Abstract
Background
Generic health-related quality of life (HRQoL) scales are increasingly being used to assess the effects of new treatments in schizophrenia. The objective of this study is to better understand the usefulness of generic and condition specific HRQoL scales in schizophrenia by analyzing their correlates.
Methods
Data formed part of the Pattern study, an international observational study among 1379 outpatients with schizophrenia. Patients were evaluated with the Mini International Neuropsychiatric Inventory, the Clinical Global Impression-Schizophrenia (CGI-SCH) Scale and the Positive and Negative Syndrome Scale (PANSS) and reported their HRQoL using the Schizophrenia Quality of Life Scale (SQLS), the Short Form-36 (SF-36), and the EuroQol-5 Dimension (EQ-5D). The two summary values of the SF-36 (the Mental Component Score and the Physical Component Score, SF-36 MCS and SF-36 PCS) were calculated.
Results
Higher PANSS positive dimension ratings were associated with worse HRQoL for the SQLS, EQ-5D VAS, SF-36 MCS, and SF-36 PCS. Higher PANSS negative dimension ratings were associated with worse HRQoL for the EQ-5D VAS, SF-36 MCS, and SF-36 PCS, but not for the SQLS or the EQ-5D tariff. PANSS depression ratings were associated with lower HRQoL in all the scales. There was a high correlation between the HRQoL scales. However, in patients with more severe cognitive/disorganized PANSS symptoms, the SQLS score was relatively higher than the EQ-5D tariff and SF-36 PCS scores.
Conclusion
This study has shown substantial agreement between three HRQoL scales, being either generic or condition specific. This supports the use of generic HRQoL measures in schizophrenia.
Clinicaltrials.gov identifier
NCT01634542 (July 6, 2012, retrospectively registered).
Introduction
Schizophrenia is a severe, disabling, stigmatizing and frequently chronic condition which impacts on the social, occupational and everyday functioning of the individuals who suffer from it. Assessing health-related quality of life (HRQoL) in schizophrenia is necessary to understand which aspects of the disorder are meaningful for the patients themselves.
Instruments that measure HRQoL in mental health have been increasingly introduced to clinical practice as a good method to monitor treatment results, functioning, and quality of life.Citation1,Citation2 Treatment for schizophrenia should aim at improving patients’ health-related quality of life (HRQoL), as this may be associated with increased satisfaction with care and higher adherence.Citation3 Moreover, identifying the factors that are correlated with reduced quality of life is essential to optimize the treatment and the management of comorbidities. Accordingly, it is necessary to incorporate self-reported HRQoL measures when assessing treatment outcomes among patients with schizophrenia. These HRQoL instruments ask patients to report their perspective on their health status.
There is currently a debate about which measurements better reflect the HRQoL of patients with schizophrenia.Citation4 HRQoL can be measured either with generic or with condition specific HRQoL scales. Generic scales have been developed to measure HRQoL across different health conditions while condition-specific scales focus on the symptoms and difficulties experienced by individuals presenting a particular health problem.
The capacity of generic HRQoL scales to reliably assess QoL has been questioned.Citation5,Citation6 In their review of the validity of generic HRQoL measures in schizophrenia, Papaioannou et al.Citation5 cast doubts upon the EQ-5D and the SF-36, the most frequently used generic HRQoL scales, being sensitive to symptom severity of the disorder. Specifically, when assessing the relationship between HRQoL and positive and negative symptoms of schizophrenia they found ten studies suggesting uncertain or no evidence of an association, whereas only four revealed moderate, to strong, correlations between these symptoms and generic HRQoL measures. They also reported that the correlation of generic HRQoL scales with depression symptoms seemed to be stronger than the correlation of generic HRQoL scales with positive and negative symptoms of schizophrenia. Additionally, previous studies have also provided inconsistent findings on the relationship between generic HRQoL measures and patient functioning: four studies found a strong relationship between HRQoL and functioningCitation7–Citation10 and four described uncertain or no evidence of such a relationship.Citation11–Citation14 However, the relative lack of agreement between HRQoL scales and symptom severity and functioning should be expected given that HRQoL covers many more dimensions than these two concepts.Citation15 In spite of being an extensive and comprehensive review, Papaioannou et al work has also some shortcomings. For example, the included studies had very heterogeneous patient populations, in the sense that patients were in different stages of illness and treatment settings.
More recently, Mulhern et al used seven existing datasets to analyze the psychometric properties of the EQ-5D and the SF-36 in the assessment of individuals with mental disorders. Two of these studies included about three hundred individuals with schizophrenia.Citation16,Citation17 They found unclear evidence that generic HRQoL scales were valid in schizophrenia and concluded that there was some support for the construct validity of generic HRQoL scales in assessing patients with schizophrenia, but that responsiveness was low.Citation18
Finally, the utility of HRQoL scales in schizophrenia has also been criticized on other grounds, which are common for generic and disorder-specific scales. In particular, many clinicians think that patients may frequently be too unwell, psychotic, or disturbed to be reliable in the self-assessment of their health status. Additionally, self-reported health measures may be of special concern in individuals with cognitive impairment, as those suffering from schizophrenia. There is limited data on the validity and consistency of HRQoL questionnaires in individuals with cognitive problems.Citation19 Finally, HRQoL may be influenced by expectations and adaptation to illness mechanisms in individuals with chronic conditions, who may tend to rate HRQoL higher than individuals with the same disability but caused by more acute illness.Citation20
In spite of these limitations, the use of generic HRQoL scales in schizophrenia has relevant support. Regulatory bodies in the USA, the UK, and France endorse the inclusion of HRQoL measures in assessing treatment effects in individuals with chronic health conditions.Citation19 Since health-policy decisions are taken for all health conditions, these agencies rely mostly on the generic HRQoL measures such as the EQ-5DCitation21 and the SF-36Citation22 For these reasons, generic HRQoL scales are increasingly being used to test the effectiveness of new treatments.
