?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
A retrospective chart review was undertaken in a private clinic to examine the clinical outcomes for patients with an eating disorder comorbid with depression or bipolar illness who underwent a referenced electroencephalographic (EEG) database analysis to help guide medication selection.
Method
We examined 33 charts for patients with the primary psychiatric diagnosis of an eating disorder and comorbid major depressive disorder or bipolar disorder who underwent a quantitative EEG database assessment to provide additional information for choices of medication. The current analysis includes data from 22 subjects who accepted treatments based on information from the referenced-EEG medication database. Hamilton Depression Rating Scale, Clinical Global Impression-Severity, Clinical Global Impression-Improvement, and hospitalization data were examined for these patients.
Results
Patients whose EEG data was used for clinical treatment reported significant decreases in associated depressive symptoms (HDRS scores), overall severity of illness (Clinical Global Impression-Severity), and overall clinical global improvement (Clinical Global Impression- Improvement). This cohort also reported fewer inpatient, residential, and partial hospitalization program days following referenced-EEG compared with the two-year period prior to treatment.
Conclusion
These findings are consistent with previously reported data for patients with eating disorders and suggest the need for future studies using EEG data correlated with those from other patients with similar quantitative EEG features.
Introduction
The pharmacologic treatment of eating disorders has offered few options for this difficult-to-treat population. A large number of psychotropic medications from multiple drug classes have been studied as potential treatments for anorexia nervosa, but none has consistently proven to be efficacious.Citation1 While one early trial reported that fluoxetine prevented relapse in weight-restored patients with anorexia nervosa,Citation2 multiple later studies, involving larger sample sizes, failed to replicate a benefit of selective serotonin reuptake inhibitors as a group in preventing relapse in weight-restored adolescents and adults with the disease.Citation3,Citation4 Currently, there is no drug approved by the US Food and Drug Administration (FDA) for the treatment of anorexia nervosa or eating disorder not otherwise specified (EDNOS). Only one medication, fluoxetine, has been approved by the FDA for the treatment of bulimia nervosa;Citation5 it has been shown to decrease binging and purging behaviors significantly in patients with bulimia nervosa.Citation6 Because research has shown that eating disorders are associated with high mortality,Citation7–Citation10 improved pharmacotherapy is desperately needed.
Despite the current lack of empirically derived guidance for choosing medications, psychiatrists prescribe a wide range of psychotropic medications for patients with eating disorders. The high morbidity and mortality rates associated with eating disorders underscore the importance of finding successful and timely treatment. Frequent relapses and prolonged recovery periods contribute to the chronic nature of eating disorders.Citation11 Compared with the general population, mortality rates are elevated in eating disorders. An examination of 6009 records spanning 30 years from the Swedish Cause-of-Death RegisterCitation12 found that people with anorexia nervosa have a six-fold increase in mortality, and that suicide was the most frequent cause of death. In a review of almost two decades of records from patients evaluated at an outpatient eating disorders clinic,Citation1 crude mortality rates were found to be 3.9% for bulimia nervosa, 4.0% for anorexia nervosa, and 5.2% for EDNOS. These rates were similar to the 5.9% rate in a study showing that the all-cause mortality rates for anorexia nervosa are up to 12 times higher than those normally seen in 15–24-year-old females and more than twice what is found in studies of hospitalized female psychiatric patients.Citation10
Twenty-four hour supervised care is a common treatment modality for patients with eating disorders. Up to 50% of patients with eating disorders require hospitalization for stabilization at some point in care.Citation13 Inpatient hospitalization choices remain costly and somewhat controversial, and the evidence for increased treatment efficacy of inpatient hospitalization is questionable.
The frequent comorbidity of other Axis I diagnoses further complicates the treatment of eating disorders. The rates of co-occurring mood, anxiety, and substance abuse in patients with eating disorders have been found to vary widely, depending on the methodology used and the characteristics of the study sample.Citation14 Mood disorders occur more frequently than anxiety disorders, and depression is the most common comorbid condition present. Lifetime estimates of depression have been reported to be as high as 88.9%.Citation15 The severity of depression and anxiety symptoms was found to be greater in underweight patients, and lessened when weight was restored.Citation16 The symptoms of mood disorders and eating disorders often affect one another. For example, depressive symptoms in adolescents predicted future bulimic eating pathology, which, in turn, can lead to worsening of depressive symptoms.Citation17
Without evidence-based research to support pharmacotherapy for eating disorders, physician choices are little more than educated guesses. Techniques that can successfully optimize pharmacotherapy by avoiding unnecessary and costly medication trials can benefit patients and their families, who also suffer when their family members are ill.Citation18 In addition, finding the most beneficial treatment in an efficient manner can serve to curb health care costs. Per patient, the cost of treatment for anorexia nervosa and bulimia nervosa have been found to be comparable with the cost of treating schizophrenia.Citation19
An objective new tool that uses quantitative, normative, and referenced electroencephalographic (EEG) sampling databases can assist physicians in determining medication selection. This technology was pioneered in the late 1980s and compares drug-free quantitative EEG features for individual patients with a database of patients with similar EEG patterns and with known outcomes after pharmacologic interventions. Based on specific EEG data elements, this technology can provide, before their patient begins treatment, quantitative EEG historic outcome data for medications likely to be effective, thereby reducing the need for “trial-and-error” prescribing. See the appendix to this paper for a detailed description of referenced-EEG technology.
