Abstract
Objective
To investigate the relationship between Hamilton Depression Rating Scale (HAM-D) score and psychiatrists’ judgment of working ability in patients with major depressive disorder (MDD) and painful physical symptoms.
Methods
This was a prospective, observational, 12-week study in patients who received duloxetine or a selective serotonin reuptake inhibitor. Patients were ≥20 years old, resided in Japan, and had at least moderate depression (Quick Inventory of Depressive Symptomatology ≥16) and at least moderate painful physical symptoms (Brief Pain Inventory-Short Form average pain ≥3). The main outcome in this post-hoc analysis was the HAM-D17 cutoff best corresponding with patients’ working ability according to the investigator’s judgment. Area under the receiver-operator curve was used to determine the time point with the strongest relationship between HAM-D17 and working ability. The optimal HAM-D17 cutoff was determined based on the maximum of sensitivity (true positive rate) minus ([1 minus specificity] [true negative rate]). For the evaluation of binary data, a mixed effects model with repeated measures analysis was used.
Results
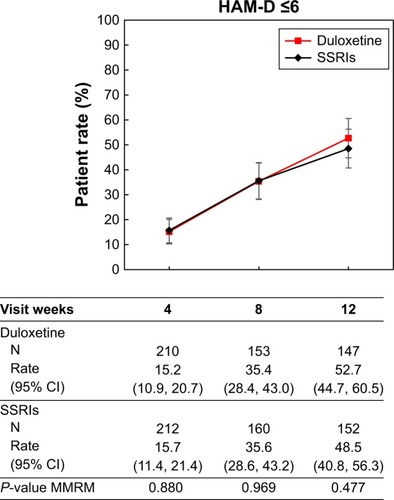
For the estimation of the HAM-D17 cutoff, the area under the receiver-operator curve was maximal at 12 weeks, when a HAM-D17 score of 6 resulted in the best correspondence with working ability in the combined study population. At 12 weeks, a HAM-D17 score of 6 also resulted in the maximum predictive ability in each of the two treatment groups separately. For predicted working ability at 12 weeks, 52.7% of duloxetine-treated patients achieved the HAM-D17 cutoff of ≤6, whereas 48.5% of SSRIs-treated patients achieved HAM-D17 ≤6 (P=0.477).
Conclusion
In this study of patients with major depressive disorder and painful physical symptoms, a HAM-D17 score ≤6 corresponded best with patients’ working ability. This finding is consistent with previous studies showing that a HAM-D17 cutoff of ≤7 may overestimate functional recovery from MDD.
Plain language summary
The Hamilton Depression Rating Scale (HAM-D) is often used to assess depressive symptoms in clinical studies. While a HAM-D 17 item (HAM-D17) cutoff of ≤7 has generally been used to define remission, many patients below that cutoff do not consider themselves ready to return to work. In the present analysis, we assessed the relationship between HAM-D17 scores and psychiatrists’ assessments of patients’ working ability. This analysis was performed on data from a 12-week observational study of duloxetine or a selective serotonin reuptake inhibitor in patients with both major depressive disorder (MDD) and painful physical symptoms (PPS). Based on the data at 12 weeks, when the largest HAM-D17 improvement was seen, we found that a HAM-D17 cutoff of ≤6 had the best correspondence with patients’ working ability. This was true in the combined treatment groups, and also when assessed for each treatment group separately. These findings are consistent with previous studies showing that a HAM-D17 cutoff of ≤7 may be too high in terms of corresponding best with functional recovery from MDD.
Introduction
MDD is a chronic, prevalent, psychiatric disease with a substantial disease burden on health, social, and economic status.Citation1–Citation3 The loss of productivity due to absenteeism (prolonged absence from work) and presenteeism (working despite medical illness) is profound, and therapeutic approaches toward reinstatement (return to work) have been investigated.Citation4,Citation5 However, patients with MDD often relapse,Citation2 making it difficult to return to work. While remission is the best indicator that patients may return to work, currently no biomarkers have been validated for the diagnosis of MDD and/or its remission.Citation6 Given the lack of available biomark-ers, numerous subjective measurement scales have been developed to assess various aspects of MDD.Citation7 Some clinical scales, such as the Hamilton Depression Rating ScaleCitation8 and Montgomery-Asberg Depression Rating Scale,Citation9 which count the number of existing symptoms and their severity, have been commonly used across many studies and clinical trials of antidepressants for patients with MDD.
