 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
Investigation of the longitudinal effect of schizophrenia on changes in various brain-metabolite levels and their relationships with cognitive deficits that have not been fully explained yet.
Methods
Five years subsequent to their first examination for their first episode of schizophrenia, eleven patients from an original group of 30 were reexamined. Their cognitive functions were assessed with the Wisconsin Card Sorting Test. Magnetic resonance imaging and proton magnetic resonance spectroscopy were performed on a 1.5 T scanner. Voxels of 8 cm3 were positioned in the left frontal lobe, left temporal lobe, and the left thalamus. The study had a naturalistic design, and patients were treated with various antipsychotics.
Results
No significant statistical differences between the baseline and follow-up in N-acetylaspartate (NAA:creatine plus phosphocreatine [Cr] and NAA/H2O) levels were observed in any region of interest. We found a significant statistical correlation between 5-year difference in frontal NAA/Cr levels and duration of the last antipsychotic treatment in this period (R=0.908, P=0.012). We found a trend (P=0.068) toward lower choline-containing compounds (Cho/Cr ratio) in the temporal lobe over 5 years and a trend (P=0.079) in higher glutamate–glutamine– GABA (Glx/H2O) levels in the left thalamus. The patients showed social and clinical improvement at follow-up examination, and there were no changes in Wisconsin Card Sorting Test results.
Conclusion
The observed tendency toward decline in choline ratio might have been due to decreased temporal cell density or impaired neuron-membrane or myelin functions. A tendency for higher Glx levels suggest the involvement of thalamus dysfunction in the chronic schizophrenia process. The lack of NAA decrease might have been due to effective antipsychotic treatment. Further longitudinal studies on large patient groups are required to confirm these metabolic changes in schizophrenia.
Introduction
A few complex theories explaining the neurobiological processes of schizophrenia have been developed based on growing evidence, mostly from neuroimaging studies. The neurodegenerative theory of schizophrenia is based on Kraepelin’s observation on the progressive illness process. LiebermannCitation1 proposed the hypothesis that schizophrenia involves a limited neurodegenerative process reflected by psychotic symptoms, and moreover such a process is most active in the early stages of illness. According to the neurodevelopmental theory of schizophrenia, etiologic and pathogenic factors occur early in brain development, and symptom occurrence is connected with the normal maturation of brain areas affected by early developmental pathology, particularly the dorsolateral prefrontal cortex.Citation2 WoodsCitation3 proposed a progressive developmental mechanism of schizophrenia that can reconcile neuropathological and imaging data, while being compatible with early onset and late deterioration. With regard to these three theories, time and duration of disease are very important variables for morphological and neurochemical brain changes in schizophrenia.
Magnetic resonance spectroscopy (MRS) may allow for testing the neurobiological models of pathogenesis of psychiatric diseases.Citation4–Citation9 This method enables in vivo evaluation of chemical tissue composition and identification of chemical compounds. An N-acetylaspartate (NAA) signal is the most visible in proton spectra (1H MRS). The level of NAA increases during brain growth, and reflects the development of dendrites, synapses, and neuronal somata.Citation5 A decrease in NAA concentration in stroke may indicate neuronal loss or dysfunction. The relationship between NAA levels and duration of schizophrenia has been analyzed in cross-sectional studies. A significant inverse relationship between NAA and creatine–phosphocreatine (Cr) in the left dorsolateral prefrontal cortex and disease duration has been observed.Citation9 The mean duration of illness in that study was 6 years, so it suggests that reduced NAA concentration may occur primarily in the early years of the illness.Citation9 Other frequently checked spectroscopic brain metabolites include: glutamate–glutamine (Glx; combined signal, with minor contribution from GABA), choline-containing compounds (Cho; a measure of cellular density), Cr, a marker of cellular energy level, and myoinositol (mI; a marker of brain osmotic balance and glial cells).Citation5–Citation9
Most studies in this area have been cross-sectional, while longitudinal studies allow for distinguishing the progressive aspects of illness from static ones, but few studies have been reported. Longitudinal studies (lasting up to 24 months)Citation11,Citation12 point to a global or frontal brain NAA decrease or increase in the cingulate cortex and to a glutamate increase in the thalamus and cingulate, whereas a longitudinal study lasting 80 monthsCitation10 on schizophrenia duration/treatment, found no changes in NAA and decreased glutamate levels in the thalamus. Glutamatergic alterations and gray-matter loss in schizophrenia in longitudinal studies are consistent with neurodegeneration;Citation10 nevertheless, results regarding NAA levels were variable in these studies: a 2-year reduction or no NAA differences in a longer study.Citation10–Citation12 As earlier longitudinal studies found changes only in glutamate levels, we decided to check and reexamine patients after 5 years of illness to determine whether other progressive metabolite changes would be observed in the frontal and temporal lobes, as well as in the thalamus. Our hypothesis was that NAA levels would be decreased.