Early studies suggested that referenced-EEGs could assist clinicians in finding more efficacious medications, especially for treatment-refractory patients. In one study, 81 severely ill patients with anorexia nervosa, bulimia nervosa, or EDNOS underwent referenced-EEG analysis, and their medications were subsequently switched to those suggested by the referenced-EEG report. After 10 months, more than 70% of the patients with anorexia nervosa demonstrated weight gain and marked improvement in their depression symptoms. Citation20 In a more recent multicenter, randomized trial,Citation21 referenced-EEG assisted treatment was compared with optimized treatment based on the STAR*D study guidelines funded by the National Institutes of HealthCitation22 in patients with treatment-refractory major depressive disorder. Referenced-EEG assisted medication selection led to statistically better outcomes compared with the control group. The improvement in the referenced-EEG group over the control group was significant as early as two weeks after starting medications selected through referenced-EEG analysis, and the superior improvement continued throughout the 12-week study.
In order to explore further the potential of referenced-EEG for improving clinical outcomes when used for selecting pharmacotherapies in eating disorders, we performed an uncontrolled, retrospective chart review of clinical cases which used referenced-EEG in eating disorders with comorbid depression. We hypothesized that referenced-EEG assisted medication selection would improve overall clinical outcomes (including reducing symptoms of eating disorders and associated mood symptoms). This study was designed to evaluate referenced-EEG as an informational tool to assist the physician in selecting an effective medication. Because there are innumerable combinations of therapies available from the referenced-EEG report, this study was not intended to evaluate any one medication against another, but instead looks at referenced-EEG itself as a tool to improve outcomes after previous medication failures in this difficult population. It compares the referenced-EEG based therapeutic outcomes against the patient’s previous therapeutic outcomes prior to referenced-EEG, regardless of the medications being used. In this model, statistical differences between outcomes before and after referenced-EEG can be attributed to the information provided by referenced-EEG.
Methods
Participants
This retrospective chart review included all adolescent and adult patients who received a fee-for-service referenced-EEG at a Boston area clinic between October 10, 2003 and April 1, 2009 and agreed to follow the medication plan derived from the referenced-EEG. Additionally, each participant had received a primary psychiatric diagnosis of an eating disorder (ie, anorexia nervosa, bulimia nervosa, or EDNOS) and had comorbid major depressive disorder or bipolar disorder in the depressive stage as diagnosed by a board-certified psychiatrist. Clinical measures, both pre-referenced and post-referenced-EEG, included Hamilton Depression Rating Scale (HDRS,)Citation23 Clinical Global Impression-Severity (CGI-S)Citation24 and Clinical Global Impression-Improvement (CGI-I)Citation24 scores. The CGI evaluations were based on improvement of mood as well as eating disorder symptoms. Specifically, this study investigated whether referenced-EEG assisted medication selection resulted in improvement of depressive symptoms as measured by HDRS, as well as overall clinical improvement measured by CGI-S and CGI-I scores.
The psychiatric clinic does not routinely collect weight data. All patients were monitored by their nutritionists and primary care physicians, who routinely weighed patients to assess the criterion for hospital readmission if necessary. Monitoring weight data for interim medication follow-up visits often interferes with treatment. Patients with eating disorders are overly focused on weight, and the potential of being weighed by multiple professionals in different offices is not standard treatment.
Hospitalization data were collected on the number of days of full inpatient, partial inpatient, and residential hospitalization. Pre-referenced-EEG hospitalization data were collected for a period of up to two years, as recalled retrospectively by the patient. Post-referenced-EEG hospitalization data were collected for a variable period of 2–5 years. To account for the variability in the follow-up period, pretreatment and post-treatment hospitalization data were calculated as average days per month for each person. An institutional review board provided written approval of this retrospective chart review.
Data analysis
Data were extracted from the files for three intervals, ie, pretreatment through baseline (which corresponded to the referenced-EEG recording date), the clinical visit closest to eight weeks following the prescription of referenced-EEG based medications, and the visit closest to six months after baseline. Extracted variables included HDRS (21-item) raw scores, CGI-I and CGI-S scores rated by the treating professional, and hospitalization data. Hospitalization data, representing the two years prior to initiation of referenced-EEG assisted therapy, were drawn from the baseline session clinical interview in the patient’s chart, and from a background form completed by the patient. The treating professionals also reviewed the charts for the prescribed medications during the six months after using the referenced-EEG neurometric database.
Raw data were recorded on spreadsheets and changes in scores were calculated by subtracting the raw scores for eight-weekly and six-monthly visits from the baseline scores for HDRS, CGI-S, and CGI-I. For the CGI-I, a score of 4 (no change) was assigned for each person as a baseline score.
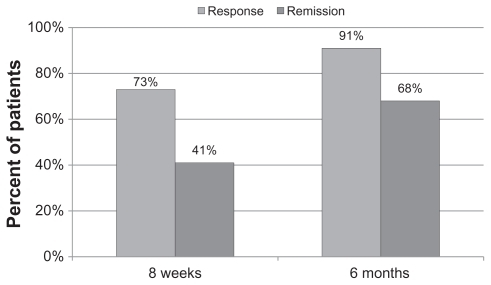
Categoric variables were also defined for the analysis as follows: depression response was defined as HDRS score improvement of 50% or more from baseline. Depression remission was defined as an HDRS score < 8 at any time post-treatment. CGI-I response was defined as scores of 1 or 2 and CGI-I remission was defined as a score of 1 post-treatment.
The CGI-S, CGI-I, hospitalization days, and HDRS scores were recorded in the chart at each visit. Additional information obtained from the patient’s chart included primary and secondary diagnoses, age at time of referenced-EEG, history of failed medication trials, and previous and subsequent hospitalizations if available.