Based on such established scales and the conceptualization of disease status, symptomatic resolution was believed to be close to remission, which is generally defined by a HAM-D17 score ≤7.Citation10 Early clinical trials for patients with depression reported that remission would be followed by functional improvement, which is a critical component of recovery;Citation11,Citation12 however, some studies have described discordant results between functional recovery and remission of depressive symptoms.Citation13–Citation17 For example, according to a study by Zimmerman et al,Citation16 about half of remitted patients evaluated based on a HAM-D17 score ≤7 did not consider themselves to be remitted. With regard to reinstatement, patients who considered themselves to be in remission reported significantly better work performance than patients who did not consider themselves in remission.Citation16 These results suggest that remission defined by a HAM-D17 score ≤7 may not represent a sufficiently stringent criterion for reinstatement.
In addition to depressive symptoms per se, PPS are often observed in MDD, and their presence is associated with more severe depression.Citation18 At least moderately PPS are also more common in Japanese patients with partial remission than in those with complete remission (based on a HAM-D17 score of ≤7).Citation19 In a recent study of Japanese patients, the presence of PPS after 12 weeks of antidepressant treatment was significantly associated with failure to recover complete social and occupational functioning after 12 additional weeks of treatment, even among patients who had complete remission of MDD based on a HAM-D17 score of ≤7.Citation20
The present analysis assessed data from a 12-week, prospective, observational trial of patients with MDD and PPS to investigate improvement of PPS, depression, function, and quality-of-life between groups treated with duloxetine or selective serotonin reuptake inhibitors (SSRIs).Citation21,Citation22 In addition to the primary study outcomes, data were also collected on patients’ ability to work based on investigators’ judgment. In the present post-hoc analysis, we investigated the relationship between the psychiatrists’ objective judgment for working ability and HAM-D scores by attempting to identify the cutoff value of the HAM-D17 score corresponding to working ability.
Methods
Study design
This was a prospective, observational, 12-week study in patients with MDD and PPS who received monotherapy with duloxetine (n=273) or an SSRI (n=250; SSRIs were escitalopram, sertraline, paroxetine, or fluvoxamine). Full study methods and results of the primary efficacy outcomes and subgroup analyses have been reported previously.Citation21,Citation22 In brief, patients were ≥20 years old, resided in Japan, and presented with an episode of MDD without psychotic traits as defined by the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision.Citation23 Patients had been diagnosed with at least moderate levels of both depression (≥16, Quick Inventory of Depressive Symptomatology)Citation24 and PPS (average pain ≥3, Brief Pain Inventory-Short Form).Citation25 Patients were excluded from participating in the study for any of the following: Prior diagnosis of schizophrenia, bipolar disorder, or other psychotic disorder; current diagnosis of dysthymic disorder or adjustment disorder; PPS that originated from organic disease aside from MDD; or treatment with opioids for PPS.
The study was conducted at 39 psychiatry and psychosomatic outpatient/inpatient clinic/hospital sites. The first and last patient visits were on February 13, 2014 and February 26, 2016, respectively.
Measures
Outcome measures for the current analysis included the HAM-D17, rated according to the Structured Interview Guide for the HAM-D17 for depressive symptoms, and working ability, as defined by the patient being able to work according to the investigators’ judgment (yes/no). Both the HAM-D17 and working ability were assessed at weeks 4, 8, and 12.
Statistical methods
The relationship between HAM-D17 and working ability was assessed for each corresponding visit evaluation (eg, Week 4 HAM-D17 and Week 4 working ability). Receiver-operating characteristic curves (ROCs) plotted sensitivity versus specificity. Sensitivity was the proportion of patients who achieved the given HAM-D17 score or less in patients with judgment of the ability to work of “Yes” (positive outcome). Specificity was the proportion of patients who did not achieve the given HAM-D17 score in patients with judgment of the ability to work of “No”. The optimal cutoff was determined based on the maximum value of sensitivity (true positive rate) minus ([1 minus specificity] [true negative rate]), which provides equal weighting to detecting true positives and true negatives. Binary data were analyzed using a mixed effects model with repeated measures. The fixed effects model included treatment (duloxetine or SSRI), propensity score, baseline score visit, the visit-by-treatment interaction, the visit-by-propensity score interaction, and the visit-by-baseline score interaction. Statistical analyses were performed using SAS version 9.13 or above (SAS Institute Inc., Cary, NC, USA).