Methods
Subjects
The first examination was performed during patients’ first hospitalization at the Department of Psychiatry of the Medical University of Białystok and at the Psychiatric Hospital in Choroszcz. Subjects were included in the study from consecutively admitted patients. The original group consisted of 30 patients with first-episode schizophrenia.Citation13 For a current sample of eleven patients, there were five women and six men, with mean age 23.5±2.61 years and mean duration of illness 6 months. At baseline, patients were given the diagnosis of schizophrenia according to ICD10 criteria: paranoid type (n=9) and undifferentiated type (n=2). One patient was scanned as neuroleptic-naïve and ten received stable doses of antipsychotics (five risperidone, four olanzapine, one haloperidol). Mean antipsychotic doses (chlorpromazine equivalents) were 358 mg/day,Citation14 and the mean number of days on antipsychotic drugs was 50 days until the neuroimaging examination. The subgroup of eleven patients did not differ from the initial group of 30 patients in terms of sex, age, education, length of illness, severity of clinical symptoms, doses of antipsychotics, or length of treatment. All the examined patients were right-handed, determined with the Edinburgh Handedness Inventory.
The follow-up examination was performed 5 years after baseline examination (mean 67.1±12.42 months, 48–87 months). Patients were scanned and underwent clinical and cognitive examination. From the initial group of 30 patients, eleven were contacted and responded (the remaining 19 were lost to medical observation). These were inpatients (n=3) and outpatients (n=8). The mean age of patients was 28.7±2.68 years and mean length of illness 5.5±1.2 years. Ten patients met the criteria for schizophrenia – eight paranoid type and two undifferentiated type – and one for schizoaffective disorder. All patients were treated with antipsychotic medication (atypical and typical antipsychotics in combination: clozapine, olanzapine, risperidone, quetiapine, zuclopenthixol, haloperidol, perphenazine). The mean duration of treatment with the last medication before the second examination was 10 months, and the mean dose (chlorpromazine equivalents) was 376 mg/day.Citation14 Some patients were also treated with mood stabilizers (n=3) and antidepressants (n=4). Clinical symptoms at baseline and follow-up were assessed by a battery of psychiatric measures: Positive and Negative Syndrome Scale (PANSS),Citation15 Calgary Depression Scale for Schizophrenia (CDSS),Citation16 Clinical Global Impression (CGI),Citation17 and Global Assessment of Functioning (GAF). Study-exclusion criteria were central nervous system organic damage confirmed on routine neurological and MR examinations, present and active alcohol and other psychoactive substance dependence, and contraindications to conduct MR examinations.
This study was carried out in accordance with the recommendations of the Declaration of Helsinki and the Bioethical Committee of the Medical University of Białystok. The protocol was approved by the Bioethical Committee of the Medical University of Białystok. All subjects provided written informed consent in accordance with the Declaration of Helsinki to participate in the baseline assessment and after 5 years at follow-up.