The CGI-S scale is a seven-point Likert scale. Clinicians rated the severity of a patient’s illness (in this case an eating disorder) on a scale between 1 and 7, where “1” means “not at all ill” and “7” means “among the most extremely ill” patients. The CGI-I is a similar seven-point Likert scale, where “1” means “very much improved”, “4” means “no change”, and “7” means “very much worse”. The CGI-S and CGI-I are well accepted outcome measures in psychiatry. The HDRS is a well validated depression rating scale, and we used the 21-item version of the HDRS to rate depressive symptoms, including insomnia, appetite, suicidal ideation, and energy level.
Hospitalizations were based upon information contained in the patient chart and were classified according to the level of care, ie, full hospitalizations, residential admissions, and partial hospitalizations. In the US, inpatient hospitalization costs were estimated to be approximately $2000 per day, and outpatient partial hospitalization costs were approximately $800 per day.Citation25 Residential care, a level of care which fits between inpatient hospitalization and partial hospitalization, is estimated to cost approximately 956 dollars/day.Citation26 Adjusting for inflation, these figures (in dollars/day) are currently estimated at $2310 inpatient hospitalization, $1033 for residential care, and $925 for partial hospitalization. To determine the estimated savings, the daily costs for the 24 months prior to referenced-EEG were calculated and the costs for the post-referenced-EEG care was also calculated and subtracted from the estimated pretreatment cost.
Given the small sample size, changes in primary variables (HDRS, CGI-S, and CGI-I) from baseline to eight weeks and six months were analyzed using the nonparametric Wilcoxon rank-sum (Mann–Whitney) test. Statistical significance was defined at the two-tailed 0.05 level. Analyses were performed only in the primary cohort of 22 subjects with eating disorders. Due to the small sample size, no analyses were planned or conducted for smaller population subgroups of anorexia nervosa (n = 11), bulimia nervosa (n = 4), or EDNOS (n = 7).
Results
Charts of 33 patients who elected to undergo referenced-EEG assessment between October 10, 2003 and April 1, 2009 were reviewed. Eleven patients were excluded from the analysis for the following reasons: seven patients decided not to follow the referenced-EEG-prescribed medications and withdrew without providing efficacy data; one patient failed to return for any follow-up sessions due to travel issues; two patients began abusing alcohol or cannabis during post-referenced-EEG treatment, thereby confounding efficacy results; and one patient violated the pre-referenced-EEG drug washout requirement and was not medication-free prior to the referenced-EEG, rendering referenced-EEG measures invalid.
The 22 patients who received and followed referenced- EEG-based medications averaged 24.8 ± 8.0 years of age (median 21.3 years). Demographic information on age, number of failed medications, current primary and secondary diagnoses, and medications used in the study are presented in –.
Table 1 Patient demographics by gender
Table 2 Diagnoses by patient
Table 3 Medications selected using referenced electroencephalograms
Patient assessment data for the eight-week time point averaged 59.6 ± 9.7 days, and assessment data for the six-month time point averaged 181 ± 12.6 days after prescription of medications aided by the referenced-EEG analysis. Most patients (n = 18) had previously failed other treatments during an average illness duration of 9.1 ± 7.1 (range 1–24) years. These subjects had failed treatments with one or more medications (average 5.7). The failed therapies included 39 different medications, ranging from St John’s wort to haloperidol, including many of the typical medications currently being prescribed in psychiatry (serotonin and/or noradrenaline reuptake inhibitors, atypicals, stimulants, monoamine oxidase inhibitors, lithium, anticonvulsants, tricyclics, and others). Four subjects were treatment-naïve, three of whom were aged 18 years or younger.
Average age of onset of eating disorders symptoms was 15.6 ± 5.6 years. Four (18%) were adolescents aged 14–16 years, and 18 (82%) were adults aged 18 years or older. Patients had a primary diagnosis of anorexia nervosa (11/22, 50%), bulimia nervosa (4/22, 18%), or EDNOS (7/22, 32%). Each patient carried more than one Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) DSM-IV Axis I diagnosis. The primary comorbid diagnosis for each patient included either major depressive disorder (18/22, 82%) or bipolar disorder (4/22, 18%). Additionally, 12 individuals were diagnosed with comorbid obsessive-compulsive disorder, three with attention deficit disorder, five with past alcohol abuse/dependence, six with generalized anxiety disorder, and one with post-traumatic stress disorder.
The medications that were prescribed based on review of the referenced-EEG report were from four different classes, ie, anticonvulsants, antidepressants, stimulants, and beta-blockers (see ). In this sample of patients with resistant eating disorders, only one was treated with a single medication (bupropion). The remainder of the patients were treated with combinations of medications from the four categories listed in the referenced-EEG report. Treatment always began with medications that were based on the report, ie, either monotherapies or combinations derived from information on the referenced-EEG report. In total, antidepressants were used in 59% of subjects, anticonvulsants in 82% of subjects, stimulants in 50% of subjects, and beta-blockers in 9% of subjects. Due to known drug intolerance in a few patients, medications that were identified by name on the referenced-EEG report were not used. Instead, the investigator selected substitutes within the same drug class for the initial treatment, ie, duloxetine (n = 2) and oxcarbazepine (n = 3). After the initial treatment with referenced-EEG assisted therapies, the treating physician used clinical judgment to add atypical antipsychotic medications, which were not based on the referenced-EEG report (aripiprazole [n = 1] and quetiapine [n = 4]). Atypical antipsychotics were prescribed for additional symptom relief usually related to intense obsessive ruminations around food, weight, and/or body image. Two patients required additional medications that were clinically indicated to maintain sobriety (disulfiram, n = 1) and to treat attention deficit/hyperactivity disorder (guanfacine, n = 1).
