administration; mPFC, medial prefrontal cortex; mRNA, messenger ribonucleic acid; mins, minutes; NMDA, N-methyl-D-aspartate; NAc, nucleus accumbens; OFC, orbitofrontal cortex; P21, post-natal day 21; P42, post-natal day 42; PFC, prefrontal cortex; SENACYT, Secretaria Nacional de Ciencia, Tecnologia e Innovacion (English: National Secretariat of Science, Technology and Innovation); 5-HT, serotonin; 5-HT1A, serotonin 1A receptor; 5-HT2A, serotonin 2A receptor; sc, subcutaneous administration; SNI, Sistema Nacional de Investigacion (English: National System of Investigation); STN, subthalamic nucleus; VTA, ventral tegmental area; vmPFC, ventromedial prefrontal cortex.
Acknowledgments
We would like to thank Open Access Publishing Fund from Florida State University Libraries for it’s funding. Dr. G. Quintero Garzola belongs and is backed by the SNI (Sistema Nacional de Investigacion) from SENACYT. SENACYT is a public organization located in Panama.
Disclosure
The author reports no conflicts of interest in this work.
References
- Lemieux A, al’Absi M. Stress psychobiology in the context of addiction medicine: from drugs of abuse to behavioral addictions. Prog Brain Res. 2016;223:43–62. doi:10.1016/bs.pbr.2015.08.00126806770
- Grant JE, Odlaug BL, Chamberlain SR. Neural and psychological underpinnings of gambling disorder: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:188–193. doi:10.1016/j.pnpbp.2015.10.00726497079
- Alguacil LF, González-Martin C. Target identification and validation in brain reward dysfunction. Drug Discov Today. 2015;20(3):347–352. doi:10.1016/j.drudis.2014.10.01425541474
- Winstanley CA, Clark L. Translational models of gambling-related decision-making. Curr Top Behav Neurosci. 2016;28:93–120. doi:10.1007/7854_2015_501427418069
- Norbury A, Husain M. Sensation-seeking: dopaminergic modulation and risk for psychopathology. Behav Brain Res. 2015;288:79–93. doi:10.1016/j.bbr.2015.04.01525907745
- Quintero GC. A biopsychological review of gambling disorder. Neuropsychiatr Dis Treat. 2017;13:51–60. doi:10.2147/NDT.S11881828096672
- Potenza MN, Kosten TR, Rounsaville BJ. Pathological gambling. Jama. 2001;286(2):141–144.11448261
- National Opinion Research Center. Gambling Impact and Behavior Study. Chicago: NORC Chicago; 1999 Available from: http://www.norc.org/PDFs/publications/GIBSFinalReportApril1999.pdf. Accessed November 20, 2018.
- Lorains FK, Cowlishaw S, Thomas SA. Prevalence of comorbid disorders in problem and pathological gambling: systematic review and meta-analysis of population surveys. Addiction. 2011;106(3):490–498. doi:10.1111/j.1360-0443.2010.03300.x21210880
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: APA; 2013.