MR imaging and 1H MRS
MR imaging and MRS examinations were performed at the Department of Radiology, Medical University of Białystok, on a 1.5 T scanner (Eclipse; Picker International, Highlands Heights, OH, USA) with a standard circularly polarized head coil. A description of this method has been provided by Galińska et al.Citation18 T1-weighted fast scans and conventional fast spin–echo T2-weighted series were obtained. 1H MRS examinations were carried out by means of single-voxel point-resolved spectroscopy (PRESS) sequences with the parameters TR=1,500 ms, TE=35 ms (TE1=~17 ms), number of excitations 192, and 3,906 kHz bandwidth. Voxels of 2×2×2 cm3 were positioned in the left frontal lobe, left temporal lobe, and the left thalamus. In order to shorten the time of scanning, left-sided voxels were analyzed. A trained investigator located voxels by eye by means of T1-weighted sections in sagittal, coronal, and axial planes, and the inclusion of cerebrospinal fluid was minimized. The left frontal lobe voxel was localized in a region that included superior and middle frontal gyrus, above the anterior horns of the lateral ventricles, and comprised mostly of white matter and cortex. The left temporal lobe voxel was localized in the region that included the middle and inferior temporal gyrus. The left thalamus voxel included mostly thalamus tissue and small portions of different structures, eg, the posterior limb of the internal capsule (). For the follow-up scan, we took into consideration the voxel position at the initial scan. This was done individually for each patient. Next, the signal over the voxel was shimmed to within a line width of 3–7 Hz and transmitter-pulse power optimized by automated procedures. The multiply optimized insensitive suppression– train method was applied to suppress the signal from water.Citation19
Figure 1 Voxel location: left frontal lobe (A), left temporal lobe (B), left thalamus (C), and 1H spectrum – raw and fitted data for each region.
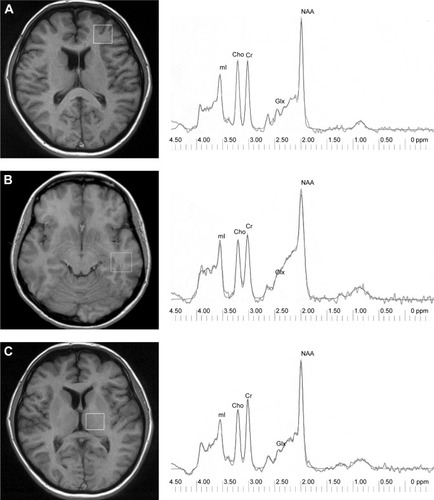
Via 2.0C software provided by Picker was used to analyze spectroscopic data. 1H MRS data were zero-filled to 8,192 points and residual water resonances removed using time-domain high-pass filtering. Exponential Gaussian transformation was applied as a time-domain apodizing Gaussian filter. Next, data were Fourier-transformed and phase-corrected. After the application of a Legendre polynomial function to approximate the baseline, automated curve fitting was performed using an iterative, nonlinear least–squares fitting procedure by means of the Levenberg–Marquardt algorithm. Line shapes of the simulated peaks used in the fitting process were fixed with 85% Gaussian and 15% Lorentzian fractions. Simulated peaks were created using a table of brain metabolites within Via 2.0. Only spectra with the best fit (exactly overlapping and adjusted raw spectrum lines) were included. Metabolites assessed were NAA at 2.01 ppm, Glx in the area from 2.11 ppm to 2.45 ppm, Cho at 3.22 ppm, Cr at 3.03 ppm, and mI at 3.56 ppm (). Then, metabolite to creatine ratios were analyzed. The ratio of metabolites to unsuppressed water signals was also calculated:
Cognitive functions
Cognitive functions were tested by the Wisconsin Card Sorting Test (WCST), which is considered a measure of “executive function” and assesses abstract reasoning ability and flexibility in problem-solving.Citation20 We used the WCST Computer Version 2 Research Edition,Citation21 and analyzed the following measures: total errors, perseverative errors, nonperseverative errors, categories completed, and trials to complete first category.