Given the relevance of assessing QoL in schizophrenia, the fact that generic scales are used to determine if a given treatment impacts on HRQoL in schizophrenia, coupled with the inconsistent literature findings on HRQoL determinants, and the need to better understand the meaning and correlates of generic HRQoL measures in schizophrenia, our study aims to: a) analyze the relationship between generic and schizophrenia-specific HRQoL scales in patients with schizophrenia and b) describe the clinical factors that influence HRQoL in these patients.
Materials and Methods
Study Design
The objective of these post-hoc analyses of the Pattern studyCitation23 is to increase the understanding of the usefulness of two generic HRQoL scales (SF-36 and EQ-5D) in evaluating patients with schizophrenia. We will analyze the socio-demographic and clinical correlates associated with the rating of these two scales, especially how they relate to symptom severity of schizophrenia. We will also compare them to one condition specific HRQoL (Schizophrenia Quality of Life Scale). Data for these analyses were obtained from the Pattern study, a multicentric observational study that assessed the care and clinical outcomes of outpatients with schizophrenia in 8 countries (Argentina, Brazil, Canada, France, Germany, Italy, Spain, and the United Kingdom).
Individuals with schizophrenia of at least 18 years old who were receiving care in an outpatient setting were eligible for study entry. Participants had to meet diagnostic criteria for schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision or the International Classification of Diseases, 10th Revision.Citation24 To confirm the diagnosis, a reduced version of the Mini International Neuropsychiatric InventoryCitation25 was administered by the participating psychiatrists. Individuals with a recent psychotic exacerbation (three months prior to baseline), those who were enrolled in an interventional study at baseline, and those who were unable or unwilling to comply with the study protocol were not eligible for inclusion. For this study, a recent psychotic exacerbation was defined as hospitalization or increased psychiatric care in order to avoid hospitalization. No other exclusion criteria were applied in order to achieve a high generalizability of the findings.
Recruitment and evaluation were conducted by psychiatrists working in outpatient facilities and the psychiatrist’s teams. Patients were recruited using a sequential selection procedure. From the list of patients being treated at each of the participating clinical sites, those individuals who fulfilled the inclusion criteria and did not have any exclusion criteria were invited to participate. Patient care was solely at the discretion of the treating psychiatrist. There were no instructions regarding treatment in the study protocol. The protocol and consent procedures were approved by all local institutional review boards/ethics committees before study initiation. All patients voluntarily participated in the study and provided informed consent.
Patient Assessment
Participating psychiatrists with, in some cases, the collaboration of adequately trained mental health professionals of their teams, evaluated the patients using an electronic hand-held tablet. This assessment included socio-demographic and clinical variables, Positive and Negative Syndrome Scale (PANSS),Citation26 and the Clinical Global Impression-Schizophrenia (CGI-SCH) Scale. Participating psychiatrists and the members of their teams who completed assessments were provided with training in the use of the questionnaires. PANSS dimensions were calculated based on Lindenmayer et al.’s five factors:Citation27 positive, negative, cognitive/disorganized, affective, and executive.
Patients also used an electronic hand-held tablet to record the patient-reported outcome (PRO) questionnaires data. To assure that both assessments were independent, PRO questionnaires were administered prior to the completion of other study assessments. Patients completed the EuroQol-5 Dimension (EQ-5D)Citation21 questionnaire, the Short Form-36 (SF-36),Citation22 and the Schizophrenia Quality of Life Scale (SQLS).Citation9
The SF-36 is a generic health status instrument that measures eight dimensions, namely general health, bodily pain, physical functioning, role-physical, mental health, vitality, social functioning, and role-emotional. Out of the items, a physical and a mental health summary score can be calculated (SF-36 physical component score, SF-36 PCS, and SF-36 mental component score, SF-36 MCS). The calculation is such that the population mean of these summary scores is 50, with higher ratings indicating a better HRQoL.
The EQ-5D questionnaire has two parts. The first part consists of five questions on different aspects of HRQoL (mobility, self-care, usual activities, pain, and anxiety/depression). This first part allows the calculation of a health preference measure or tariff with values ranging from 0 (death) to 1 (perfect health). The second part is a visual analog scale (EQ-5D VAS) which invites the responder to mark their current quality of life on a line from 0 mm (worst imaginable health state) to 100 mm (best imaginable health state). The EQ-5D population tariffs and SF-36 mental component score (SF-36 MCS) and physical component score (SF-36 PCS) were calculated using the UK validation studies.Citation28,Citation29 Both, the SF-36 and the EQ-5D have been found to have good validity and reliability in several populations.Citation30–Citation34
The Schizophrenia Quality of Life Scale assesses HRQoL in three areas: a) psychological features, which comprises various emotional problems such as feeling lonely, depressed, or hopeless, and difficulties in social situations or being worried about the future; b) motivation and energy, which includes problems with motivation and activity such as lacking the drive to do things and engaging in activities that cause positive experiences; and c) symptoms and side effects, such as problems with sleeping, blurred vision, dizziness, muscle twitches, and dry mouth, which can be associated with medication.Citation9 The rating of each of the three scales is recalculated to have a range from 0 to 100 with higher ratings meaning worse health status. The scale includes 30 items. The internal reliability of the SQLS scale ranges from 0.78 to 0.93 for the three subscales (psychosocial, motivation and energy and symptoms and side-effects).Citation9
Statistical Analysis
The analyses comprised patients who fulfilled all the eligibility criteria for the cross-sectional phase (N=1,379). Descriptive analyses were conducted on socio-demographic, clinical and HRQoL variables. Internal consistency of the scales was estimated using the Cronbach alpha statistic.Citation35 A Cronbach alpha greater than 0.7 has been considered satisfactory.Citation36 Scatter plots of the correlations between the HRQoL scales and sub-scales were provided. Pearson correlation coefficients between the pairs of HRQoL scales were calculated. The analyses of the baseline factors associated with HRQoL ratings were estimated using linear regression models. Given that many individuals had a rating of 1 in the EQ-5D tariff, Tobit regression was used to adjust for ceiling effects in this analysis.Citation37 Sex, age, and country were included in all regression models regardless of their statistical significance. The rest of the covariates were chosen based on the relevance and significance in the descriptive analyses. The following variables were tested: age at onset, time since onset, PANSS (overall and positive, negative, cognitive/disorganized, affective and excitement), number of co-morbidities, type of co-morbidity (cardiovascular, endocrine/metabolic, musculoskeletal, gastrointestinal, obesity, genitourinary, respiratory, neurological in extremities, cancer, hematopoietic, dermatological, and others), extrapyramidal symptoms, tardive dyskinesia and akathisia, and substance use (including past or current use of alcohol, recreational drugs, and other substances). All statistical analyses were done with SAS version 9.