- Goulet-Kennedy J, Labbe S, Fecteau S. The involvement of the striatum in decision making. Dialogues Clin Neurosci. 2016;18(1):55–63.27069380
- Banz BC, Yip SW, Yau YH, Potenza MN. Behavioral addictions in addiction medicine: from mechanisms to practical considerations. Prog Brain Res. 2016;223:311–328. doi:10.1016/bs.pbr.2015.08.00326806783
- Levy DJ, Glimcher PW. The root of all value: a neural common currency for choice. Curr Opin Neurobiol. 2012;22(6):1027–1038. doi:10.1016/j.conb.2012.06.00122766486
- Meng YJ, Deng W, Wang HY, et al. Reward pathway dysfunction in gambling disorder: a meta-analysis of functional magnetic resonance imaging studies. Behav Brain Res. 2014;275:243–251. doi:10.1016/j.bbr.2014.08.05725205368
- Potenza MN. Neurobiology of gambling behaviors. Curr Opin Neurobiol. 2013;23(4):660–667. doi:10.1016/j.conb.2013.03.00423541597
- van Den Bos R, Davies W, Dellu-Hagedorn F, et al. Cross-species approaches to pathological gambling: a review targeting sex differences, adolescent vulnerability and ecological validity of research tools. Neurosci Biobehav Rev. 2013;37(10 Pt 2):2454–2471. doi:10.1016/j.neubiorev.2013.07.00523867802
- Anselme P. Dopamine, motivation, and the evolutionary significance of gambling-like behaviour. Behav Brain Res. 2013;256:1–4. doi:10.1016/j.bbr.2013.07.03923896052
- Cocker PJ, Winstanley CA. Irrational beliefs, biases and gambling: exploring the role of animal models in elucidating vulnerabilities for the development of pathological gambling. Behav Brain Res. 2015;279:259–273. doi:10.1016/j.bbr.2014.10.04325446745
- Silveira MM, Malcolm E, Shoaib M, Winstanley CA. Scopolamine and amphetamine produce similar decision-making deficits on a rat gambling task via independent pathways. Behav Brain Res. 2015;281:86–95. doi:10.1016/j.bbr.2014.12.02925529186
- Yates JR, Batten SR, Bardo MT, Beckmann JS. Role of ionotropic glutamate receptors in delay and probability discounting in the rat. Psychopharmacology. 2015;232(7):1187–1196. doi:10.1007/s00213-014-3747-325270726
- Gueye AB, Trigo JM, Vemuri KV, Makriyannis A, Le Foll B. Effects of various cannabinoid ligands on choice behaviour in a rat model of gambling. Behav Pharmacol. 2016;27(2–3Spec Issue):258–269. doi:10.1097/FBP.000000000000022226905189
- Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2005;312(3):875–883. doi:10.1124/jpet.104.07797415525797
- Gamaleddin I, Zvonok A, Makriyannis A, Goldberg SR, Le Foll B. Effects of a selective cannabinoid CB2 agonist and antagonist on intravenous nicotine self administration and reinstatement of nicotine seeking. PLoS One. 2012;7(1):e29900. doi:10.1371/journal.pone.002990022291896
- Navarrete F, Rodriguez-Arias M, Martin-Garcia E, et al. Role of CB2 cannabinoid receptors in the rewarding, reinforcing, and physical effects of nicotine. Neuropsychopharmacol. 2013;38(12):2515–2524. doi:10.1038/npp.2013.157
- Melis M, Pistis M. Endocannabinoid signaling in midbrain dopamine neurons: more than physiology? Curr Neuropharmacol. 2007;5(4):268–277. doi:10.2174/15701590778279361219305743
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29(4):225–232. doi:10.1016/j.tins.2006.01.00816483675
- Maldonado R, Berrendero F, Ozaita A, Robledo P. Neurochemical basis of cannabis addiction. Neuroscience. 2011;181:1–17. doi:10.1016/j.neuroscience.2011.02.03521334423
- Gong JP, Onaivi ES, Ishiguro H, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071(1):10–23. doi:10.1016/j.brainres.2005.11.03516472786
- Zhang HY, Gao M, Liu QR, et al. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci U S A. 2014;111(46):E5007–E5015. doi:10.1073/pnas.141321011125368177
- Di Ciano P, Pushparaj A, Kim A, et al. The impact of selective dopamine D2, D3 and D4 ligands on the rat gambling task. PLoS One. 2015;10(9):e0136267. doi:10.1371/journal.pone.013626726352802