Results
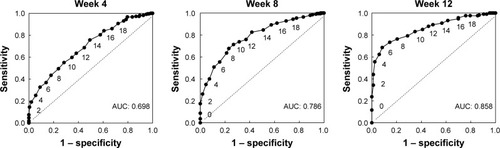
First, in order to estimate the cutoff for working ability, we examined the relationship between the patients’ working ability and HAM-D17 score at 4, 8, and 12 weeks of post-treatment for the combined treatment population. As shown in the ROCs in , the area under the curve (AUC) increased, starting at 4 weeks, and was maximal at 12 weeks. We therefore estimated the cutoff based on data from the 12-week visit. At 12 weeks, the cutoff of a HAM-D17 score of 6 resulted in the maximum value for sensitivity minus (1 minus specificity) of 0.602 ().
Table 1 Sensitivity and specificity of HAM-D17 predictions for ability to work by study week in the combined population
Figure 1 Receiver-operator curves at 4, 8, and 12 weeks, plotting each HAM-D17 score in the total patient population. Data labels indicate HAM-D17 scores.
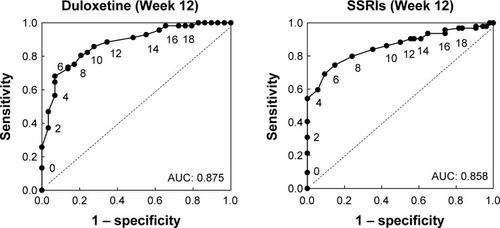
As a sensitivity analysis of the HAM-D17 cutoff estimated for the combined treatment groups, we next examined the ROC curves in each of the treatment groups separately at 12 weeks (; ). Consistent with the results shown in , at 12 weeks a HAM-D17 score of 6 resulted in the maximum score of sensitivity minus (1 minus specificity) in each of the two treatment groups separately.
Figure 2 Receiver-operator curves plotting each HAM-D17 score at 12 weeks for duloxetine- or SSRI-treated patients. Data labels indicate HAM-D17 scores. Abbreviations: AUC, area under the curve; HAM-D, Hamilton Depression Rating Scale; SSRIs, selective serotonin reuptake inhibitors.
Given that the result for the combined population was confirmed for each treatment arm separately, we estimated that a HAM-D17 score ≤6 had the best predictive value for patients’ working ability. We therefore estimated the percentage of patients who reached HAM-D17 ≤6 in the duloxetine and SSRI treatment groups at each time point (). At 12 weeks, 52.7% of duloxetine-treated patients achieved HAM-D17 ≤6, whereas 48.5% of SSRI-treated patients achieved HAM-D17 ≤6; the difference in the proportion of patients responding was not significantly different between treatment groups (P=0.477).
Discussion
In the present analysis, we assessed the relationship between HAM-D17 scores and working ability in an observational clinical trial comparing duloxetine versus SSRI monotherapy in Japanese patients with MDD and PPS. At 12 weeks, in both the combined treatment group and in each treatment group separately, a HAM-D17 score of ≤6 corresponded most closely with investigators’ judgment of patients’ working ability at the same time point.
A HAM-D17 score of 7 has historically been considered the cutoff value for remission;Citation10 however, recently the idea that symptomatic improvement does not fully represent functional recovery has caused this notion to be revisited.Citation13–Citation17 Romera et alCitation27 examined the relationship between symptomatic improvement, assessed using the HAM-D17, and working ability, as assessed by objective evaluation using the Social and Occupational Functioning Assessment Scale (SOFAS) score. That analysis determined that a HAM-D17 score ≤5 maximized sensitivity and specificity for identifying normal social and occupational function (based on a SOFAS score ≥80). In another study using data from a post-marketing study of paroxetine in Japanese patients, Sawamura et alCitation28 examined the relationship between HAM-D17 scores and quality-of-life scores on the 36-item Short Form Health Survey (SF-36). In that study, a HAM-D17 score ≤4 was the best candidate for indicating recovery of social function, and improvements in the physical function and bodily pain domains were also significantly associated with this cutoff value.
The present findings are important in that absenteeism and presenteeism due to MDD are common problems worldwide, and result in substantial social costs.Citation4,Citation5 In daily practice, psychiatrists must make judgments about whether patients responding to out-patient treatment for MDD have recovered sufficiently to return to work; however, a proportion of those patients will still have impaired occupational function, as other studies have reported.Citation29,Citation30 Although scoring systems to evaluate functioning such as SOFAS and the SF-36 are available and well-validated, they may be time-consuming and are not widely used in clinical practice. In the present study of patients with MDD and PPS, we demonstrated that the clinicians’ objective judgment for working ability would be compatible with a HAM-D17 score ≤6. This result is consistent with the findings noted above that many patients with a HAM-D17 score of ≤7 have not achieved complete functional recovery, although those studies generally pointed to even lower HAM-D cutoffs as corresponding best with full recovery.