Hamilton Depression Rating Scale
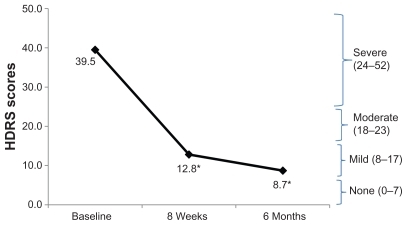
Results showed a statistically significant improvement in HDRS scores ( and ) from pretreatment to both eight weeks and six months after receiving the prescribed medications. Pretreatment HDRS scores ranged from 27 to 47, indicating that patients were suffering from severe depression in addition to an eating disorder. With the addition of pharmacotherapy guided by referenced-EEG, the average HDRS scores at eight weeks dropped significantly, although one patient remained in the severely depressed (24–52) range (her score dropped from 45 to 25), and five patients remained in the moderately depressed range (18–24 HDRS points). Five patients reported remission of depressive symptoms at eight weeks (). The balance of patients rated their depressive symptoms in the mild range (7–17). By six months, 11 patients reported complete remission of depression symptoms, while two remained moderately depressed, and the remaining nine reported mild depression symptoms.
Figure 2 HDRS – proportion of subjects with response and remission.
Abbreviation: HDRS, Hamilton Depression Rating Scale.
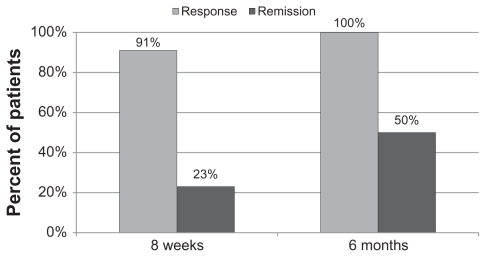
Table 4 Efficacy results
Clinical Global Impression: Severity
Initial average CGI-S scores of 5.5 ± 0.6 indicate that this cohort was rated between 5 (markedly ill) and 6 (severely ill) prior to initiation of pharmacotherapy treatment. When taking prescribed medications guided by the referenced-EEG, the cohort experienced a significant reduction in CGI-S scores by an average of 2.8 ± 0.9 points. This took the cohort, on average, to a rating of between 2 (borderline mentally ill) and 3 (mildly ill). At six months, clinicians ratings improved again by an average of 3.2 ± 1.0 points, in the same range. At eight weeks, 10 of the 22 patients were rated as 2 or 1 (mildly ill or not at all ill), and at six months 12 of the patients were rated as 2 or 1.
Clinical Global Impression: Improvement
The CGI-I scores are a rating of global clinical improvement. CGI-I is not typically rated at baseline because there is no improvement to rate. For analysis, a score of 4 (no change) was assigned as the CGI-I at baseline. Change, if any, was measured relative to that initial score. Patients in this cohort who received a CGI-I score of 2 or 1 (much or very much improved) were defined as having clinical response. The number of patients who responded (received a CGI-I score of 1 or 2) after eight weeks of treatment was 16 (73%). At six months, 20 of the 22 patients (91%) responded based on the CGI-I (see ).
Hospitalization data
Hospitalization data for the 24 months prior to referenced-EEG assisted prescribing was available for all patients (). Two patients reported no psychiatric hospitalization prior to or after beginning referenced-EEG-based pharmacotherapy. In total, 18 patients (82%) had pre-referenced-EEG inpatient hospitalizations and only seven (32%) required hospitalization in the variable 2–5- year post-referenced-EEG period. Estimated hospitalization cost data for the participants are shown in . Prior to the referenced-EEG assisted treatment, this cohort had 45 separate inpatient hospitalizations which accounted for 434 days of care or 9.7 days per episode. The inpatient hospitalization days of care per month for the 24 months prior to referenced-EEG were 0.82 days per person. The number of inpatient hospitalization days post- referenced-EEG was reduced to 136, representing 0.26 days per person. Reductions can also be seen in residential and partial hospitalization levels of care.
Figure 4 Estimated cost of hospitalization (2010 US dollars).
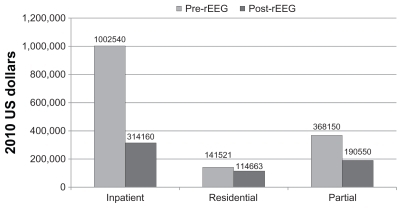
Table 5 Hospitalization days and estimated costs
Discussion
Our retrospective chart analysis of clinical cases indicates that referenced-EEG may be a useful metric tool for clinicians making medication recommendations for refractory patients with eating disorders and comorbid depression or bipolar depression. This pilot study, in conjunction with previously presented work,Citation20 suggests that further controlled studies of referenced-EEG are warranted in patients with eating disorders.
The patients who underwent referenced-EEG analysis in our cohort had previously failed to achieve improvement with more traditional care. As these data indicate, referenced-EEG may be useful in selecting more efficacious agents in treatment-resistant patients with significant levels of comorbid diagnoses. When utilized in a setting in which the results of the referenced-EEG are integrated with other components of multidisciplinary treatment this information can be invaluable in providing effective treatment to those suffering with an eating disorder and comorbid depression.