Results
Patient Characteristics
A total of 1,433 patients were included in the study. They came from 40 study sites in eight countries (Argentina N=110, Brazil N=100, Canada N=117, France N=237, Germany N=250, Italy N=219, Spain N=207, and the United Kingdom N=139). More than two third of the patients (71%) were male and the mean age was about forty years old (). Four out of ten patients had one or more co-morbid physical disorders and about five percent had a current substance use problem. The mean PANSS overall score was 78 points. The SF-36 physical component score was 50, similar to the population mean, whereas the SF-36 mental component was 41.
Table 1 Sociodemographic and Clinical Data
Internal Consistency
Cronbach’s alphas were estimated for the scales. The value for the SQLS was 0.92. The value for the EQ-5D was 0.65. For the SF-36 subscales, values were 0.90 for Physical functioning, 0.91 for Role physical, 0.83 for Bodily pain, 0.77 for General health, 0.76 for Vitality, 0.77 for Social functioning, 0.88 for Role emotional, and 0.82 for Mental health.
Factors Associated with Health-Related Quality of Life
Females consistently reported worse quality of life except for the EQ-5D tariff, which did not show gender differences (). Higher PANSS positive dimension ratings were associated with worse HRQoL for the SQLS, EQ-5D VAS, SF-36 MCS, and SF-36 PCS. Higher PANSS negative dimension ratings were associated with worse HRQoL for the EQ-5D VAS, SF-36 MCS, and SF-36 PCS, but not for the SQLS or the EQ-5D tariff. PANSS depression ratings were associated with lower HRQoL ratings in all the scales. The other factors presented a less consistent pattern across the different HRQoL scales. Current substance use was associated to lower EQ5D tariff, obesity with lower SF-36 MCS ratings but higher SF-36 PCS, the number of physical comorbidities with both lower EQ5D tariff and SF-36 PCS, the number of hospitalizations only with lower EQ5D VAS.
Table 2 Multiple Regression Models on Factors Associated with Each of the QoL Scales. Figures are Coefficients (95% CI)
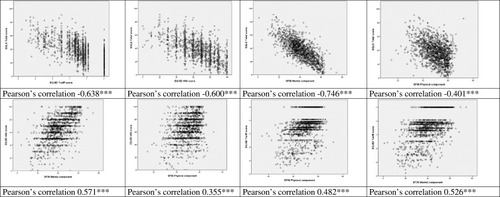
Relationships Among Health-Related Quality of Life Scales
There was a high correlation between the different HRQoL scales (). The highest correlation () was between the SQLS total score and the SF-36 MCS (Pearson Correlation Coefficient, PCC 0.746), and the lowest between the EQ5D-VAS and the SF-36 PCS (PCC 0.355). Not included in the figure are the correlations between EQ-5D VAS and EQ-5D tariff (0.516, p<0.001) and between SF-36 MCS and SF-36 PCS (0.076, p<0.01).
Table 3 Association Between QoL Measures
Discussion
This study has shown substantial agreement between three health-related quality of life scales, two generic, EQ-5D and SF-36, and one condition-specific, SQLS, in a large cross-national sample of patients with schizophrenia. We have shown that the three scales also tend to show analogous gender differences and vary similarly with the intensity of the different symptom dimensions in schizophrenia. This provides some support for the use of generic HRQoL measures in schizophrenia.
Our finding of depressive symptoms as a key determinant of HRQoL in people with schizophrenia is consistent with many other QoL studiesCitation38–Citation41 and confirm they are negative predictors of HRQoL, with respect to both mental health and physical components. Previous results have showed that the effect of depressive symptoms is mostly present on the mental health component,Citation42 but our findings are still consistent with the literature as Meijer et al showed that the mental health component may have an impact on the physical component.Citation43 The longitudinal study of QoL in older adults (≥55 years old) from Cohen et alCitation44 has suggested that the relationship between QoL and depressive symptoms is bidirectional, conversely to other clinical symptoms, as the increased social isolation because of the decrease in interactions with friends and peers, reduces self-reported life satisfaction, and the lower quality of life impacts on the feelings of the individual. Further studies on the relationship between depressive symptoms and QoL are warranted.