The presence of PPS in MDD is very common,Citation18,Citation31 but the assessment of treatment outcomes in clinical practice for patients with MDD and PPS has not been fully investigated, particularly regarding measures of social functioning. We have previously observed a close relationship between MDD, PPS, and social functioning, including working ability, in a prospective observational study for 24 weeks.Citation19,Citation20 Notably, MDD patients with complete remission (defined as HAM-D17 ≤7) at 12 weeks were more likely to achieve recovery of social functioning (judged by SOFAS ≥80) at 24 weeks compared to those with partial remission (defined as HAM-D17 between 8 and 18, inclusive),Citation20 which clearly suggests that symptomatic improvement is related to functional recovery. Importantly, with respect to PPS, completely-remitted patients with PPS at 12 weeks were less likely to achieve a SOFAS ≥80 score at 24 weeks compared to completely-remitted patients without PPS at 12 weeks. It is also important to note that the HAM-D17 scale does not directly address PPS, as the only question on the instrument that specifically assesses physical symptoms is question 13, which refers to “general somatic symptoms” and thus does not distinguish between PPS and other physical symptoms. Given the relationship among PPS, MDD, and social functioning, and the lack of a direct measure of PPS in the HAM-D17 instrument, it is notable that we showed a HAM-D17 cutoff corresponding to occupational recovery in MDD with PPS. In other words, the presence of PPS as a comorbid symptom in subjects of this study might play a role in making the HAM-D17 cutoff value lower than 7.
It is possible that the recovery of social functioning in patients with MDD might occur over an extended period of time. In a previous study in which two measures of social functioning (Global Assessment Scale and Social Adjustment Scale-Self Report) were monitored over 2 years,Citation32 the measures showed continued amelioration from baseline to remission, remission to recovery, and after sustained recovery. In the current study, at 12 weeks, of 141 patients with a HAM-D17 score ≥7, 76 patients (53.9%) were judged as Ability to work=No; of 15 patients with a HAM-D17 score of 7, five patients (33.3%) were judged as Ability to work=No. We could not investigate the temporal relationship between remission and recovery as our results were limited to only up to 12 weeks; however, these data suggest that a full recovery of social functioning might take longer than 12 weeks.
Previous research has examined functional recovery following treatment for MDD in numerous studies, including some that assessed response to different classes of agents such as serotonin-norepinephrine reuptake inhibitors versus SSRIs.Citation33 Although results comparing between treatments varied, in general, functional recovery was seen across agents. In a randomized study of patients with severe MDD, treatment with duloxetine 60–120 mg daily resulted in significantly greater improvement in the Sheehan Disability Scale (SDS) compared to treatment with generic SSRIs.Citation34 In a study comparing duloxetine 80 or 120 mg/day to paroxetine 20 mg QD, both treatments were superior to placebo for improvements on the SDS; however, no specific information comparing the active treatments for SDS improvement was provided.Citation35 Finally, a meta-analysis of the norepinephrine reuptake inhibitor reboxetine versus SSRIs showed no significant difference in functional improvements between the active treatment groups based on the Social Adaptation Self-evaluation Scale.Citation36 In the present study, we compared the proportion of patients who achieved the cutoff of a HAM-D17 score of ≤6 in patients treated with duloxetine or with SSRIs. As shown in , improvements were seen in both groups, with no significant difference between treatment arms.
Limitations
Limitations of the current study included the following: this was a post-hoc analysis, and the ability to work was not a primary or secondary study endpoint. As such, the indicator of “ability to work” was based on investigators’ judgment and we did not collect actual data regarding patients’ reinstatement, nor was the assessment by clinicians of ability to work validated as being comparable among investigators. In addition, the HAM-D17 may have been administered by the same investigator who rated patients’ working ability; therefore, HAM-D17 scores may have influenced the investigators’ assessment of working ability. It should also be noted that this study enrolled patients with MDD and at least moderate PPS, and results of this study cannot necessarily be generalized to MDD patients without PPS. Finally, this was an observational study without treatment randomization.
