Abstract
Objective
Periodic leg movement in sleep (PLMS) is common among patients with obstructive sleep apnea (OSA). The PLMS frequency changes after continuous positive airway pressure (CPAP) titration. This study investigated the effects of two PLMS diagnostic criteria on PLMS prevalence and the restless leg syndrome (RLS) detection rate in patients with OSA before and after CPAP titration.
Methods
This retrospective study included patients with OSA who received polysomnography (PSG) and successful CPAP titration from December 2012 to December 2014. Their clinical variables and sleep parameters were evaluated using the PLMS diagnostic criteria: PLMS index (PLMI) ≥5 and ≥15. PLMS prevalence and the RLS detection rate were analyzed according to the PLMI before and after CPAP.
Results
In patients with OSA with a PLMI of ≥5 and ≥15 after PSG with CPAP titration, the PLMS prevalence was 20.1% (76/378) and 4.5% (17/378), respectively, which revealed CPAP titration increased PLMI. Moreover, in terms of PLMI ≥5 and ≥15, PSG with CPAP titration led to significantly higher PLMS prevalence than PSG alone (20.1% vs 7.1% and 4.5% vs 0.8%, respectively; both P<0.001). PLMI ≥5 also demonstrated a higher RLS detection rate than PLMI ≥15 did (69.2% vs 15.4%; P=0.016).
Conclusion
In patients with OSA, CPAP titration increases PLMS prevalence and the PLMI regardless of whether PLMI is ≥5 or ≥15. The use of the current diagnostic criteria, PLMI ≥15, for PLMS may lead to underestimation of PLMS prevalence and the RLS detection rate in patients with OSA.
Introduction
Periodic leg movement in sleep (PLMS) involves repetitive leg movement at night, which is recorded through polysomnography (PSG). Periodic leg movement (PLM) consists of ≥4 consecutive events of 0.5–10-second-long stereotypical leg movements (LMs) occurring at a 5–90-second interval in sleep.Citation1 Periodic limb movement disorder (PLMD) is characterized by the presence of PLMS accompanied by insomnia. PLMD may also be associated with daytime hypersomnia or unrefreshing sleep after the exclusion of other sleep disorders.Citation2 PLMS is frequently present in patients with restless legs syndrome (RLS). A study found that more than 80% of patients with RLS had PLMS.Citation3 The International Restless Legs Syndrome Study Group (IRLSSG) reported that PLMS is a supportive criterion for RLS diagnosis.Citation4
Before 2005, a PLMS index (PLMI) of ≥5 was considered clinically significant.Citation5 According to this criterion, studies reported that PLMS prevalence was 4–11% in adultsCitation6 and the PLMS prevalence increased with age.Citation7 In 2005, the American Academy of Sleep Medicine (AASM) established the current PLMI cut-off of ≥15 as the PLMD criterion.Citation8 However, clinically, the differences in the PLMS diagnostic criteria used may influence PLMS prevalence and the RLS detection rate.
In PSG studies, PLMS is usually concurrently noted with obstructive sleep apnea (OSA). Moreover, patients with sleep-disordered breathing may present with RLS. However, very few studies have examined PLMS prevalence and RLS detection rate in patients with OSA. A retrospective study in Canada reported PLMS in 48% of patients with OSA.Citation9 Moreover, a small prospective study found that RLS occurred in 8.3% of patients with OSA in the United States.Citation10 The occurrence and clinical relevance of patients with OSA coexisting with PLMS remain unclear.
Continuous positive airway pressure (CPAP) is the most effective therapy for OSA. Some studies have noted that administering CPAP therapy to patients with OSA may increase PLMS prevalence.Citation11 Studies also indicate that the presence of PLMS represents persistent sleep-disordered breathing.Citation12 By contrast, another investigation reported a decrease in the PLMI after CPAP therapy.Citation13 Recent studies have suggested that OSA masks post-CPAP titration PLMS.Citation14,Citation15 Thus, PLMS prevalence and frequency in relation to CPAP titration in patients with OSA remains unclear.
The main purpose of this study was to evaluate two PLMS diagnostic criteria and their effects on PLMS prevalence and the RLS detection rate in patients with OSA before and after CPAP titration.