Findings on the association of positive symptoms on QoL have been varied and contradictory, while some have found associations,Citation41,Citation43–Citation48 many have not.Citation38,Citation49,Citation50 Our results show that positive symptoms are a negative predictor of both mental and physical components of quality of life. One of the reasons most studies have not found significant results for positive symptoms might be due to the different QoL measures used: Eack and Newhill’s meta-analysis suggested that disparate findings might be due to positive symptoms having strongest relationship with HRQoL, smaller associations with subjective QoL and general well-being, and no relationship with objective QoL.Citation51
The association between negative symptoms and QoL has been found varying and contrasting too, probably due to the way negative symptoms have been assessed. The assessment of QoL may also be relevant in explaining these diverse results. Subjective and objective HRQoL measures may have different relationships with negative symptoms.Citation52 Also, it has been found that apart from the low correlation between subjective QoL and objective QoL, their relationships with patient characteristics varies, which suggests that they may be separate and possibly complementary constructs.Citation53 Hence, both types of QoL data are essential. The present study shows that negative symptoms are significant predictor of lower HRQoL. Negative symptomatology represents a highly personal and social burden for a large number of individuals with schizophrenia, since they are unable to live independently and manage everyday social situations. This is more relevant if we consider that negative symptoms are associated with a limited response to pharmacotherapies, thus, remain an area of unmet therapeutic need.Citation54
A higher cognitive symptom severity score in the PANSS scale was associated with a higher EQ-5D rating and with a higher SF-36 rating. Thus, in our study, patients with more cognitive problems reported a higher quality of life on the EQ-5D and the SF-36 scales. This is not consistent with previous studies. For example, population studies have found that mild cognitive impairment is associated with lower health-related quality of life.Citation55,Citation56 The results of studies with patients with schizophrenia are comparable. Apteinin et alCitation57 in a sample of 38 patients with schizophrenia, found that cognitive deficits, specifically in executive function and working memory, were associated with lower self-reported quality of life, in particular in the social sphere. Analogous results were reported by Savilla et al in a sample of 57 individuals with schizophrenia.Citation58 However, several of these studies did not adjust for the severity of other psychiatric symptoms. Our results do take into account the severity of other symptoms of schizophrenia. Some might be skeptical about the reliability and validity of self-rated instruments in people with schizophrenia due to cognitive deficits. A meta-analysis revealed that neurocognitive deficits have an insignificant relationship with subjective QoL (client satisfaction)Citation59 whereas three recent studies suggest that cognitive deficits, when assessed from the patients’ point of view, show a significant relationship with QoL.Citation60 This warrants the need to further investigate the role cognition plays in QoL of patients with schizophrenia.Citation40 Another possible explanation for our findings is that patients with cognitive problems are less aware of their problems. To elucidate the influence of cognitive dysfunction on QOL, further studies using neuropsychological tests are necessary.Citation61
It is worth noting that approximately forty percent of the participants in this study reported one or more co-morbid physical disorders. This is in line with past literature, where chronic medical conditions, especially cardiovascular diseases and diabetes, often co-occur with schizophrenia or severe mental illnesses.Citation62–Citation64 Furthermore, somatic comorbidity resulted to be associated with poorer HRQoL on EQ5D TARIFF and SF-36 PCS scales. The study from Sexton at al. has shown that chronic medical conditions affect QoL via physical impairment (increased deficits in physical body function and activity), which is directly and indirectly associated with reduced positive affect.Citation65 Recently, the prevalence of a comorbid condition (at least one condition) in patients with Schizophrenia has been estimated to be approximately 80%. Furthermore, while an increase in the prevalence of comorbid conditions was generally observed across all age categories, an age-specific increase was found in the schizophrenia group as compared to controls with regard to suicide attempts, diabetes, and epilepsy. These differences were highly pronounced at younger ages and decreased with increasing age.Citation66 The magnitude of the problem underlines the need for regular screening, comprehensive assessment, preventive pharmacotherapy, and targeted somatic comorbidity management.
Other results in the present study are in line with previous literature, as current substance use and a higher number of hospitalization have been associated with lower HRQoL.Citation67,Citation68 Regarding obesity, the present study found an association with absence of obesity and a lower score on SF-36 MCS, contrary to a higher score on SF-36 PCS: while the association between obesity and a lower physical component HRQoL has been widely documented,Citation69 the association between obesity and a higher mental component HRQoL is uncommon. However, obesity can cause developmental problems, such as poor cognitive function,Citation70 which, in turn, is associated with higher HRQoL in our study. In conclusion, the presence of different clinical factors that affect the health-related quality of life in people with schizophrenia encourages the adoption of an integrated recovery-oriented model that considers wider outcomes than schizophrenia core symptoms as the main goal of treatment.
The analysis of the internal consistency of the scales also deserves some comment. The internal consistency of the SQLS and the scales of the SF-36 were good. However, the Cronbach alpha of the EQ-5D was below the value of 0.7, which is considered acceptable in group comparisons. This may be explained by the EQ-5D being mostly an inventory of symptoms assessing different dimensions of HRQoL. Although previous studies have found good reliability measures for the EQ5D,Citation71 other studies have found lower values than 0.7Citation72
In evaluating these findings, we need to take into account a number of limitations. First, this analysis may be criticized on the bases that we are comparing two generic HRQoL scales whose theoretical construct is not exactly the same as the condition-specific scale. While the first two include both mental and physical health problems, the SQLS specifically assesses HRQoL associated with the symptoms of schizophrenia and its treatment and how they impair HRQoL. However, since there is still a debate about using generic or condition specific HRQoL scales when assessing treatment effects, we think our results are informative. Second, as mentioned above, we present a cross-sectional analysis, and sensitivity to change of the scales was not assessed. Third, we only evaluated three scales, two generic and one condition-specific, among the many that are available. Results with other scales could have been different. Since these scales and other HRQoL scales may also lack a common conceptual base agreement may vary depending on the specific comparison. Fourth, we included in the study a community sample of patients with stable symptoms, and the results may be different in patients presenting a psychotic exacerbation. Fifth, there may be many other aspects beyond those analyzed in this study when choosing a preference-based measure.Citation73 Sixth, the calculation of the PANSS factors was based on the work by Lindenmayer et alCitation27 but other models could also have been used.Citation74 Seventh, we have not adjusted for multiple testing. Eight, we had limited information on contextual factors that may influence the quality of life, such as psychological aspects and living conditions.Citation75,Citation76 Finally, we have accepted two types of diagnostic criteria (ICD and DSM) in the inclusion of patients with schizophrenia. These diagnostic criteria differ in significant aspects such as the minimum duration of the disorder to fulfill the diagnostic criteria.