Conclusion
In the present study of patients with both MDD and PPS, the balance of sensitivity versus specificity for investigators’ judgment of the ability to work was optimal at a HAM-D17 score ≤6. This finding is consistent with previous studies showing that a HAM-D17 cutoff of ≤7 may not best represent functional recovery from MDD.
Ethics approval and consent
The study was conducted in accordance with good post-marketing study practicesCitation26 and applicable Japanese laws and regulations. The Japanese Ministry of Health, Labor and Welfare reviewed and approved the protocol. Patients provided written informed consent before enrollment.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
Medical writing assistance was provided by Thomas Melby of Syneos Health, funded by Eli Lilly Japan K.K. This study was funded by Eli Lilly Japan K.K.
Supplementary material
Table S1 Sensitivity, specificity, and AUC of HAM-D17 predictions for ability to work at Week 12 for duloxetine and SSRIs
Disclosure
AK, HI, and SF are full-time employees of Eli Lilly Japan K.K.; AK and SF are stockholders of Eli Lilly and Company; TT and SH are full-time employees and stockholders of Shionogi & Co., Ltd. TT also owns stock in Takeda Pharmaceutical Company Limited; RE is a full-time employee and stakeholder of Eli Lilly and Company. TO has received speaker’s honoraria from Eli Lilly Japan K.K., Otsuka Pharmaceutical Co., Ltd., and Pfizer Japan Inc. The authors report no other conflicts of interest in this work.
References
- KesslerRCAngermeyerMAnthonyJCLifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey InitiativeWorld Psychiatry20076316817618188442
- MuellerTILeonACKellerMBRecurrence after recovery from major depressive disorder during 15 years of observational follow-upAm J Psychiatry199915671000100610.1176/ajp.156.7.100010401442
- BrometEAndradeLHHwangICross-national epidemiology of DSM-IV major depressive episodeBMC Med201199010.1186/1741-7015-9-9021791035
- de GraafRTuithofMvan DorsselaerSten HaveMComparing the effects on work performance of mental and physical disordersSoc Psychiatry Psychiatr Epidemiol201247111873188310.1007/s00127-012-0496-722434047
- EkmanMGranströmOOmérovSJacobJLandénMThe societal cost of depression: evidence from 10,000 Swedish patients in psychiatric careJ Affect Disord2013150379079710.1016/j.jad.2013.03.00323611536
- FonsekaTMMacQueenGMKennedySHNeuroimaging biomark-ers as predictors of treatment outcome in major depressive disorderJ Affect Disord2018233213510.1016/j.jad.2017.10.04929150145
- MöllerHJStandardised rating scales in psychiatry: methodological basis, their possibilities and limitations and descriptions of important rating scalesWorld J Biol Psychiatry200910162610.1080/1562297080226460618663668
- HamiltonMA rating scale for depressionJ Neurol Neurosurg Psychiatry196023566214399272
- MontgomerySAAsbergMA new depression scale designed to be sensitive to changeBr J Psychiatry1979134382389444788
- FrankEPrienRFJarrettRBConceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrenceArch Gen Psychiatry19914898518551929776
- MillerIWKeitnerGISchatzbergAFThe treatment of chronic depression, part 3: psychosocial functioning before and after treatment with sertraline or imipramineJ Clin Psychiatry199859116086199862607
- PapakostasGIPetersenTDenningerJWPsychosocial functioning during the treatment of major depressive disorder with fluoxetineJ Clin Psychopharmacol200424550751115349006
- HirschfeldRMDunnerDLKeitnerGDoes psychosocial functioning improve independent of depressive symptoms? A comparison of nefazodone, psychotherapy, and their combinationBiol Psychiatry200251212313311822991
- van der VoortTYSeldenrijkAvan MeijelBFunctional versus syndromal recovery in patients with major depressive disorder and bipolar disorderJ Clin Psychiatry2015766e809e81410.4088/JCP.14m0954826132690
- ZimmermanMMcGlincheyJBPosternakMAFriedmanMBoerescuDAttiullahNDiscordance between self-reported symptom severity and psychosocial functioning ratings in depressed outpatients: implications for how remission from depression should be definedPsychiatry Res2006141218519110.1016/j.psychres.2005.05.01616499976
- ZimmermanMMartinezJAAttiullahNWhy do some depressed outpatients who are in remission according to the Hamilton Depression Rating Scale not consider themselves to be in remission?J Clin Psychiatry201273679079510.4088/JCP.11m07203.22569085