- Cohen AI, Todd RD, Harmon S, O’Malley KL. Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc Natl Acad Sci USA. 1992;89(24):12093–12097. doi:10.1073/pnas.89.24.120931334557
- Valerio A, Belloni M, Gorno ML, Tinti C, Memo M, Spano P. Dopamine D2, D3, and D4 receptor mRNA levels in rat brain and pituitary during aging. Neurobiol Aging. 1994;15(6):713–719.7891826
- O’Malley KL, Harmon S, Tang L, Todd RD. The rat dopamine D4 receptor: sequence, gene structure, and demonstration of expression in the cardiovascular system. New Biol. 1992;4(2):137–146.1554689
- Meador-Woodruff JH, Grandy DK, Van Tol HH, et al. Dopamine receptor gene expression in the human medial temporal lobe. Neuropsychopharmacol. 1994;10(4):239–248. doi:10.1038/npp.1994.27
- Meador-Woodruff JH, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ. Dopamine receptor transcript expression in striatum and prefrontal and occipital cortex. Focal abnormalities in orbitofrontal cortex in schizophrenia. Arch Gen Psychiatry. 1997;54(12):1089–1095.9400344
- Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381(6579):245–248. doi:10.1038/381245a08622768
- Khaled MA, Pushparaj A, Di Ciano P, Diaz J, Le Foll B. Dopamine D3 receptors in the basolateral amygdala and the lateral habenula modulate cue-induced reinstatement of nicotine seeking. Neuropsychopharmacol. 2014;39(13):3049–3058. doi:10.1038/npp.2014.158
- Heidbreder C. Novel pharmacotherapeutic targets for the management of drug addiction. Eur J Pharmacol. 2005;526(1–3):101–112. doi:10.1016/j.ejphar.2005.09.03816253234
- Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564(2):203–219.1839781
- Diaz J, Pilon C, Le Foll B, et al. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci. 2000;20(23):8677–8684.11102473
- Nakajima S, Gerretsen P, Takeuchi H, et al. The potential role of dopamine D(3) receptor neurotransmission in cognition. Eur Neuropsychopharmacol. 2013;23(8):799–813. doi:10.1016/j.euroneuro.2013.05.00623791072
- Le Foll B, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. Sep. 2005;49(4):525–541.
- Pes R, Godar SC, Fox AT, et al. Pramipexole enhances disadvantageous decision-making: lack of relation to changes in phasic dopamine release. Neuropharmacology. 2017;114:77–87. doi:10.1016/j.neuropharm.2016.11.01427889491
- Fitoussi A, Le Moine C, De Deurwaerdere P, et al. Prefronto-subcortical imbalance characterizes poor decision-making: neurochemical and neural functional evidences in rats. Brain Struct Funct. 2015;220(6):3485–3496. doi:10.1007/s00429-014-0868-825134683
- Tremblay M, Cocker PJ, Hosking JG, Zeeb FD, Rogers RD, Winstanley CA. Dissociable effects of basolateral amygdala lesions on decision making biases in rats when loss or gain is emphasized. Cogn Affect Behav Neurosci. 2014;14(4):1184–1195. doi:10.3758/s13415-014-0271-124668615
- Zeeb FD, Winstanley CA. Functional disconnection of the orbitofrontal cortex and basolateral amygdala impairs acquisition of a rat gambling task and disrupts animals’ ability to alter decision-making behavior after reinforcer devaluation. J Neurosci. 2013;33(15):6434–6443. doi:10.1523/JNEUROSCI.3971-12.201323575841
- Bhatti M, Jang H, Kralik JD, Jeong J. Rats exhibit reference-dependent choice behavior. Behav Brain Res. 2014;267:26–32. doi:10.1016/j.bbr.2014.03.01224657593
- Cocker PJ, Hosking JG, Murch WS, Clark L, Winstanley CA. Activation of dopamine D4 receptors within the anterior cingulate cortex enhances the erroneous expectation of reward on a rat slot machine task. Neuropharmacology. 2016;105:186–195. doi:10.1016/j.neuropharm.2016.01.01926775821
- Zeeb FD, Baarendse PJ, Vanderschuren LJ, Winstanley CA. Inactivation of the prelimbic or infralimbic cortex impairs decision-making in the rat gambling task. Psychopharmacology. 2015;232(24):4481–4491. doi:10.1007/s00213-015-4075-y26387517
