Abstract
Background
Numerous studies have demonstrated an association between C-reactive protein (CRP) levels and schizophrenia. However, the findings on psychotic severity and cognition remain inconsistent. The relationship between CRP and formal thought disorder in subdomains remains unclear.
Methods
We enrolled stable patients (defined as those who had no treatment changes during the 4-week period before evaluation) with a diagnosis of schizophrenia or schizoaffective disorder, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. We used the 30-item Thought and Language Disorder (TALD) scale to evaluate thought and language dysfunction over four subscales. We assessed psychotic symptoms using the Positive and Negative Syndrome Scale (PANSS). We collected fasting venous blood and measured plasma CRP levels.
Results
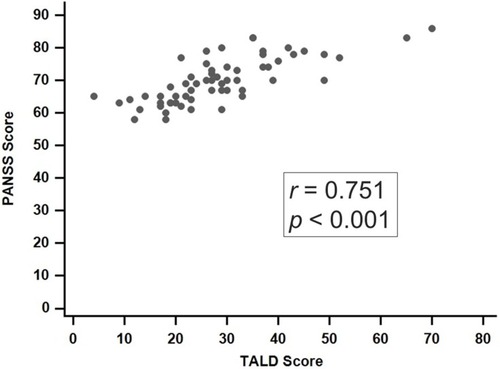
We enrolled 60 patients with schizophrenia. All patients received TALD and PANSS evaluation, and 33 of them had their CRP levels checked. The multivariate regression analysis indicated that CRP levels were significantly associated with the total score on the TALD (t=2.757, P=0.010) and the TALD Objective Positive subscale (t=2.749, P=0.011), after sex, age, duration of illness (in years), and use of atypical antipsychotics were adjusted for. Additionally, CRP was significantly associated with the PANSS positive subscale (t=2.102, P=0.045). A significantly positive correlation was observed between the total scores on the TALD scale and PANSS (ρ =0.751, P<0.001).
Conclusion
Our results suggest that abnormal CRP levels are significantly associated with formal thought and language dysfunction in the Objective Positive subdomain and positive psychotic symptoms.
Introduction
C-reactive protein (CRP) is the primary inflammation biomarker in clinical practice.Citation1 Numerous studies have identified an association between chronic inflammation and schizophrenia.Citation2,Citation3 A meta-analysis included 26 longitudinal or cross-sectional trials and reported moderately increased CRP levels in patients with schizophrenia irrespective of antipsychotic use.Citation2 The CRP levels were also reported to increase with the severity of positive symptoms but not with negative symptoms.Citation2 By contrast, other studies have demonstrated that abnormally elevated CRP levels are not associated with the severity of psychotic symptoms.Citation4–Citation8 Although inconsistent, these findings suggest that CRP is associated with positive but not negative symptoms; these effects may be confounded by factors such as different psychotic phases (eg, acute or chronic phase).Citation9 For instance, three trials with community-dwelling stabilized outpatients observed no association between elevated CRP and psychotic severity.Citation6,Citation10,Citation11
Many studies have suggested that high CRP levels are also associated with varying levels of cognitive performance impairment in patients with schizophrenia.Citation4,Citation9,Citation12–Citation14 Moreover, abnormally elevated CRP levels are associated with different cognitive function impairments, including impairments in short-term memory,Citation15 abstract reasoning, working memory, learning ability, semantic memory, mental flexibility, visual attention, and processing speed.Citation14 However, other studies have observed no significant correlations between abnormal CRP levels and cognitive functions.Citation16–Citation18 Boozalis et al observed that CRP was correlated with negative symptoms but not cognitive function.Citation18
Formal thought disorder (FTD), a core schizophrenia symptom, is characterized by disorganized speech and a deficit in the ability to organize thought; FTD results from the inappropriate use of aspects of language (particularly, semantics). Previous studies have demonstrated that FTD is a marker of psychotic severityCitation19,Citation20 and impaired cognition in schizophrenia.Citation21 A recent meta-analysis reported that neurocognitive and linguistic capabilities are correlated with positive and negative FTD.Citation22 However, the relationship between CRP and FTD remains unclear. Moreover, a study recommended objective and subjective FTD assessment in addition to the assessment of positive and negative subdomains.Citation23 On the basis of these studies, we hypothesize that CRP is associated with FTD in different subdomains. Therefore, in this study, we investigated the relationship between CRP levels and the 30-item Thought and Language Disorder (TALD) scale scores. We studied the objective and subjective subdomains as well as the positive and negative subdomains in patients with schizophrenia.
Methods
Study population
The Institutional Review Board of China Medical University Hospital approved this study (CMUH107-REC3-055). We followed the Declaration of Helsinki recommendations. In this study, all participants provided written informed consent.