Methods
Participants
This retrospective study included 443 patients with an apnea–hypopnea index (AHI) of ≥5. The patients underwent both baseline PSG and a second-night PSG for manual CPAP titration between December 2012 and December 2014. The patients were excluded if CPAP titration failed. Patients were also excluded if they used dopaminergic agents or antidepressants, which could affect PLMS before baseline PSG. Before data review, this study was approved by The Institutional Review Board of Chang Gung Memorial Hospital (IRB/CGMH No. 201600860B0) and the inform consent to review their medical records was not required. Patient confidentiality was maintained as no patients’ identifiers were collected and the private will be carefully protected. All research process was in accordance with the Declaration of Helsinki.
The age, gender, body mass index (BMI), neck circumstance (NC), and medical history of the patients were recorded. Detailed sleep parameters were measured, including the total sleep time (TST), sleep efficiency, slow wave sleep (SWS) and rapid-eye movement (REM) percentages, mean and minimal oxyhemoglobin saturation, and mean desaturation. The daytime sleepiness severity was measured on the Epworth Sleepiness Scale (ESS).Citation16 RLS was diagnosed according to the five clinical criteria defined by the IRLSSG in 2014.Citation17
PSG and CPAP titration
Standard overnight PSG was performed using a computerized PSG system (N7000 Embla, Broomfield, USA). The recorded parameters were as follows: electroencephalograms (EEGs), bilateral electrooculograms (EOGs), submental and bilateral anterior tibialis electromyograms (EMGs), electrocardiograms (ECGs), the nasal and oronasal airflow (by using nasal pressure monitor and thermistor), arterial oxygen saturation (through finger probe pulse oximetry), chest and abdominal movements (through inductance plethysmography), body position, and sound intensity. Sleep stages were scored manually in 30-second epochs by using the AASM scoring criteria.Citation18 CPAP titration was administered using AutoSet Spirit S8 (ResMed, Sydney, Australia) in the sleep laboratory on a separate night. In the CPAP titration study, manual CPAP titration was performed to determine an optimal CPAP level.Citation19 The optimal CPAP level was defined as the lowest effective pressure to eliminate most respiratory events, including apnea, hypopnea, and snoring in all body positions and all sleep stages, particularly in the supine position and REM sleep, respectively.
Scoring
Obstructive apnea was defined as the absence of airflow for at least 10 seconds in the presence of respiratory effort, whereas central apnea was defined as the absence of airflow without concurrent respiratory effort. Hypopnea was considered when more than a 50% decrease in airflow occurred for more than 10 seconds, followed by at least 3% oxygen desaturation or EEG arousal.Citation18 The AHI was defined as the average number of apneas and hypopneas per hour of sleep. LM caused an 8-μV increase in the EMG voltage of the right and left anterior tibialis above the resting EMG voltage. The increase lasted 0.5–10 seconds. LM with EEG arousal was also calculated during sleep, which allowed a LM arousal index (LMAI) to be generated. LMs occurring in a wide time window from 0.5 seconds before the start of a respiratory event (apnea or hypopnea) until 0.5 seconds after its end were not counted. PLM was defined as a minimum of four consecutive LM events with a 5–90-second interval during sleep. The PLMI was scored as the number of PLM per hour of TST.Citation20 Two PLMS diagnostic criteria with a PLMI of ≥5Citation3,Citation5 and ≥15Citation2 were assessed. The RLS detection rate was defined as the percentage of patients with RLS matching the PLMS diagnostic criteria divided by the total number of patients with RLS who were diagnosed using the clinical criteria.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) for Windows (version 19.0; SPSS Inc., Chicago, IL, USA). The patient characteristics after using the PLMS diagnostic criteria (PLMI ≥5 and ≥15) are presented as a mean ± standard deviation or number (%). The Wilcoxon signed-rank tests were used to compare the sleep parameters, AHI, and PLMI of the patients at baseline and after CPAP titration. Pearson’s correlation coefficient was used to evaluate the relationship between PLMI and AHI before CPAP titration and the relationship between the increase of PLMI and the decrease of AHI after CPAP titration. McNemar’s test was used to evaluate the differences in PLMS prevalence and the RLS detection rate after using PLMI ≥5 or 15 with PSG alone and PSG with CPAP titration. The variables associated with PLMS were evaluated through multivariate logistic regression by using PLMI ≥5 and 15. A P-value of <0.05 was considered statistically significant.