The results of this review are encouraging and indicate that treating patients with the additional information conveyed by referenced-EEG may result in robust treatment responses in a group of patients who had not previously responded to trial-and-error medication selection (which is currently considered standard practice). Some patients did achieve almost complete remission of their depression. Prior to referenced-EEG, 18 subjects (82%) required hospitalization, but after referenced-EEG, only three of these 18 patients required rehospitalization within the five-year follow-up period in this study. These results are encouraging enough to suggest that the referenced-EEG may play a role in helping to select effective pharmacotherapy for eating disorder patients who have been diagnosed with comorbid major depression. Previously, medication trials have provided limited insight and guidance in the pharmacologic management of eating disorders.Citation5,Citation27–Citation30 Many have speculated that there was no use for medications in the treatment of eating disorders, particularly in the treatment of anorexia nervosa.Citation5 No single class of medications has emerged as predictably superior for all individuals, suggesting that treatment according to objective neurophysiology may be more successful than by DSM diagnosis. Indeed, a wide range of agents was recommended in this study by referenced-EEG based upon the individual patient’s EEG. The diversity of recommended medications may help explain why in general the search for a single pharmacologic agent to treat eating disorders has failed.
The medications selected from referenced-EEG correlations provided information that led to combinations from four different classes of medications. The diversity of medications successfully utilized in treatment of this dually diagnosed cohort extended beyond the drugs recommended by the literature in eating disorders.
Some medication recommendations were counterintuitive. For example, based on referenced-EEG correlations, stimulant medications were used in the treatment of nine of the 22 patients. For these nine patients, the addition of a stimulant was well tolerated and associated with positive clinical outcomes.
The decrease in depression severity (HDRS) for these patients is encouraging, especially given that depression is frequently treatment-refractory in individuals with anorexia. It is intriguing that eight of the 22 patients reported an improvement in depression symptoms on medications that did not include an antidepressant. This is also consistent with previous research utilizing referenced-EEG in persons with depression.
In addition to decreasing symptoms of depression, the data suggest a decrease in number of hospitalizations and in hospitalization days. Significant cost savings were seen following post-referenced-EEG medication changes. As stated in the Methods section of the paper, after adjusting for inflation, the cost of treatment (in US dollars/day) per subject is currently estimated at $2310 for inpatient hospitalization, $1033 for residential care, and $925 for partial hospitalization. This leads to a total pre-referenced-EEG treatment cost for these 22 subjects of $1,512,211 over the course of two years and a total post-referenced-EEG treatment cost of only $612,373 over the variable follow-up period of 2–5 years. The total pre-referenced-EEG cost per subject for one year is approximately $34,368 ($1,512,211/22 patients per two years). This total is within reason because the medical literature reports that the total treatment cost per patient for one year of treatment averages $33,105.Citation38 Therefore the total post-referenced-EEG treatment cost of $27,835 per subject over a 2–5-year range ($612,373/22 subjects) represents a significant cost saving.
The potential cost savings as a result of an effective medication regimen suggests that referenced-EEG analysis may be cost-effective. The durability of response with medications selected according to data provided by the referenced-EEG, and the broader options of medication combinations suggested by referenced-EEG analysis, portends well for advancing treatment for patients with eating disorders. People who elect to undergo the referenced-EEG procedure and follow treatment regimens based on the referenced-EEG findings are reporting that they are doing better overall. Because there are no current standard treatments for eating disorders, it suggests that patients are trapped in relatively long cycles of trial-and-error prescribing before successful therapies can be found.
The gender distribution in this study (21 females, one male) is notable. Eating disorders occur at much lower rates in men compared with women. In the scientific literature, the female to male ratio of anorexia has been reported to be 10:1. This gender divide also exists in the prevalence rate of bulimia. A review of the literatureCitation39 found annual prevalence rates of bulimia ranging from 6.8 to 13.5 persons per 100,000 people. The annual prevalence rate for males was reported as 0.8 males per 100,000 people.Citation39 The lone male participant in the current study reflects the realistic proportion of men with eating disorders in the general population.
Furthermore, studies have indicated that there are many personality and clinical similarities between men with eating disorders and women with eating disorders.Citation40 Also, few differences were found in rates of comorbidity between men and women with eating disorders, with the exception of expected gender-specific differences in the rates of alcoholism and depression.Citation41 This suggests that the nature of eating disorders is similar in both genders and the gender divide in the current study is of little consequence to the results.
There are several limitations inherent in this or any retrospective chart review that may limit the conclusions one may draw from this case series. First, this review did not systematically document research-ready data in charts, and the clinic does not routinely collect weight data on patients. Standard clinical practice does utilize the CGI-S, CGI-I, and HDRS with all patients, and these data suggest both overall improvement and specific improvement in depression symptoms. The hospitalization data included can be seen as reflecting the severity of the underlying disorder as well as the severity of illness in these patients. Second, there was no comparison group in this study, so it is not clear what the effects of treatment would have been in a parallel cohort of subjects not utilizing the referenced-EEG analysis. However, some information is provided by comparing our results with pre-referenced-EEG experience (ie, treatment failure) and historic data for hospitalizations over the two-year period prior to referenced-EEG. Third, this is a small cohort of persons who could afford the costs associated with the referenced-EEG and additional appointments, and thus these patients may not be representative of patients with eating disorders as a whole. Fourth, referenced-EEG provides information to physicians that helps identify medications based on the specific neurophysiology of each patient, ie, personalized medicine. These medications were utilized only when they were thought to be appropriate and justifiable. As would be expected from any tool used to augment decision-making in medicine, clinical judgment is critical in the treatment process. And fifth, although the focus of this study was to explore levels of depression following referenced-EEG-guided medication selection, the majority of the patients reviewed had multiple comorbid conditions in addition to a diagnosis of an eating disorder and mood disorder. The study did not control for the presence of other comorbid disorders such as obsessive compulsive disorderCitation42 or substance abuse disorder,Citation43 although common in this population. These additional comorbid conditions may have influenced the results of the study as they may have affected patient responses to the depression scales. However, to some degree, this may have been taken into account through the use of global rating scales such as the CGI-S and CGI-I. In conclusion, the use of referenced-EEG appears to add valuable information to the clinical practice of treating eating disorders in patients with comorbid major depressive disorder and bipolar disorder.