Statistical analysis
Statistical analysis was performed with the Polish version of Statistica 9.0. Metabolite ratios to creatine and water and clinical and neuropsychological results of patients at baseline and follow-up were compared using Wilcoxon’s signed-rank test, due to the small sample. Relationships among all metabolites and clinical characteristics, doses, time of treatment, and neuropsychological results at start and follow-up were analyzed with Spearman’s correlations. For these results, we also performed post hoc analyses with Bonferroni corrections. In addition, dose of antipsychotics and duration of treatment were also correlated with differences in metabolite levels (last minus first day of study) without post hoc analyses. P<0.05 was taken as significant.
Results
Mean metabolite ratios at baseline and follow-up are shown in . There were no significant differences in any region studied in NAA/Cr or NAA/H2O, the main metabolite of interest. No metabolite ratios from any of the three regions studied correlated with antipsychotic dose at baseline or follow-up. There was a trend toward a lower Cho/Cr ratio in the temporal lobe (P=0.068) and a trend (P=0.079) toward a higher Glx/H2O ratio in the left thalamus over 5 years.
Table 1 Neurochemical findings in patients with schizophrenia during index hospitalization and at 5-year follow-up
Clinical characteristics of patients and WCST results are shown in . There was a significant improvement in patients’ social, occupational, and school functioning according to the GAF scale (P<0.05). Depressive symptoms significantly decreased (P<0.05), and the general psychopathology score on the PANSS significantly improved (P<0.05). No significant differences were observed between WCST results at baseline and second evaluation. NAA/Cr ratios from the left frontal region at follow-up significantly correlated with WCST results (trials to complete first category, R=−0.942; P=0.005). Using a Bonferroni-corrected significance level of P≤0.003 (or 0.05/15), this correlation did not survive this correction. At baseline examination, we did not observe a significant correlation of the frontal NAA/Cr ratio with the WCST results. We found a significant correlation between the 5-year difference in frontal NAA levels and the duration of the last antipsychotic treatment in this period (R=0.908; P=0.012; ).
Figure 2 Correlation between 5-year difference in frontal NAA levels and duration of last antipsychotic treatment (R=0.908, P=0.012).
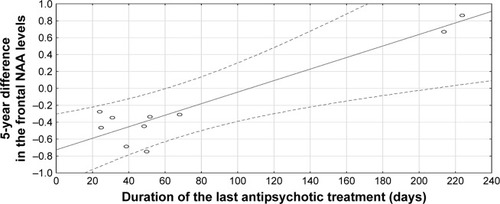
Table 2 Clinical and cognitive findings in patients with schizophrenia during index hospitalization and at 5-year follow-up
Discussion
According to our knowledge, the present study is one of few published longitudinal 1H MRS reports on schizophrenia. Aoyama et alCitation10 followed 17 patients with schizophrenia for 80 months after diagnosis, Théberge et alCitation11 examined 16 patients with a first episode before and after 10 and 30 months of antipsychotic treatment, and Bustillo et alCitation12 scanned early-schizophrenia patients and repeated 1H MRS every 6 months up to 2 years (only six patients scanned at 2 years). We examined patients for a mean 67 months (~5 years) after the onset of the illness, which is one of the longest observations.
In our study, no significant differences in NAA/Cr or NAA/H2O ratios in the left frontal lobe, left temporal lobe, or left thalamus were identified between baseline and follow-up. Previous longitudinal 1H MRS studies in schizophrenia have shown conflicting short-term results: frontal NAA was reduced within the first year of treatment,Citation22 but there were no changes in NAA in a 2-year evaluation;Citation12 NAA reduction in the left anterior cingulate and left thalamus was found only between 10-month and 80-month assessments, but not between start point and 80 months;Citation10 and in other 30-month study, NAA decrease was not found.Citation11 The results of our study and others may show metabolite alterations at different stages of illness. Probably, NAA lowering develops gradually over the duration of illness and would be more noticeable in continuing observation. On the other hand, NAA reduction may be observed only within the first year of treatment,Citation22 and potentially has a tendency to increase thereafter. It may also be that a larger group needs to be checked to show a real trend in NAA levels.