- Pittaras E, Callebert J, Chennaoui M, Rabat A, Granon S. Individual behavioral and neurochemical markers of unadapted decision-making processes in healthy inbred mice. Brain Struct Funct. 2016;221(9):4615–4629. doi:10.1007/s00429-016-1192-226860089
- Koot S, Baars A, Hesseling P, van Den Bos R, Joels M. Time-dependent effects of corticosterone on reward-based decision-making in a rodent model of the Iowa gambling task. Neuropharmacology. 2013;70:306–315. doi:10.1016/j.neuropharm.2013.02.00823474014
- Mizoguchi H, Katahira K, Inutsuka A, et al. Insular neural system controls decision-making in healthy and methamphetamine-treated rats. Proc Natl Acad Sci U S A. 2015;112(29):E3930–E3939. doi:10.1073/pnas.141801411226150496
- Ishii H, Ohara S, Tobler PN, Tsutsui K, Iijima T. Inactivating anterior insular cortex reduces risk taking. J Neurosci. 2012;32(45):16031–16039. doi:10.1523/JNEUROSCI.2278-12.201223136439
- Ishii H, Ohara S, Tobler PN, Tsutsui K, Iijima T. Dopaminergic and serotonergic modulation of anterior insular and orbitofrontal cortex function in risky decision making. Neurosci Res. 2015;92:53–61. doi:10.1016/j.neures.2014.11.00925481848
- Pushparaj A, Kim AS, Musiol M, et al. Differential involvement of the agranular vs granular insular cortex in the acquisition and performance of choice behavior in a rodent gambling task. Neuropsychopharmacol. 2015;40(12):2832–2842. doi:10.1038/npp.2015.133
- Peak JN, Turner KM, Burne TH. The effect of developmental vitamin D deficiency in male and female Sprague-Dawley rats on decision-making using a rodent gambling task. Physiol Behav. 2015;138:319–324. doi:10.1016/j.physbeh.2014.09.00725447469
- Roitman JD, Loriaux AL. Nucleus accumbens responses differentiate execution and restraint in reward-directed behavior. J Neurophysiol. 2014;111(2):350–360. doi:10.1152/jn.00350.201324174652
- Lobo DS, Aleksandrova L, Knight J, et al. Addiction-related genes in gambling disorders: new insights from parallel human and pre-clinical models. Mol Psychiatry. 2015;20(8):1002–1010. doi:10.1038/mp.2014.11325266122
- Boileau I, Payer D, Chugani B, et al. The D2/3 dopamine receptor in pathological gambling: a positron emission tomography study with [11C]-(+)-propyl-hexahydro-naphtho-oxazin and [11C]raclopride. Addiction. 2013;108(5):953–963. doi:10.1111/add.1206623167711
- Paine TA, Asinof SK, Diehl GW, Frackman A, Leffler J. Medial prefrontal cortex lesions impair decision-making on a rodent gambling task: reversal by D1 receptor antagonist administration. Behav Brain Res. 2013;243:247–254. doi:10.1016/j.bbr.2013.01.01823354057
- Paine TA, O’Hara A, Plaut B, Lowes DC. Effects of disrupting medial prefrontal cortex GABA transmission on decision-making in a rodent gambling task. Psychopharmacology. 2015;232(10):1755–1765. doi:10.1007/s00213-014-3816-725420610
- Baarendse PJ, Counotte DS, O’Donnell P, Vanderschuren LJ. Early social experience is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacol. 2013;38(8):1485–1494. doi:10.1038/npp.2013.47
- Cocker PJ, Dinelle K, Kornelson R, Sossi V, Winstanley CA. Irrational choice under uncertainty correlates with lower striatal D(2/3) receptor binding in rats. J Neurosci. 2012;32(44):15450–15457. doi:10.1523/JNEUROSCI.0626-12.201223115182
- Gadziola MA, Wesson DW. The neural representation of goal-directed actions and outcomes in the Ventral Striatum’s olfactory tubercle. J Neurosci. 2016;36(2):548–560. doi:10.1523/JNEUROSCI.3328-15.2016
- Phillips D, Choleris E, Ervin KS, et al. Cage-induced stereotypic behaviour in laboratory mice covaries with nucleus accumbens FosB/DeltaFosB expression. J Neurosci. 2016;301:238–242.
- Aleksandrova LR, Creed MC, Fletcher PJ, Lobo DS, Hamani C, Nobrega JN. Deep brain stimulation of the subthalamic nucleus increases premature responding in a rat gambling task. Behav Brain Res. 2013;245:76–82. doi:10.1016/j.bbr.2013.02.01123434606