Acknowledgments
The authors would like to acknowledge the work of Ken Graap and Chris Webster for their supportive role in summarizing the data in this study.
Disclosure
JMG is a consultant for and holds shares in CNS Response Inc. DAH is an employee of CNS Response Inc and holds stock in the company, and has received speaking support from Wyeth, Forest Laboratories, Cephalon, Eli Lilly, Pfizer, GlaxoSmithKline, but not within the last five years. DVI has received research support from Aspect Medical Systems, Euthymics Bioscience Inc, Forest Laboratories, Janssen Pharmaceutica, and NeoSync Inc. In the past two years, DVI has received speaker honoraria from Reed-Elsevier Medical and is a consultant to CNS Response Inc Lifetime, and has also received speaker honoraria from Eli Lilly and Co, Forest Laboratories, and Pfizer Inc. Some data entry and manuscript preparation support was provided by CNS Response Inc employees. The remaining authors report no conflicts of interest in this work.
References
- CrowSPetersonCSwansonSIncreased mortality in bulimia nervosa and other eating disordersAm J Psychiatry2009166121342134619833789
- KayeWNagataTWeltzinTDouble-blind placebo-controlled administration of fluoxetine in restricting- and restricting-purging-type anorexia nervosaBiol Psychiatry200149764465211297722
- HoltkampKKonradKKaiserNA retrospective study of SSRI treatment in adolescent anorexia nervosa: Insufficient evidence for efficacyJ Psychiatr Res200539330331015725429
- WalshBKaplanAAttiaEFluoxetine after weight restoration in anorexia nervosa: A randomized controlled trialJAMA2006295222605261216772623
- CrowSJMitchellJERoerigJDSteffenKWhat potential role is there for medication treatment in anorexia nervosa?Int J Eat Disord20094211818683884
- WalshBAgrasWDevlinMFluoxetine for bulimia nervosa following poor response to psychotherapyAm J Psychiatry200015781332133410910801
- CrispAHCallenderJSHalekCHsuLKLong-term mortality in anorexia nervosa. A 20-year follow-up of the St George’s and Aberdeen cohortsBr J Psychiatry19921611041071638303
- CrowSPrausBThurasPMortality from eating disorders – a 5- to 10-year record linkage studyInt J Eat Disord19992619710110349590
- HarrisECBarracloughBExcess mortality of mental disorderBr J Psychiatry199817311539850203
- SullivanPFMortality in anorexia nervosaAm J Psychiatry19951527107310747793446
- SteinhausenHWeberSThe outcome of bulimia nervosa: Findings from one-quarter century of researchAm J Psychiatry2009166121331134119884225
- PapadopoulosFCEkbomABrandtLEkseliusLExcess mortality, causes of death and prognostic factors in anorexia nervosaBr J Psychiatry2009194101719118319
- BerkmanNDBulikCMBrownleyKAManagement of Eating Disorders. Evidence Report/Technology Assessment No. 135Rockville, MDAgency for Healthcare Research and Quality Publication2006
- GodartNPerdereauFReinZComorbidity studies of eating disorders and mood disorders. Critical review of the literatureJ Affect Disord2007971–3374916926052
- FornariVKaplanMSandbergDEMatthewsMSkolnickNKatzJLDepressive and anxiety disorders in anorexia nervosa and bulimia nervosaInt J Eat Disord19921212129
- PolliceCKayeWGreenoCWeltzinTRelationship of depression, anxiety, and obsessionality to state of illness in anorexia nervosaInt J Eat Disord19972143673769138049
- PresnellKSticeESeidelAMadeleyMDepression and eating pathology: Prospective reciprocal relations in adolescentsClinical Psychol Psychother2009164357365
- KayeWEating disorders: Hope despite mortal riskAm J Psychiatry2009166121309131119952078
- Striegel-MooreRLeslieDPetrillSGarvinVRosenheckROne-year use and cost of inpatient and outpatient services among female and male patients with an eating disorder: Evidence from a national database of health insurance claimsInt J Eat Disord200027438138910744844
- SchillerMEmoryWHSuffinSCrEEG® in the treatment of eating disordersPoster presentation at the American Psychiatric Association annual meetingMay 1–6, 2004New York, NY
- DebattistaCKinrysGHoffmanDThe use of referenced EEG (rEEG) in assisting medication selection for the treatment of depressionJ Psychiatr Res2011451647520598710
- RushAJTrivediMHWisniewskiSRAcute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D reportAm J Psychiatry2006163111905191717074942
- HamiltonMA rating scale for depressionJ Neurol Neurosurg Psychiatry196023566114399272
- GuyWClinical Global ImpressionsEarly Clinical Drug Evaluation Unit assessment manual for psychopharmacology, revisedRockville, MDUS Dept of Health, Education and Welfare, National Institute of Mental Health1967
- CrowSNymanJThe cost-effectiveness of anorexia nervosa treatmentInt J Eat Disord200435215516014994352
- FrischMHerzogDFrankoDResidential treatment for eating disordersInt J Eat Disord200639543444216528698
- BeckerAEGrinspoonSKKlibanskiAHerzogDBEating disordersN Engl J Med1999340141092109810194240
- BerghCEjderhamnJSoderstenPWhat is the evidence basis for existing treatments of eating disorders?Curr Opin Pediatr200315334434512806269
- BulikCMBerkmanNDBrownleyKASedwayJALohrKNAnorexia nervosa treatment: A systematic review of randomized controlled trialsInt J Eat Disord200740431032017370290
- MitchellJEde ZwaanMRoerigJLDrug therapy for patients with eating disordersCurr Drug Targets CNS Neurol Disord200321172912769809
- SuffinSCEmoryWHNeurometric subgroups in attentional and affective disorders and their association with pharmacotherapeutic outcomeClin Electroencephalography19952627683