These results may be contingent on the HRQoL scales which were chosen in the evaluation. Previous research has found that the correlations of HRQoL may differ depending on the dimensions and particular questions each of the scales include. When choosing the generic HRQoL scales, we decided to employ the most widely used scales in Europe and the USA. To decide which condition-specific HRQoL scale to employ, we looked for a scale that covered the areas described by Awad and Voruganti.Citation3 According to Awad and Voruganti, for HRQoL scales to be relevant in the assessment of patients with schizophrenia, the outcome should cover the interaction among three major determinants: psychotic symptoms and their severity, medication side effects, and psychosocial performance. We have chosen the SQLS scale as the disorder-specific scale as it includes those three dimensions.Citation9 In conclusion, these findings provide some support for the use of generic HRQoL scales in schizophrenia and underline the clinical factors that affect the health-related quality of life in people with schizophrenia: depressive symptoms, positive and negative symptoms, cognitive impairment, current substance use and somatic comorbidities. Although there is much debate on whether these scales capture the health status of individuals with schizophrenia, this study has shown that, in clinically stable patients, their ratings correlate at least as much as a condition-specific scale with the main symptom dimensions of the disorder. Our results are limited since only cross-sectional relationships were analyzed.
Conclusion
In conclusion, our study shows that generic and condition-specific quality of life scales have similar correlates in patients with schizophrenia, which supports the use of generic HRQoL scales in schizophrenia.
Abbreviations
CGI-SCH, Clinical Global Impression-Schizophrenia; EQ-5D, EuroQol-5 Dimension; EQ-5D VAS, EQ-5D visual analogue scale; HRQoL, Health-related quality of life; PANSS, Positive and Negative Syndrome Scale; PCC, Pearson Correlation Coefficient; PRO, Patient-reported outcome; SF-36, Short Form-36; SF-36 MCS, SF-36 mental component score; SF-36 PCS, SF-36 physical component score; SQLS, Schizophrenia Quality of Life Scale.
Ethics Approval
The protocol and consent procedures of the original study were approved by all local Institute Review Boards/Ethics Committees before study initiation. All patients and caregivers provided written informed consent. This project was approved by the Fundació Sant Joan de Déu Ethics Committee.
Data Sharing Statement
Roche provides qualified researchers access to individual patient level data. Details for Roche’s Data Sharing Policy are available from the corresponding author on reasonable request.
Author Contributions
CD designed the study, Conducted the statistical analysis and produced the first version of the manuscript. ACA, CB, RC, HE, JE, AM, MOK, ALN, MZ, and JMH participated in the study design, data collection and critically revised the manuscript for important intellectual content. AP and FM participated in data analysis and interpretation and revised the manuscript for important intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Acknowledgements
We want to thank all participating patients, families, and clinicians. We thank Jaume Aguado for his help with the statistical analysis and Ellen Vorstenbosch for her critical review of the manuscript.
Disclosure
Carlo Altamura has served as a consultant or advisory board member for F. Hoffmann- La Roche, Ltd., Lundbeck, Merck, Astra Zeneca, Bristol Myers Squibb, Janssen-Cilag, Sanofi, Eli Lilly, Pfizer and Otsuka. Corrado Bernasconi is a contractor of F. Hoffmann- La Roche, Ltd. Helio Elkis has received research grants from the São Paulo Research Foundation (FAPESP), Janssen-Cilag and Roche, participated on advisory boards for Janssen-Cilag and Roche, and received honoraria and travel support from Janssen-Cilag and Roche. Ashok Malla has received honoraria or participated in advisory boards or educational conferences or received research funding for investigator-initiated projects from Janssen Canada, Pfizer Canada, Bristol-Myers-Squib, F. Hoffmann- La Roche, Ltd., Otsuka, Lundbeck and Astra-Zeneca. Francesco Margari has no conflict of interest. Anna-Lena Nordstroem is an employees of F. Hoffmann- La Roche, Ltd. Mathias Zink has received unrestricted scientific grants from the European Research Advisory Board, German Research Foundation, Servier, Pfizer Pharma GmbH, Bristol-Myers Squibb GmbH & CoKGaA, further speaker and travel support from Pfizer Pharma GmbH, Bristol-Myers Squibb, Otsuka, Astra Zeneca, Eli-Lilly, Janssen Cilag, Servier, Trommsdorff and F. Hoffmann- La Roche Ltd. Marie-Odile Krebs has received honoraria and participated in advisory boards F. Hoffmann- La Roche, Ltd. Josep Maria Haro has acted as a consultant, participated in advisory boards or given educational presentations for Eli Lilly and Co., Lundbeck, Otsuka, F. Hoffmann- La Roche Ltd. and Takeda. The authors report no other conflicts of interest in this work.