Since patients with schizophrenia have been treated with antipsychotic medications during long-term studies, NAA levels can be influenced by exposure to these medications. However, studies of patients with first-episode schizophrenia revealed no significant increase in previously lowered hippocampal and medial temporal NAA levels after 3 months of treatment.Citation23,Citation24 After 12 months of treatment with atypical antipsychotics, the NAA/Cr ratio in the dorsolateral prefrontal cortex did not significantly change in antipsychotic-naïve first-episode schizophrenia patients either.Citation25 Studies of chronic patients with schizophrenia have demonstrated NAA changes associated with antipsychotic treatment.Citation26–Citation28 Therefore, type of antipsychotic treatment may have an impact on NAA levels. Increased NAA can be related to risperidoneCitation29 or clozapine treatment.Citation30 On the contrary, patients receiving typical neuroleptics show lower NAA in the thalamus compared to controls.Citation31 Moreover, there were no changes in NAA following treatment with haloperidol or quetiapine in one study.Citation12 In two studies performed by Bustillo et al,Citation12,Citation32 the antipsychotic treatment was randomized-controlled.
In our study, we did not control the regimen of treatment: we used a naturalistic design, and patients were receiving various antipsychotics (atypical and typical) due to their clinical condition. Patients in our study benefited clinically from pharmacological treatment, and at followup they exhibited improvement in social functioning and clinical symptoms (better GAF and PANSS scores). Therefore, a correlation between the 5-year difference in frontal NAA/Cr levels and duration of the last antipsychotic treatment in this period shows that the lack of significant decrease in NAA levels might have been due to effective treatment of schizophrenia. A better clinical state of schizophrenia patients at follow-up might potentially also have been due to adaptation to the disease/treatment and be the reason for the result of no longitudinal decline in cognitive processes.
Inconsistent results have been found regarding choline levels in schizophrenia.Citation33 Signals derived from choline-containing compounds are related to the concentration of phosphocholine and glycerophosphocholine, and choline level in 1H MRS is interpreted as a measure of overall cell density and alterations in neuron membrane or myelin turnover.Citation27,Citation33 Also, choline-containing compounds and mI connected with antigliadin antibodies may reflect brain inflammation in schizophrenia.Citation34 In a longitudinal study by Aoyama et al,Citation10 there was a trend of increasing thalamic choline levels at 80 months compared to the never-treated measure. In our study, we observed a trend toward a lower Cho/Cr ratio in the temporal lobe over 5 years, suggesting a longitudinal trend in cell density or impaired neuron-membrane or myelin function in this localization. In a study by Chang et al,Citation35 choline in the right frontal white matter was shown to decrease with age in elderly schizophrenic subjects.
Earlier studies also found increased glutamate levels in the thalamus and cingulate cortex in schizophrenia, whereas after 24–80 months of treatment, glutamate levels in the thalamus had decreased.Citation10,Citation11 We found a trend toward a higher Glx/H2O ratio in the left thalamus over the 5 years of our study. The different result of a glutamate increase in our study might have been due to the neurodegenerative tendency of the thalamus.Citation10 As in our study and the aforementioned,Citation10,Citation11 patients were treated with first- and second-generation antipsychotics, some differences in medications might not be excluded as a reason for these discrepancies in glutamate results.
In our study, cognitive function results did not significantly differ between the baseline examination performed in the first episode and after 5 years of schizophrenia. This is consistent with the results of other studies. Cognitive functions in patients with first-episode schizophrenia and recent-onset schizophrenia did not deteriorate over 5 years.Citation36 In addition, an improvement in verbal IQ and full-scale IQ was correlated with a reduction in negative symptoms, which supports the view that negative syndrome is more strongly related to the level of cognitive functions than psychotic or disorganized syndrome. In our study, the level of negative symptoms had a tendency to improve, but not strongly enough to improve cognitive functions. In another study, there was no decline in cognitive functions over the first 2 years after a first episode of psychosis.Citation37 In others, the cognitive decline observed in the first episode of schizophrenia remained relatively stable through at least 10 years of the illness,Citation38 executive deficits were present in first-episode psychosis, but there was no progress over 10–12 years,Citation39 and impairment in executive function was observed in schizophrenia through the various disease stages, and also the negative symptoms were able to be explained by that dysfunction.Citation40 Studies of gene interactions with symptoms severity and cognitive functioning may also explain schizophrenia pathophysiology.Citation41 We may also suggest that effective treatment might stop progression in cognitive deficit in our patients.