- BortolatoBMiskowiakKWKöhlerCACognitive remission: a novel objective for the treatment of major depression?BMC Med201614910.1186/s12916-016-0560-326801406
- BrnabicALinCMonkulESDueñasHRaskinJMajor depressive disorder severity and the frequency of painful physical symptoms: a pooled analysis of observational studiesCurr Med Res Opin201228121891189710.1185/03007995.2012.74865423145858
- HaradaESatoiYKikuchiTWatanabeKAlevLMimuraMResidual symptoms in patients with partial versus complete remission of a major depressive disorder episode: patterns of painful physical symptoms in depressionNeuropsychiatr Dis Treat2016121599160727418827
- HaradaESatoiYKugaAAssociations among depression severity, painful physical symptoms, and social and occupational functioning impairment in patients with major depressive disorder: a 3-month, prospective, observational studyNeuropsychiatr Dis Treat2017132437244510.2147/NDT.S13456629033569
- KugaATsujiTHayashiSAn observational study of dulox-etine versus SSRI monotherapy for the treatment of painful physical symptoms in Japanese patients with major depressive disorder: primary analysisNeuropsychiatr Dis Treat2017132105211410.2147/NDT.S13143828831259
- KugaATsujiTHayashiSAn observational study of duloxetine versus SSRI monotherapy in Japanese patients with major depressive disorder: subgroup analyses of treatment effectiveness for pain, depressive symptoms, and quality of lifeNeuropsychiatr Dis Treat2017132115212410.2147/NDT.S13644828831260
- Diagnostic and Statistical Manual of Mental Disorders4th ed Text RevisonWashington (DC)American Psychiatric Association2000
- RushAJTrivediMHIbrahimHMThe 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depressionBiol Psychiatry200354557358312946886
- CleelandCSRyanKMPain assessment: global use of the brief pain inventoryAnn Acad Med Singapore19942321291388080219
- Ministry of Health, Labor, and WelfareGood Post-Marketing Study Practices Ordinance No. 171 issued on December 202004
- RomeraIPérezVMenchónJMPolaviejaPGilaberteIOptimal cutoff point of the Hamilton Rating Scale for Depression according to normal levels of social and occupational functioningPsychiatry Res2011186113313710.1016/j.psychres.2010.06.02320659770
- SawamuraJIshigookaJNishimuraKRe-evaluation of the definition of remission on the 17-item Hamilton Depression Rating Scale based on recovery in health-related quality of life in an observational post-marketing studyHealth Qual Life Outcomes20181611410.1186/s12955-018-0838-629338728
- de VriesGKoeterMWNieuwenhuijsenKHeesHLScheneAHPredictors of impaired work functioning in employees with major depression in remissionJ Affect Disord201518518018710.1016/j.jad.2015.07.01326188379
- TrivediMHMorrisDWWisniewskiSRIncrease in work productivity of depressed individuals with improvement in depressive symptom severityAm J Psychiatry2013170663364110.1176/appi.ajp.2012.1202025023558394
- KishiTMatsudaYMukaiTA cross-sectional survey to investigate the prevalence of pain in Japanese patients with major depressive disorder and schizophreniaCompr Psychiatry201559919710.1016/j.comppsych.2015.02.00425724075
- FurukawaTATakeuchiHHiroeTSymptomatic recovery and social functioning in major depressionActa Psychiatr Scand2001103425726111328238
- SheehanDVNakagomeKAsamiYPappadopulosEABoucherMRestoring function in major depressive disorder: a systematic reviewJ Affect Disord201721529931310.1016/j.jad.2017.02.029.28364701
- MartinezJMKatonWGreistJHA pragmatic 12-week, ran-domized trial of duloxetine versus generic selective serotonin-reuptake inhibitors in the treatment of adult outpatients in a moderate-to-severe depressive episodeInt Clin Psychopharmacol2012271172610.1097/YIC.0b013e32834ce11b22027844
- DetkeMJWiltseCGMallinckrodtCHMcNamaraRKDemitrackMABitterIDuloxetine in the acute and long-term treatment of major depressive disorder: a placebo- and paroxetine-controlled trialEur Neuropsychopharmacol200414645747010.1016/j.euroneuro.2004.01.00215589385
- PapakostasGINelsonJCKasperSMöllerHJA meta-analysis of clinical trials comparing reboxetine, a norepinephrine reuptake inhibitor, with selective serotonin reuptake inhibitors for the treatment of major depressive disorderEur Neuropsychopharmacol200818212212710.1016/j.euroneuro.2007.07.00517719752