References
- Millier A, Clay E, Charaf I, et al. Patient reported outcomes instruments in schizophrenia : a review of psychometric properties. Open J Med Psychol. 2014;2014(1):141–156. doi:10.4236/ojmp.2014.32017
- Awada G, Voruganti LN, Heslegrave RJ. A conceptual model of quality of life in schizophrenia: description and preliminary clinical validation. Qual Life Res. 1997;6(1):21–26. doi:10.1023/a:10264093266909062438
- Awad AG, Voruganti LNP. Measuring quality of life in patients with schizophrenia: an update. Pharmacoeconomics. 2012;30(3):183–195. doi:10.2165/1159447022263841
- Seow LSE, Tan THG, Abdin E, Chong SA, Subramaniam M. Comparing disease-specific and generic quality of life measures in patients with schizophrenia. Psychiatry Res. 2019;273(January):387–393. doi:10.1016/j.psychres.2019.01.03430682561
- Papaioannou D, Brazier J, Parry G. How valid and responsive are generic health status measures, such as EQ-5D and SF-36, in Schizophrenia? A systematic review. Value Heal. 2011;14(6):907–920. doi:10.1016/j.jval.2011.04.006
- Brazier J, Connell J, Papaioannou D, et al. A systematic review, psychometric analysis and qualitative assessment of generic preference-based measures of health in mental health populations and the estimation of mapping functions from widely used specific measures. Health Technol Assess (Rockv). 2014;18(34):1–188. doi:10.3310/hta18340
- Auquier P, Simeoni MC, Sapin C, et al. Development and validation of a patient-based health-related quality of life questionnaire in schizophrenia: the S-QoL. Schizophr Res. 2003;63:137–149. doi:10.1016/S0920-9964(02)00355-912892868
- Prieto L, Sacristán JA, Hormaechea JA, Casado A, Badia X, Gómez JC. Psychometric validation of a generic health-related quality of life measure (EQ-5D) in a sample of schizophrenic patients. Curr Med Res Opin. 2004;20:827–835. doi:10.1185/03007990412500367415200739
- Wilkinson G, Hesdon B, Wild D, et al. Self-report quality of life measure for people with schizophrenia: the SQLS. Br J Psychiatry. 2000;177(JUL):42–46. doi:10.1192/bjp.177.1.4210945087
- Whitehorn D, Milliken H, Ronson K, Rui Q. 0230 THE SF-36 AS A COMPONENT OF A CLINICAL ASSESSMENT BATTERY IN EARLY PSYCHOSIS. Schizophr Res. 2006;86:S105. doi:10.1016/s0920-9964(06)70313-9
- Revicki DA, Genduso LA, Hamilton SH, Ganoczy D, Beasley CM. Olanzapine versus haloperidol in the treatment of schizophrenia and other psychotic disorders: quality of life and clinical outcomes of a randomized clinical trial. Qual Life Res. 1999;8:417–426. doi:10.1023/A:100895892584810474283
- Reine G, Simeoni M-C, Auquier P, Loundou A, Aghababian V, Lancon C. Assessing health-related quality of life in patients suffering from schizophrenia: a comparison of instruments. Eur Psychiatry. 2005;20:510–519. doi:10.1016/j.eurpsy.2005.05.00916139488
- Meijer CJ, Schene AH, Koeter MWJ. Quality of life in schizophrenia measured by the MOS SF-36 and the lancashire quality of life profile: a comparison. Acta Psychiatr Scand. 2002;105:293–300. doi:10.1034/j.1600-0447.2002.1198.x11942934
- Barton GR, Hodgekins J, Mugford M, Jones PB, Croudace T, Fowler D. Measuring the benefits of treatment for psychosis: validity and responsiveness of the EQ–5D. Br J Psychiatry. 2009;195:170–177. doi:10.1192/bjp.bp.108.05738019648552
- Katschnig H. Schizophrenia and quality of life. Acta Psychiatr Scand Suppl. 2000;102:33–37. doi:10.1034/j.1600-0447.2000.00006.x
- Gray R, Leese M, Bindman J, et al. Adherence therapy for people with schizophrenia: European multicentre randomised controlled trial. Br J Psychiatry. 2006;189:508–514. doi:10.1192/bjp.bp.105.01948917139034
- Crawford MJ, Killaspy H, Barnes TRE, et al. Group art therapy as an adjunctive treatment for people with schizophrenia: multicentre pragmatic randomised trial. BMJ. 2012;344:e846–e846. doi:10.1136/bmj.e84622374932
- Mulhern B, Mukuria C, Barkham M, et al. Using generic preference-based measures in mental health: psychometric validity of the EQ-5D and SF-6D. Br J Psychiatry. 2014;205:236–243. doi:10.1192/bjp.bp.112.12228324855127
- Boyer L, Baumstarck K, Boucekine M, Blanc J, Lançon C, Auquier P. Measuring quality of life in patients with schizophrenia: an overview. Expert Rev Pharmacoecon Outcomes Res. 2013;13(3):343–349. doi:10.1586/erp.13.1523763531
- Livneh H, Lott SM, Antonak RF. Patterns of psychosocial adaptation to chronic illness and disability: a cluster analytic approach. Psychol Health Med. 2004;9(4):411–430 20. doi:10.1080/1354850042000267030
- Rabin R, de Charro F. EQ-SD: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi:10.3109/0785389010900208711491192
- Ware J, Sherbourne C, The MOS 36-ltem short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi:10.1097/00005650-199206000-000021593914
- Haro JM, Altamura C, Corral R, et al. Understanding the impact of persistent symptoms in schizophrenia: cross-sectional findings from the pattern study. Schizophr Res. 2015;169:1–3. doi:10.1016/j.schres.2015.09.00126481614
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; Elsevier Inc 2000. doi:10.1016/B978-1-4377-2242-0.00016-X
- Lecrubier Y, Sheehan DV, Weiller E, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry. 1997;12(5):224–231. doi:10.1016/S0924-9338(97)83296-8
- Kay SR, Fiszbein AOL, Positive T. Negative Syndrome Scale for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi:10.1093/schbul/13.2.2613616518
- Lindenmayer JP, Grochowski S, Hyman RB. Five factor model of schizophrenia: replication across samples. Schizophr Res. 1995;14(3):229–234. doi:10.1016/0920-9964(94)00041-67766534
- Jenkinson C, Coulter A, Wright L. Short form 36 (SF 36) health survey questionnaire: normative data for adults of working age. BMJ. 1993;306(Sf36):1437–1440. doi:10.1136/bmj.306.6890.14378518639
- Dolan P, Gudex C, Kind P, Williams A. A social tariff for EuroQol: results from a UK general population survey. Work Pap. 1995.