An important problem in longitudinal studies is to maintain the results from the same persons in subsequent examinations. The number of patients is decreasing in long-term 1H MRS examinations.Citation12,Citation24,Citation25,Citation32 In our study, subjects from the initial group (30 patients) were followed up, and only eleven (36%) were available for a second examination 5 years after the first examination (the remaining 19 patients were lost to medical observation). This problem occurs not only in neuroimaging studies but also in longitudinal studies of cognitive functions in patients with schizophrenia.Citation38 Some factors have to be taken into consideration, eg, uncooperative patients or cooperative patients who fail to report for a follow-up or quit due to changes in their living situation. Therefore, our study has certain limitations. Firstly, the limited size of the patient group might have been insufficient to detect significant changes in NAA between two study points, because of variability measurements made by the same method in the same person.Citation42 The absence of findings may also have been due to selection bias. However, our subsample of eleven patients did not differ clinically from the original sample of 30 patients. Secondly, we do not have results from a control group. Thirdly, we did not perform segmentation within the volume of interest. This procedure was not available in our study, and thus we cannot exclude the possibility of regional variations in metabolite concentration.Citation43 Other limitations may also include low field strength (1.5 T) and no partial volume correction. The use of the unsuppressed water signal as an internal standard enhances the meaning of our results, because the observed creatine level was relatively stable. The strength of this study is its longitudinal design, which is one of the longest 1H MRS observations of schizophrenia patients in the literature.
Conclusion
Early-schizophrenia patients on neuroleptic treatment did not show significant regional NAA decline in the first 5 years of illness, which may have been due to the effect of schizophrenia treatment. A tendency shown toward lower Cho/Cr ratio in the temporal lobe over 5 years might point to a longitudinal trend in lower cell density or impaired neuron-membrane or myelin functions in the temporal lobe. A tendency toward higher glutamate levels might have been due to the involvement of thalamic dysfunction in the chronic schizophrenia process. Despite the choline and glutamate longitudinal changes/tendencies, these patients had no worsening of cognitive functions and a better clinical state. Further studies on large patient groups are required to check other long-term metabolic changes in different brain regions of schizophrenia patients.
Acknowledgments
This work was supported by a grant from the State Committee for Scientific Research (KBN, Poland): 3 PO5B 098 24.
Disclosure
The authors report no conflicts of interest in this work.