Results
Subject description
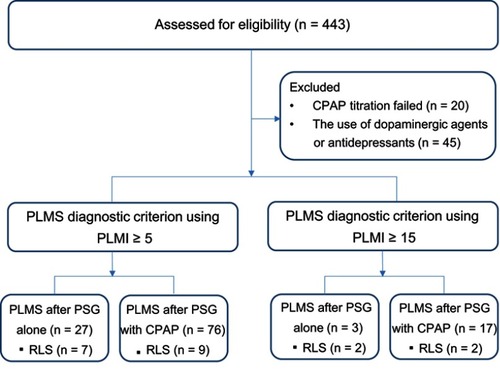
After the exclusion of 20 patients with failed CPAP titration and 45 patients with dopaminergic agent or antidepressant use, 378 patients with OSA were finally included in this study. The patients that met the PLMS diagnostic criterion of PLMI ≥5 or ≥15 at both PSG with and without CPAP titration were considered to have PLMS one time. Among the patients with PLMI ≥5, 27 (7.1%) had PLMS after PSG alone and 76 (20.1%) had PLMS after PSG with CPAP titration. Among the patients with PLMI ≥15, three (0.8%) had PLMS after PSG alone and 17 (4.5%) had PLMS after PSG with CPAP titration (). The basic patient characteristics, including the age, gender, BMI, ESS, NC, AHI, RLS, and past history are listed in . The mean age of the participants was 49.5 years, with a mean BMI of 28.5 kg/m2, and 83.1% of the patients were male.
Table 1 Patient characteristics stratified according to the PLMS diagnostic criteria (PLMI ≥5 and ≥15) after PSG with CPAP titration
Figure 1 Study flow. In this study, 443 patients with an AHI of ≥5 were enrolled. These patients underwent both baseline PSG and a second-night PSG for manual CPAP titration. Among the 443 patients, 20 and 45 were excluded because of CPAP titration failure and dopaminergic agent or antidepressant use, respectively. Finally, 378 patients were included in this study. Patients with a PLMI of ≥5 or ≥15 after PSG with CPAP titration were considered to have PLMS one time. Among the patients with PLMS, 27 (7.1%) and 76 (20.1%) had a PLMI of ≥5 after PSG alone and PSG with CPAP titration, respectively, whereas only three (0.8%) and 17 (4.5%) had a PLMI of ≥15 after PSG alone and PSG with CPAP titration, respectively.
PLMI and sleep parameters after PSG with CPAP titration
In patients with PLMI ≥5, the PLMI increased in 60 (78.9%) patients and decreased in the others (n=16, 21.1%) after CPAP titration. In patients with PLMI ≥15, the PLMI increased in 15 (88.2%) patients and decreased in the others (n=2, 11.8%) after CPAP titration. The PLMI, AHI, and sleep parameters of the patients with PLMI ≥5 and ≥15 after PSG with CPAP titration are listed in . CPAP titration significantly improved nocturnal desaturation and sleep architecture. The TST, SWS, and REM sleep of both the groups increased. Among patients with PLMI ≥5 and ≥15, compared with baseline PSG, CPAP titration reduced AHI (5.92±4.45 vs 55.68±25.26 and 4.61±3.00 vs 53.02±25.42, respectively; both P<0.001) and increased PLMI (10.09±6.98 vs 4.08±4.91, P<0.001 and 19.67±7.70 vs 5.96±6.59, P=0.002, respectively). PLMI (r=−0.611, P<0.001) was negatively correlated with AHI before CPAP and the increase of PLMI (r=0.426, P<0.001) was positively correlated with the decrease of AHI from baseline PSG to CPAP titration among OSA patients coexisting with PLMS.
Table 2 Sleep parameters of patients with a PLMI of ≥5 (n=76) and ≥15 (n=17) after PSG with CPAP titration
PLMS prevalence and the RLS detection rate
PLMS prevalence using PLMI ≥5 or ≥15 after PSG alone and PSG with CPAP titration is listed in . PLMS prevalence after PSG with CPAP titration was significantly higher than that after PSG alone irrespective of the diagnostic criteria (PLMI ≥5: 20.1% vs 7.1%; PLMI ≥15: 4.5% vs 0.8%; both P<0.001). Moreover, different PLMS diagnostic criteria significantly affected PLMS prevalence both after PSG alone and PSG with CPAP titration.
Table 3 PLMS prevalence stratified according to the PLMS diagnostic criteria (PLMI ≥5 and ≥15) after PSG alone and PSG with CPAP titration
The RLS detection rate was also influenced by the PLMS diagnostic criteria (). In total, 13 patients were diagnosed as having RLS according to the IRLSSG diagnostic criteria. Of them, seven (53.8%) and two (15.4%) had a PLMI of ≥5 and ≥15 after PSG alone, respectively; however, the results were not significant. By contrast, nine (69.2%) and two (15.4%) patients had a PLMI of ≥5 and ≥15 after PSG with CPAP titration, respectively, and the results were significant.