- Linde L, Sørensen J, Ostergaard M, Hørslev-Petersen K, Hetland ML. Health-related quality of life: validity, reliability, and responsiveness of SF-36, 15D, EQ-5D [corrected] RAQoL, and HAQ in patients with rheumatoid arthritis. J Rheumatol. 2008;35(8):1528–1537.18484697
- Fransen M, Edmonds J. Reliability and validity of the EuroQol in patients with osteoarthritis of the knee. Rheumatol. 1999;38:807–813. doi:10.1093/rheumatology/38.9.807
- Jenkinson C, Wright L. Coulter a. Criterion validity and reliability of the SF-36 in a population sample. Qual Life Res. 1994;3(1):7–12. doi:10.1007/BF006478438142947
- Pitkanen A, Valimaki M, Endicott J, et al. Assessing quality of life in patients with schizophrenia in an acute psychiatric setting: reliability, validity and feasibility of the EQ-5D and the Q-LES-Q. Nord J Psychiatry. 2012;66(1):19–25. doi:10.3109/08039488.2011.59309921770824
- Tunis SL, Croghan TW, Heilman DK, Johnstone BM, Obenchain RL. Reliability, validity, and application of the medical outcomes study 36-item short-form health survey (SF-36) in schizophrenic patients treated with olanzapine versus haloperidol. Med Care. 1999;37(7):678–691. doi:10.1097/00005650-199907000-0000810424639
- Cronbach LJ, Meehl PE. Construct validity in psychological tests. Psychol Bull. 1955;52:281–302. doi:10.1037/h004095713245896
- Bland JM, Altman DG. Statistics notes: cronbach’s alpha. BMJ. 1997;314:572. doi:10.1136/bmj.314.7080.5729055718
- McDonald JF, Moffitt RA. The Uses of Tobit Analysis. Rev Econ Stat. 1980;62:318. doi:10.2307/1924766
- Chou CY, Ma MC, Yang TT. Determinants of subjective health-related quality of life (HRQoL) for patients with schizophrenia. Schizophr Res. 2014;154:83–88. doi:10.1016/j.schres.2014.02.01124613000
- Arraras JI, Ibañez B, Pereda N, Iribarren S, Basterra I. The association of clinical insight and depression with quality of life in schizophrenia. Psychiatry Res. 2019;(February):0–1. doi:10.1016/j.psychres.2019.02.069
- Vrbova K, Prasko J, Ociskova M, et al. Quality of life, self-stigma, and hope in schizophrenia spectrum disorders: a cross-sectional study. Neuropsychiatr Dis Treat. 2017. doi:10.2147/NDT.S122483
- Hasan AAH. The correlation between the quality of life and clinical variables among outpatients with schizophrenia. Psychiatry Res. 2019;271(September2018):39–45. doi:10.1016/j.psychres.2018.09.06230465980
- Huppert JD, Weiss KA, Lim R, Pratt S, Smith TE. Quality of life in schizophrenia: contributions of anxiety and depression. Schizophr Res. 2001;51:171–180. doi:10.1016/S0920-9964(99)00151-611518637
- Meijer CJ, Koeter MWJ, Sprangers MAG, Schene AH. Predictors of general quality of life and the mediating role of health related quality of life in patients with schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 2009;44:361–368. doi:10.1007/s00127-008-0448-418974910
- Cohen CI, Vengassery A, Garcia Aracena EF. A longitudinal analysis of quality of life and associated factors in older adults with schizophrenia spectrum disorder. Am J Geriatr Psychiatry. 2017;25:755–765. doi:10.1016/j.jagp.2017.01.01328431868
- Fervaha G, Agid O, Takeuchi H, Foussias G, Remington G. Clinical determinants of life satisfaction in chronic schizophrenia: data from the CATIE study. Schizophr Res. 2013;151:203–208. doi:10.1016/j.schres.2013.10.02124183751
- Wartelsteiner F, Mizuno Y, Frajo-Apor B, et al. Quality of life in stabilized patients with schizophrenia is mainly associated with resilience and self-esteem. Acta Psychiatr Scand. 2016;134:360–367. doi:10.1111/acps.1262827497263
- Nakagawa S, Hayashi N. Clinical correlates of objective and subjective quality of life among middle-aged and elderly female inpatients with chronic schizophrenia. Asian J Psychiatr. 2013;6:389–393. doi:10.1016/j.ajp.2013.03.01524011685
- Xiang YT, Hou YZ, Yan F, et al. Quality of life in community-dwelling patients with schizophrenia in China. J Nerv Ment Dis. 2012;200:584–587. doi:10.1097/NMD.0b013e31825bfc7122759934
- Karow A, Moritz S, Lambert M, Schoder S, Krausz M. PANSS syndromes and quality of life in schizophrenia. Psychopathology. 2005;38:320–326. doi:10.1159/00008892116224206
- Alessandrini Lançon C, Fond G, Faget-Agius C, et al. A structural equation modelling approach to explore the determinants of quality of life in schizophrenia. A Struct Equ Model Approach Explor Determ Qual Life Schizophr. 2016;171(1–3):SP–27–34.