References
- LiebermanJAIs schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspectiveBiol Psychiatry199946672973910494440
- WeinbergerDRImplications of normal brain development for the pathogenesis of schizophreniaArch Gen Psychiatry19874476606693606332
- WoodsBTIs schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenic mechanismAm J Psychiatry1998155121661167010.1176/ajp.155.12.16619842773
- DagerSROskinNMRichardsTLPosseSResearch applications of magnetic resonance spectroscopy (MRS) to investigate psychiatric disordersTop Magn Reson Imaging2008192819610.1097/RMR.0b013e318181e0be19363431
- DemougeotCMarieCGiroudMBeleyAN-acetylaspartate: a literature review of animal research on brain ischaemiaJ Neurochem200490477678310.1111/j.1471-4159.2004.02583.x15287882
- Galińska-SkokBMałusAKonarzewskaBCholine compounds of the frontal lobe and temporal glutamatergic system in bipolar and schizophrenia proton magnetic resonance spectroscopy studyDis Markers201810.1155/2018/3654894
- WaszkiewiczNGalińska-SkokBNestsiarovichANeurobiological effects of binge drinking help in its detection and differential diagnosis from alcohol dependenceDis Markers201810.1155/2018/5623683
- IwataYNakajimaSPlitmanEMihashiYCaravaggioFChungJKNeurometabolite levels in antipsychotic-naïve/free patients with schizophrenia: a systematic review and meta-analysis of 1H-MRS studiesProg Neuropsychopharmacol Biol Psychiatry2018308634035210.1016/j.pnpbp.2018.03.016
- MolinaVSánchezJReigSN-acetyl-apartate levels in the dorsolateral prefrontal cortex in the early years of schizophrenia are inversely related to disease durationSchizophr Res2005732–320921910.1016/j.schres.2004.02.00115653263
- AoyamaNThébergeJDrostDJGrey matter and social functioning correlates of glutamatergic metabolite loss in schizophreniaBr J Psychiatry2011198644845610.1192/bjp.bp.110.07960821628707
- ThébergeJWilliamsonKEAoyamaNLongitudinal grey-matter and glutamatergic losses in first-episode schizophreniaBr J Psychiatry200719132533410.1192/bjp.bp.106.03367017906243
- BustilloJRRowlandLMJungRProton magnetic resonance spectroscopy during initial treatment with antipsychotic medication in schizophreniaNeuropsychopharmacology200833102456246610.1038/sj.npp.130163118094668
- GalińskaBSzulcATarasówERelationship between frontal N-acetylaspartate and cognitive deficits in first-episode schizophreniaMed Sci Monit200713Suppl 1111617507879
- WoodsSWChlorpromazine equivalent doses for the newer atypical antipsychoticsJ Clin Psychiatry200364666366712823080
- KaySRFiszbeinAOplerLAThe Positive and Negative Syndrome Scale (PANSS) for schizophreniaSchizophr Bull19871322612763616518
- AddingtonDAddingtonJMaticka-TyndaleEAssessing depression in schizophrenia: The Calgary Depression ScaleBr J Psychiatry Suppl199322394410.1192/S0007125000292581
- GuyWECDEU Assessment Manual for PsychopharmacologyWashington, DCUS Department of Health, Education, and Welfare1976
- GalińskaBSzulcATarasówEDuration of untreated psychosis and proton magnetic resonance spectroscopy (1H-MRS) findings in first-episode schizophreniaMed Sci Monit200915CR8288
- MurdochJBLampmanDABeyond Wet and Dry: Optimized Pulses for Water Suppression. Society of Magnetic Resonance in MedicineNew YorkTwelfth Annual Meeting19931191
- HeatonRKCheluneGJTalleyJLKayGGCurtissGWisconsin Card Sorting Test Manual Revised and ExpandedOdessa (FL)Psychological Assessment Resources1993
- HeatonRKWisconsin Card Sorting Test: Computer Version 2 Research EditionOdessa (FL)Psychological Assessment Resources19901993
- BustilloJRLaurielloJRowlandLMLongitudinal follow-up of neurochemical changes during the first year of antipsychotic treatment in schizophrenia patients with minimal previous medication exposureSchizophr Res2002582–331332112409172
- FannonDSimmonsATennakoonLSelective deficit of hippocampal N-acetylaspartate in antipsychotic-naive patients with schizophreniaBiol Psychiatry200354658759813129653