Table 4 The RLS detection rate stratified according to the PLMS diagnostic criteria (PLMI ≥5 and ≥15) after PSG alone and PSG with CPAP titration
Factors associated with PLMS
The results of the multivariate logistic regression of the factors associated with PLMS after using the two diagnostic criteria (PLMI ≥5 and ≥15) are presented in . For PLMI ≥5, RLS (OR: 15.101, 95% CI: 3.965–57.515; P<0.001) and chronic kidney disease (OR: 3.633, 95% CI: 1.084–12.172; P=0.037) were independently associated with PLMS; however, no variables were associated with PLMS for PLMI ≥15.
Table 5 Multivariate analyses of the PLMS associated characteristics for PLMI ≥5 and ≥15
Discussion
In a sample of consecutive patients with OSA, different PLMS diagnostic criteria and CPAP application had a significant influence on PLMS prevalence and the RLS detection rate. The PLMS prevalence and frequency were higher when PSG was combined with CPAP titration compared with when PSG was used alone. PLMS prevalence and the RLS detection rate were higher when the PLMI was ≥5 than when the PLMI was ≥15.
PLMS appears to be more common in patients with OSA than in the general population. PLMS prevalence in OSA patients with a PLMI of ≥5 was 48% in CanadaCitation9 and 33% in the United States.Citation21 However, very few studies have examined PLMS prevalence in Asian patients with OSA. In our study, 20.1% of patients with OSA had PLMS with a PLMI of ≥5. These data reveal a lower PLMS prevalence in patients with OSA in Taiwan than in Western countries. By using the PLMS diagnostic criterion of PLMI ≥15, Ren et al reported that PLMS prevalence was 20.1% in a sample of 364 patients with OSA in China.Citation22 In our study, PLMS prevalence in patients with a PLMI of ≥15 was 4.5% lower than the values reported by Ren et al However, we could not clearly elucidate the reason for this relatively low PLMS prevalence. In a Japanese study, among patients with PLMI ≥15, women had a higher PLMS prevalence than men.Citation23 Our study had a higher men/women ratio (4.9/1) than did the study of Ren et al (men/women =1/1). Therefore, different gender ratios may have led to the relatively low PLMS prevalence in our study.
A large multinational study that included 15,391 adults from the United States and five European countries reported the RLS prevalence was 7.2% in the general population.Citation24 However, the RLS prevalence in the Taiwanese population (1.57%) has been reported to be much lower than that in Caucasians due to genetic differences.Citation25 Our study revealed a higher RLS prevalence (3.4%) in patients with OSA than in the general population in Taiwan. Moreover, clinicians consider that PLMS occurrence is likely associated with RLS after the exclusion of other precipitating factors because PLMS is noted in most patients with RLS. In our study, we examined the RLS detection rate in PLMS patients by using two criteria and found that only two out of 13 patients with RLS had a PLMI of ≥15, whereas nine out of 13 patients with RLS had a PLMI of ≥5. Moreover, RLS was an independent factor when the PLMS diagnostic criterion of PLMI ≥5 was used, whereas no such relationship was seen for PLMI ≥15. Although RLS should be diagnosed with clinical criteria, using the diagnostic criterion of PLMI ≥15 in clinical practice can influence the RLS detection rate. Various specialists arrange PSG for different sleep disorders and they may not routinely evaluate RLS symptoms that would miss RLS diagnosis. In our study, PLMI ≥5 demonstrated a higher RLS detection rate than PLMI ≥15 (69.2% vs 15.4%). If PLMS is discovered through PSG with CPAP titration, it will remind the clinician to check clinical symptoms and increase the RLS detection rate. Therefore, our study want to illustrate the PLMI ≥5 or ≥15, CPAP titration, and routinely evaluate RLS symptoms are important factors to affect the detection rate of PLMS and RLS.