- SuffinSCEmoryWHGutierrezGA QEEG database method for predicting pharmacotherapeutic outcome in refractory major depressive disordersJ Am Physicians Surgeons2007124104108
- DeBattistaCHoffmanDSchillerMIosifescuDReferenced-EEG (rEEG) guidance of medications for treatment resistant depressed patients – a pilot studyPoster 228, presented at US Psychiatric and Mental Health CongressOctober 30–November 2, 2008San Diego, CA
- TrivediMPigottTStegmanKTMAP Procedural Manual; Depression Module; Physician Algorithm Implementation ManualUniversity of Texas Southwestern Medical School at Dallas121998 Available from: http://en.wikipedia.org/wiki/Texas_Medication_Algorithm_ProjectAccessed June 14, 2011
- GreenblattJSussmanCJamesonMKasumovaGReferenced-EEG® guided medication predictions in treatment-refractory eating disorder patientsPoster presented at the annual meeting of the American Psychiatric AssociationMay 3–8, 2008Washington, DC
- ShafferJHMilnerJESchillerMJrEEG-guided pharmacotherapy for severely ill dually diagnosed patientsPoster 684 presented at the 158th annual meeting of the American Psychiatric AssociationMay 21–26, 2005Atlanta, GA
- SchillerMJReferenced-EEG® guided pharmacotherapy of dually-diagnosed patientsPoster presented at the College on Problems in Drug Dependence annual meetingJune 14–19, 2008San Juan, Puerto Rico
- LockJCouturierJAgrasWSCosts of remission and recovery using family therapy for adolescent anorexia nervosa: A descriptive reportEat Disord200816432233018568922
- HoekHWvan HoekenDReview of the prevalence and incidence of eating disordersInt J Eat Disord200334438339614566926
- WoodsideDBBulikCMThorntonLPersonality in men with eating disordersJ Psychosom Res200457327327815507254
- WoodsideDBGarfinkelPELinEComparisons of men with full or partial eating disorders, men without eating disorders, and women with eating disorders in the communityAm J Psychiatry2001158457057411282690
- Salbach-AndraeHLenzKSimmendingerNKlinkowskiNLehmkuhlUPfeifferEPsychiatric comorbidities among female adolescents with anorexia nervosaChild Psychiatry Hum Dev200839326127217987378
- The National Center on Addiction and Substance Abuse at Columbia UniversityFood for Thought: Substance Abuse and Eating DisordersNew York, NYThe National Center on Addiction and Substance Abuse at Columbia University2003
Appendix:
Reference electroencephalographic methodology
EEG collection and Z scores
The first step of the assessment is to collect 19-channel, awake, eyes-closed, digital electroencephalographic (EEG) recordings on 6–92-year-old patients who have either washed out their medication for five half-lives or who are currently medication-free. The results are reviewed in raw form by an electroencephalographer to ensure that no other pathology is present. The EEG is then screened to remove any “artifacts” that may exist in the EEG record. These artifacts include muscle twitches, eye blinks, and periods of drowsiness.
Neurometric analysis involves computation of a series of measures that mathematically describe the EEG. These measures are then compared with a database of “normal” EEGs. The software computes approximately 1200 measures derived from the EEG component wavelengths and amplitudes. These measures fall into four main categories, ie, power, coherence, symmetry, and frequency. Power is the sum of the amplitudes of the wavelengths in each band, computed on an absolute and relative basis. Relative power indicates the percentage of total power in each band. Coherence measures the synchronization of electrical activity between two channels. In mathematical terms, coherence is the phase shift between similar wavelengths at the two channels. Symmetry measures the ratio of power between a symmetrical pair of electrodes and, lastly, frequency measures the average frequency of the EEG component wavelengths with each band.
Most neurometric features are highly non-Gaussian in their characteristics. For this reason, the neurometrics are log-transformed to make the distributions more normal (Gaussian) in nature. Many quantitative EEG features also vary consistently with age. To account for the difference between the age of the patient and the age of the subjects in the normative database, these quantitative EEG features are age-regressed using a linear regression equation to yield a “standard-age” quantitative EEG feature. The comparison of the actual values of the neurometric variables with norms is expressed as a Z score which is defined as:
Development of pattern variables
Neurometric analysis outputs approximately 2400 variables (known as univariables) that describe the EEG. To make this data utilizable, reference-EEG transforms this data into a smaller set of multivariables (or pattern variables). These pattern variables preserve the information contained in the set of quantitative EEG univariables while retaining some degree of physical interpretation. As such, the data are not simply “mined” to come up with combinations of variables that are indicative of one state or another; instead they are combined according to anatomical location. In some cases, factor analysis is employed to give greatest weight to those univariables that preserve the largest amount of total information of all the univariables in an anatomic group. In other cases, the univariables in an anatomic group are combined in a nonlinear fashion to increase the separation of observed clusters within the data. At present, there are 74 pattern variables.