- WoodSJBergerGEWellardRMA 1H MRS investigation of the medial temporal lobe in antipsychotic-naïve early-treated first episode psychosisSchizophr Res20081021–316317010.1016/j.schres.2008.03.01218456460
- GrošićVGrošićPFKalemberPThe effect of atypical anti-psychotics on brain N-acetylaspartate levels in antipsychotic-naïve first-episode patients with schizophrenia: a preliminary studyNeuropsychiatr Dis Treat2014101243125310.2147/NDT.S6141525045268
- BertolinoACallicottJHMattayVSThe effect of treatment with antipsychotic drugs on brain N-acetylaspartate measures in patients with schizophreniaBiol Psychiatry2001491394611163778
- SzulcAGalińska-SkokBWaszkiewiczNProton magnetic resonance spectroscopy changes after antipsychotic treatmentCurr Med Chem201320341442723157634
- ChoeBYSuhTSShinKSLeeCWLeeCPaikIHObservation of metabolic changes in chronic schizophrenia after neuroleptic treatment by in vivo hydrogen magnetic resonance spectroscopyInvest Radiol19963163453528761867
- SzulcAGalińskaBTarasówEThe effect of risperidone on metabolite measures in the frontal lobe, temporal lobe and thalamus in schizophrenic patients. A proton magnetic resonance spectroscopy (1H MRS) studyPharmacopsychiatry200538521421910.1055/s-2005-87315616189748
- ErtrugrulAVolkan-SalanciBKorayBThe effect of clozapine on regional cerebral blood flow and brain metabolite ratios in schizophrenia: relationship with treatment responsePsychiatry Res2009174212112910.1016/j.pscychresns.2009.04.00719837567
- SzulcAGalińskaBTarasówEN-acetylaspartate (NAA) levels in selected areas of the brain in patients with chronic schizophrenia treated with typical and atypical neuroleptics: a proton magnetic resonance spectroscopy (1H MRS) studyMed Sci Monit200713Suppl 1172217507880
- BustilloJRRowlandLMMullinsP1H MRS at 4 Tesla in minimally treated early schizophreniaMol Psychiatry201015662963610.1038/mp.2009.12119918243
- MaddockRJBuonocoreMHMR spectroscopic studies of the brain in psychiatric disordersCurr Top Behav Neurosci20121119925122294088
- RowlandLMDemyanovichHKWijtenburgSAEatonWWRodriguezKGastonFAntigliadin antibodies (AGA IgG) are related to neurochemistry in schizophreniaFront Psychiatry2017810410.3389/fpsyt.2017.0029728674504
- ChangLFriedmanJErnstTZhongKTsopelasDDavisKBrain metabolite abnormalities in the white matter of elderly schizophrenic subjects: implication for glial dysfunctionBiol Psychiatry200762121396140410.1016/j.biopsych.2007.05.02517693392
- GoldSArndtSNopoulosPO’LearyDSAndreasenNCLongitudinal study of cognitive function in first-episode and recent-onset schizophreniaAm J Psychiatry199915691342134810.1176/ajp.156.9.134210484943
- AddingtonJSaeediHAddingtonDThe course of cognitive functioning in first episode psychosis: changes over time and impact on outcomeSchizophr Res2005781354310.1016/j.schres.2005.05.00815978781
- HoffALSvetinaCShieldsGStewartJDeLisiLETen year longitudinal study of neuropsychological functioning subsequent to first episode of schizophreniaSchizophr Res2005781273410.1016/j.schres.2005.05.01015964177
- StirlingJWhiteCLewisSNeurocognitive function and outcome in first-episode schizophrenia: a 10-year follow-up of epidemiological cohortSchizophr Res2003652–3758614630300
- OrellanaGSlachevskyAExecutive functioning in schizophreniaFront Psychiatry201343510.3389/fpsyt.2013.0003523805107
- KandratsenkaHNestsiarovichAGoloenkoIAssociation of mir137 with symptom severity and cognitive functioning in belarusian schizophrenia patientsFront Psychiatry2018929510.3389/fpsyt.2018.0029530026708
- VenkatramanTNHamerRMPerkinsDOSongAWLiebermanJASteenRGSingle-voxel 1H PRESS at 4.0 T: precision and variability of measurements in anterior cingulated and hippocampusNMR Biomed200619448449110.1002/nbm.105516763968
- AuerDPWilkeMGrabnerAHeidenreichJOBronischTWetterTCReduced NAA in the thalamus and altered membrane and glial metabolism in schizophrenic patients detected by 1H-MRS and tissue segmentationSchizophr Res2001521–2879911595395