- Eack SM, Newhill CE. Psychiatric symptoms and quality of life in schizophrenia: a meta-analysis. Schizophr Bull. 2007;33(5):1225–1237. doi:10.1093/schbul/sbl07117204532
- Savill M, Orfanos S, Reininghaus U, Wykes T, Bentall R, Priebe S. The relationship between experiential deficits of negative symptoms and subjective quality of life in schizophrenia. Schizophr Res. 2016;176:387–391. doi:10.1016/j.schres.2016.06.01727328889
- Narvaez JM, Twamley EW, McKibbin CL, Heaton RK, Patterson TL. Subjective and objective quality of life in schizophrenia. Schizophr Res. 2008;98(1–3):201–208. doi:10.1016/j.schres.2007.09.00117919890
- Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):214–219. doi:10.1093/schbul/sbj05316481659
- Teng E, Tassniyom K, Lu P. Reduced quality of life ratings in mild cognitive impairment: analyses of subject and informant responses (P04.208). Neurology. 2012;78(MeetingAbstracts 1):P04.208-P04.208. doi:10.1212/WNL.78.1_MeetingAbstracts.P04.208
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med. 2002;64(3):510–519. doi:10.1097/00006842-200205000-0001612021425
- Alptekin K, Akvardar Y, Kivircik Akdede BB, et al. Is quality of life associated with cognitive impairment in schizophrenia? Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):239–244. doi:10.1016/j.pnpbp.2004.11.00615694230
- Savilla K, Kettler L, Galletly C. Relationships between cognitive deficits, symptoms and quality of life in schizophrenia. Aust N Z J Psychiatry. 2008;42(6):496–504. doi:10.1080/0004867080205051218465376
- Tolman AW, Kurtz MM. Neurocognitive predictors of objective and subjective quality of life in individuals with schizophrenia: a meta-analytic investigation. Schizophr Bull. 2012;38:304–315. doi:10.1093/schbul/sbq07720624752
- Caqueo-Urízar A, Boyer L, Baumstarck K, Gilman SE. Subjective perceptions of cognitive deficits and their influences on quality of life among patients with schizophrenia. Qual Life Res. 2015;24:2753–2760. doi:10.1007/s11136-015-1019-226038220
- Takeda T, Nakataki M, Ohta M, et al. Negative and positive self-thoughts predict subjective quality of life in people with schizophrenia. Neuropsychiatr Dis Treat. 2019;Volume 15:293–301. doi:10.2147/NDT.S190381
- Casey DA, Vickar G, Shihabuddin L, Northcott C, Rodriguez M. Schizophrenia: medical illness, mortality, and aging. Int J Psychiatry Med. 2011;41:245–251. doi:10.2190/pm.41.3.c22073763
- Lee J, Nurjono M, Wong A, Salim A. Prevalence of metabolic syndrome among patients with schizophrenia in Singapore. Ann Acad Med Singapore. 2012;41(10):457–462.23138143
- Fagiolini A, Goracci A. The effects of undertreated chronic medical illnesses in patients with severe mental disorders. J Clin Psychiatry. 2009;70:22–29. doi:10.4088/JCP.7075su1c.0419570498
- Sexton E, King-Kallimanis BL, Layte R, Hickey A. How does chronic disease status affect CASP quality of life at older ages? Examining the WHO ICF disability domains as mediators of this relationship. Aging Ment Heal. 2015;19:622–633. doi:10.1080/13607863.2014.955457
- Bitter I, Czobor P, Dossenbach M, Volavka J. Effectiveness of clozapine, olanzapine, quetiapine, risperidone, and haloperidol monotherapy in reducing hostile and aggressive behavior in outpatients treated for schizophrenia: a prospective naturalistic study (IC-SOHO). Eur Psychiatry. 2005;20(5–6):403–408. doi:10.1016/j.eurpsy.2005.01.00916084068
- Lozano ÓM, Rojas AJ, Fernández Calderón F. Psychiatric comorbidity and severity of dependence on substance users: how it impacts on their health-related quality of life? J Ment Heal. 2017;26:119–126. doi:10.1080/09638237.2016.1177771
- Alshowkan A, Curtis J, White Y. Quality of life for people with schizophrenia : a literature review. Arab J Psychiatry. 2012;23(2):122–131.
- Strassnig M, Brar JS, Ganguli R. Body mass index and quality of life in community-dwelling patients with schizophrenia. Schizophr Res. 2003;62:73–76. doi:10.1016/S0920-9964(02)00441-312765746
- Yu ZB, Han SP, Cao XG, Guo XR. Intelligence in relation to obesity: a systematic review and meta-analysis. Obes Rev. 2010. doi:10.1111/j.1467-789X.2009.00656.x
- Kontodimopoulos N, Pappa E, Niakas D, Yfantopoulos J, Dimitrakaki C, Tountas Y. Validity of the EuroQoL (EQ-5D) instrument in a Greek general population. Value Heal. 2008;11:1162–1169. doi:10.1111/j.1524-4733.2008.00356.x
- Khanna R, Jariwala K, Bentley JP. Psychometric properties of the EuroQol Five Dimensional Questionnaire (EQ-5D-3L) in caregivers of autistic children. Qual Life Res. 2013;22:2909–2920. doi:10.1007/s11136-013-0423-823615959
- Brazier J, Deverill M. A checklist for judging preference-based measures of health related quality of life: learning from psychometrics. Health Econ. 1999;8(1):41–51.10082142
- Perez J, Russo DA, Stochl J, et al. Comparison of high and low intensity contact between secondary and primary care to detect people at ultra-high risk for psychosis: study protocol for a theory-based, cluster randomized controlled trial. Trials. 2013;14(1):222. doi:10.1186/1745-6215-14-22223866815
- Ruggeri M, Nosè M, Bonetto C, et al. Changes and predictors of change in objective and subjective quality of life: multiwave follow-up study in community psychiatric practice. Br J Psychiatry. 2005;187:121–130. doi:10.1192/bjp.187.2.12116055822
- Zeng Y, Zhou Y, Lin J, Zhou Y, Yu J. Generic and disease-specific quality of life and its predictors among Chinese inpatients with schizophrenia. Psychiatry Res. 2015;228(3):724–728. doi:10.1016/j.psychres.2015.05.03326077848