Correlation of pattern variables with known patient outcomes
The reference-EEG variables for historical patients with known positive and negative clinical outcomes to various psychotropic medications are examined in order to develop a model that will allow the prospective determination of likely patient medication responsivity to these medications. The variables are examined by stratifying the distribution according to the individual medication responsivities represented. Before utilizing this apparent relationship, the appropriateness of the pattern variables are checked. Tests of skewness and kurtosis are conducted for each of the pattern variables to ensure that the original variable distribution is Gaussian. Having ensured a Gaussian distribution, mathematics can be applied that provide a comparison of other patients with similar patterns demonstrating whether the pattern variable value for the current test in question belongs to the distribution represented by a particular medication or belongs to the distribution defined by some other group (the rest of the population). This procedure is done for all medications represented in the database and for all of the pattern variables that serve as indicators for those medications. The weightings then are averaged to calculate a “score” for each medication.
Calibration against patient records
The final step is to calibrate this score against actual patient records to determine what level of score translates into a specified likelihood response to the medication. For purposes of communication, at the time of this study, three levels of responsiveness were created. The first is “sensitive” or “S”. This level indicates that the indicated medication, or group of medications, produced a positive outcome to treatment in 80% or more of cases. “Intermediate” or “I”, the second level, indicates that the responsivity was in less than 80% of cases but more than 35% of cases. The third level, “resistive” or “R”, indicates that a response to the medication is seen in fewer than 35% of cases. In other terms, if we formulate H0 (the null hypothesis) in such a way that H0 is true if the patient is not actually responsive to the medication, then the model is calibrated to allow for a type I error rate of no more than 20% in the region indicated as “S” and a type II error rate of no more than 35% in the region designated as “R”.
To calibrate the report generator model against these standards, the outcomes database is queried for all patient responses that were not used in the construction of the actual model. This dataset is known as the validation sample. This sample is then divided into two subsets, the first of which is known as the tuning sample and the second is the final validation sample. To complete the model development, the scoring model is run against the tuning sample and the resulting distribution of scores is compared against the known responses. Thresholds for scores are then empirically set to implement the standards of S, I, and R described earlier, and which are common in such medical reports as, for example, antibiotic sensitivity results. Final validation of the model is made by running the processes, complete with the thresholds that were set, against the final validation sample. In order to preserve the fully prospective nature of this validation, no adjustment of the model parameters, including the thresholds, is made after this process. If the results of this “run” meet the specifications for the previous clinical correlations, the model is then ready to be used for new patients.
The reference-EEG methodology does not take into account the diagnosis of the patient when offering objective data on any specific medication. Response research has shown, and industry experience corroborates, that diagnosis is a poor predictor of the treatment most likely to be successful for the individual patient. This is one of the fundamental improvements that shared quantitative EEG features correlated to long-term clinical outcomes brings to the practice of psychiatry.
This process can provide objective neurophysiologic data to assist a physician in avoiding the unnecessary risk that comes with the practice of trial and error psychopharmacology, which is also seen through the efficiency of treating a patient, thus reducing suffering and medical costs. The report is unique to each patient’s quantitative EEG features.
Previous clinical evidence
Using EEG features and patterns differs from a standard quantitative EEG in that it references the quantitative EEG to a normative and then symptomatic database. This may thus have the advantage of providing information about medication to aid in selection before treatment is initiated. This approach is based on correlations between particular EEG data and medication class response in samples of patients with affective and attentional symptoms.Citation31 The authors extended this model by creating a database from patients seen in clinical practice. Since the first publication of this work,Citation31 the database has continued to expand. At the time of the study it contained unmedicated EEGs from more than 1800 patients while comparing it with EEGs from a normative age-corrected database approved by the US Food and Drug Administration. For these same 1800+ patients, they also collected the outcomes (average length of time 405 days) on more than 17,000 medication trials/intervals over time (positive, negative, or neutral) from which they built a usable database of 74 pattern variables. At that time, well over 7500 patients had accessed the correlation database. It provides a large collection of outcome data enabling clinical correlations between the patterns found in a patient’s EEG and a long-term treatment response to many medications. Small, preliminary studies have suggested a potential role in the use of this type of information for additional data about medication selection for depression, to name just one psychiatric disorder. A small, prospective, randomized, controlled study enrolled and completed 25 weeks of follow-up on two groups of treatment-resistant depressed patients (n = 6 control, n = 7 experimental) at a Veterans Administration hospital.Citation32 The trial used these quantitative EEG features to guide prescribing of psychotropic medications, while the control group received essential treatment as usual. The results indicated that six of seven subjects augmented with EEG data received ratings of moderate to marked improvement, in contrast with a single subject in the control group. When unblinded, that same subject was treated successfully with the medication that was consistent with the EEG patterns in the database. Another pilot studyCitation33 was conducted to compare this same methodology with the Texas Medication Algorithm ProjectCitation34 (TMAP) algorithm for patients with treatment-resistant depression. This small (n = 18) 10-week study found that quantitative EEG variables resulted in statistically greater change from baseline scores for the Quick Inventory of Depressive Symptomatology-Self Report-16 (QIDS-SR16) and the Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q-SF) than TMAP-guided therapy. It also found that five subjects in the TMAP group received a successful TMAP therapy that was identical to what would have been prescribed with the information obtained for the quantitative EEG features from the database. Recently, a larger study was conducted using a modified algorithm developed from the results of the STAR*D study as the control.Citation21 This multicenter (12 sites) randomized, single-blind, controlled study of 114 treatment-resistant subjects (89 evaluable) demonstrated significantly greater improvement on both primary endpoints (QIDS-SR16 and Q-LES-Q-SF, ie, the same as in the STAR*D study) of P < 0.0002 compared with control, as well as statistical superiority in nine of 12 secondary endpoints. Pilot studies using these quantitative EEG variables in eating disordersCitation20,Citation35 and substance abuseCitation36,Citation37 demonstrated similar promising results.