Numerous studies have discussed the etiology of the change in PLMI from baseline PSG to CPAP titration, and various theories have been proposed. A 1989 study indicated that CPAP therapy can worsen PLMS.Citation11 Subsequent studies have found that the influence of CPAP on PLMI may be related to the score unmasking PLMS when respiratory events are adequately controlled by CPAP.Citation14,Citation15 By contrast, Yamashiro and Kryger reported that PLMI decreased after CPAP titration.Citation13 A recent investigation hypothesized that CPAP therapy improves residual respiratory-effort related arousals, which may lead to decreased PLMI.Citation14 In our study, only small proportion of patients were decreased PLMI and high proportional patients were increased PLMI after CPAP titration. It means that the mechanism of the score unmasking PLMS and CPAP therapy improves residual respiratory-effort related arousals are existence in different type of OSA patients. Nevertheless, the PLMI increased from the baseline PSG to CPAP titration with a reduction in AHI in patients with both OSA and PLMS regardless of PLMI ≥5 or ≥15. A negative correlation was demonstrated between PLMI and AHI before CPAP titration and a positive correlation was demonstrated between the increase of PLMI and the decrease of AHI after CPAP titration. Those data support that the mechanism of the score unmasking PLMS seems play more important role. These findings are consistent with that of Hedli et al,Citation15 which indicates that respiratory events may mask PLMS, which appears with CPAP therapy. Furthermore, the current AASM scoring criteria emphasize that PLM should only be counted if the PLM is spontaneous and not caused by respiratory events.Citation20 Thus, LM events occurring within 0.5 seconds of apnea or hypopnea are considered as respiratory-related LMs and deleted during manual scoring. PLMS prevalence in patients with OSA may be underestimated in baseline PSG. The real presentation of PLMS should occur after a treatment for OSA, such as CPAP, is implemented.
The recognition and distribution of true PLMS and respiratory event-associated LMs in patients with OSA have been debated considerably. Recent studies have suggested that the elimination of all LMs associated with respiratory events is possibly an incorrect practice because the distribution of respiratory-related LMs increases mainly over an interval of −2 to 10.25 seconds around the end of respiratory events.Citation26,Citation27 Manconi et al reported that respiratory-related LMs were not augmented at the beginning or middle of respiratory events but clustered only around the end of respiratory events.Citation28 These findings suggest that the duration and distribution of respiratory-related LMs are different compared with the AASM criteria for scoring LMs. After the application of the AASM criteria, many cases of PLMS can be eliminated from the counts that have not been eliminated under CPAP therapy because of the disappearance of most respiratory events.
The analysis of hypersomnia symptoms indicated that ESS was not an independent factor associated with PLMS occurrence based on either PLMI ≥5 or ≥15 as the PLMS diagnostic criterion in patients with OSA. Chervin et al and Haba-Rubio et al have also reported that concurrent OSA and PLMS are not associated with increased hypersomnia.Citation29,Citation30 We propose that PLMS is not treated even if the PLMI increases after CPAP therapy without any clinical symptoms. Further investigation is required for confirming whether concurrent PLMS and OSA results in additive consequences, such as cardiovascular events.
This study has certain limitations. First, we did not investigate PLMD. The diagnosis of PLMD requires the exclusion of other sleep disorders. However, all these sleep disorders cannot be identified through a simple chart review. Thus, we did not include PLMD in this study. Second, this study was a retrospective one and we did not obtain follow-up data after long-term CPAP therapy. Additional prospective studies are required to assess the effect of long-term CPAP on PLMS in patients with OSA. Third, the common medical or behavioral condition (eg, myalgia, venous stasis, leg edema, arthritis, leg cramps, positional discomfort, habitual foot tapping) can be mistaken for RLS. A retrospective study with chart review may not exclude RLS-mimics completely.
Conclusion
CPAP titration increases the prevalence of PLMS, which suggests that OSA masks PLMS. PLMS prevalence and the RLS detection rate were higher when the PLMI was ≥5 than when the PLMI was ≥15. Therefore, the current AASM criteria for scoring LMs and the PLMS diagnostic criterion with PLMI ≥15 require reconsideration.
Acknowledgments
The authors thank all the physicians and members of the sleep center in Chang Gung Memorial Hospital, Keelung, Taiwan.
Disclosure
The authors report no conflicts of interest in this work.
References
- Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med. 2006;7(2):175–183. doi:10.1016/j.sleep.2006.01.00116459136
- Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. CHEST. 2014;146(5):1387–1394. doi:10.1378/chest.14-097025367475
- Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12(1):61–65. doi:10.1002/mds.8701201118990055
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119.14592341
- Coleman RM, Pollak CP, Weitzman ED. Periodic movements in sleep (nocturnal myoclonus): relation to sleep disorders. Ann Neurol. 1980;8(4):416–421. doi:10.1002/ana.4100804137436384
- Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev. 2006;10(3):169–177. doi:10.1016/j.smrv.2005.12.00316762807
- Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53(1):547–554.12127170
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. Diagnostic and Coding Manual. 1st ed. Westchester (IL): American Academy of Sleep Medicine; 2005.
- Al-Alawi A, Mulgrew A, Tench E, Ryan CF. Prevalence, risk factors and impact on daytime sleepiness and hypertension of periodic leg movements with arousals in patients with obstructive sleep apnea. J Clin Sleep Med. 2006;2(3):281–287.17561540
- Lakshminarayanan S, Paramasivan KD, Walters AS, Wagner ML, Patel S, Passi V. Clinically significant but unsuspected restless legs syndrome in patients with sleep apnea. Mov Disord. 2005;20(4):501–503. doi:10.1002/mds.2036615597337
- Fry JM, DiPhillipo MA, Pressman MR. Periodic leg movements in sleep following treatment of obstructive sleep apnea with nasal continuous positive airway pressure. CHEST. 1989;96(1):89–91. doi:10.1378/chest.96.1.892661161
- Seo WH, Guilleminault C. Periodic leg movement, nasal CPAP, and expiratory muscles. CHEST. 2012;142(1):111–118. doi:10.1378/chest.11-156322241760
- Yamashiro Y, Kryger MH. Acute effect of nasal CPAP on periodic limb movements associated with breathing disorders during sleep. Sleep. 1994;17(2):172–175. doi:10.1093/sleep/17.2.1728036372
- Baran AS, Richert AC, Douglass AB, May W, Ansarin K. Change in periodic limb movement index during treatment of obstructive sleep apnea with continuous positive airway pressure. Sleep. 2003;26(6):717–720. doi:10.1093/sleep/26.6.71714572125
- Hedli LC, Christos P, Krieger AC. Unmasking of periodic limb movements with the resolution of obstructive sleep apnea during continuous positive airway pressure application. J Clin Neurophysiol. 2012;29(4):339–344. doi:10.1097/WNP.0b013e318262456722854768
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi:10.1093/sleep/14.6.5401798888
- Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med. 2014;15(8):860–873. doi:10.1016/j.sleep.2014.03.02525023924
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi:10.5664/jcsm.217223066376
- Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171.18468315
- American Academy of Sleep Medicine. American Academy of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester: American Academy of Sleep Medicine; 2007.
- Javaheri S, Abraham WT, Brown C, Nishiyama H, Giesting R, Wagoner LE. Prevalence of obstructive sleep apnoea and periodic limb movement in 45 subjects with heart transplantation. Eur Heart J. 2004;25(3):260–266. doi:10.1016/j.ehj.2003.10.03214972428
- Ren R, Huang G, Zhang J, et al. Age and severity matched comparison of gender differences in the prevalence of periodic limb movements during sleep in patients with obstructive sleep apnea. Sleep Breath. 2016;20(2):821–827. doi:10.1007/s11325-015-1231-x26174846
- Aritake-Okada S, Namba K, Hidano N, et al. Change in frequency of periodic limb movements during sleep with usage of continuous positive airway pressure in obstructive sleep apnea syndrome. J Neurol Sci. 2012;317(1–2):13–16. doi:10.1016/j.jns.2012.03.01322498043
- Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165(11):1286–1292. doi:10.1001/archinte.165.11.128615956009
- Chen NH, Chuang LP, Yang CT, et al. The prevalence of restless legs syndrome in Taiwanese adults. Psychiatry Clin Neurosci. 2010;64(2):170–178. doi:10.1111/j.1440-1819.2010.02067.x20447013
- Fulda S, Heinzer R, Haba-Rubio J. Characteristics and determinants of respiratory event associated leg movements. Sleep. 2017.
- Manconi M, Zavalko I, Bassetti CL, Colamartino E, Pons M, Ferri R. Respiratory-related leg movements and their relationship with periodic leg movements during sleep. Sleep. 2014;37(3):497–504. doi:10.5665/sleep.348424587572
- Manconi M, Zavalko I, Fanfulla F, Winkelman JW, Fulda S. An evidence-based recommendation for a new definition of respiratory-related leg movements. Sleep. 2015;38(2):295–304. doi:10.5665/sleep.441825325500
- Chervin RD. Periodic leg movements and sleepiness in patients evaluated for sleep-disordered breathing. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1454–1458. doi:10.1164/ajrccm.164.8.201106211704595
- Haba-Rubio J, Staner L, Krieger J, Macher JP. Periodic limb movements and sleepiness in obstructive sleep apnea patients. Sleep Med. 2005;6(3):225–229. doi:10.1016/j.sleep.2004.08.00915854852