We recruited patients, from a chronic ward and an outpatient clinic, with a diagnosis of either schizophrenia or schizoaffective disorder. We obtained demographic data on age, sex, age of onset, duration of illness, years of education, and body mass index (BMI).
Inclusion criteria
We enrolled stable patients (defined as patients with no treatment changes during a 4-week period before evaluation) with the diagnosis of schizophrenia or schizoaffective disorder, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Diagnosis was confirmed by trained psychiatrists.
Exclusion criteria
We excluded patients with a history of neurological disorders, including stroke, epilepsy, and the effects of head injury. Patients with any current major infectious or inflammatory diseases, particularly autoimmune diseases such as lupus and rheumatoid arthritis, were excluded. We also excluded patients who used corticosteroids or nonsteroidal anti-inflammatory drugs.
Sample collection and CRP measurement
We collected fasting venous blood and measured plasma CRP levels. In this study, we used a particle-enhanced immunoturbidimetric assay (IMMULITE1; Diagnostic Products Corp., Los Angeles, California, USA) to measure high-sensitivity CRP. The coefficient of variation was 5% at 0.02 mg/dL CRP.
Thought language function and psychotic symptoms
We used the 30-item TALD scaleCitation23 to evaluate thought and language dysfunction. The TALD scale comprises four subscales: Objective Positive, Objective Negative, Subjective Positive, and Subjective Negative. We used the Positive and Negative Syndrome Scale (PANSS) to assess psychotic symptoms.Citation24 The PANSS comprises three subscales: positive, negative, and general.
Statistical analysis
We provided descriptive statistics of the mean, median and standard deviation. A multivariate regression analysis was used to evaluate the associations between the CRP and TALD scale scores and between the CRP and the PANSS scores. We measured the correlation between the TALD scale and PANSS and calculated Spearman’s rank correlation. We performed the analyses using IBM SPSS, version 20 (IBM Corp., Armonk, NY, USA), and statistical significance was defined as a two-tailed P<0.05.
Results
Our sample comprised 60 patients with schizophrenia. The demographic and clinical characteristics of the sample are detailed in . There were 51.7% men in the sample population. The mean age of the patients was 44.78±9.88 years, and the mean age during schizophrenia onset was 25.40±8.17 years. The mean duration of illness was 19.38±8.019 years, and the mean PANSS total score was 69.98±7.02. Among the 60 subjects, 57 patients were diagnosed as having schizophrenia and 3 as having schizoaffective disorder. All patients were interviewed with the TALD scale and received a PANSS evaluation, and 33 of them had their CRP levels checked. All patients were receiving antipsychotic treatment when they were enrolled. The major antipsychotics used were second-generation antipsychotics (in 52/60 or 86.67% of patients), and the three leading second-generation antipsychotics were olanzapine, aripiprazole, and risperidone. Among the 60 patients, one was enrolled from an outpatient clinic, and the other 59, from a chronic ward.
Table 1 Clinical and demographic characteristics of individuals with schizophrenia (N=60)
CRP and thought and language dysfunction
The multivariate regression analysis indicated that CRP levels were significantly associated with scores on the TALD scale (t=2.757, P=0.010), after sex, age, duration of illness (in years), and use of atypical antipsychotics were adjusted for. Additionally, in the analysis of the four TALD subscales, CRP was significantly associated with the Objective Positive subscale of the TALD (t=2.749, P=0.011), but associations with the other three TALD subscales were nonsignificant ().
Table 2 Multivariate regression analysis
CRP and psychotic symptoms
In this study, CRP levels were associated with PANSS scores, but the association was nonsignificant (t=1.744, P=0.092), after sex, age, duration of illness (in years), and use of atypical antipsychotics were adjusted for. Additionally, in the analysis of the four PANSS subscales, CRP was significantly associated with the positive subscale of the PANSS (t=2.102, P=0.045), but the association with the other two PANSS subscales was nonsignificant ().
Thought, language dysfunction and psychotic symptoms
A significantly positive correlation was observed between the total score on the TALD and PANSS total score (rho=0.751, P<0.001; ). In the subscale analysis, the TALD Objective Positive subscale was significantly correlated with three subscales of the PANSS (ρ=0.481, 0.540, and 0.585, respectively, P<0.001). The TALD Subjective Positive subscale was significantly correlated with the PANSS positive subscale (ρ=0.264, P=0.042), and the TALD Subjective Negative subscale was significantly correlated with both the PANSS positive and negative subscales (ρ=0.528, P<0.001; ρ=0.335, P=0.009; ).
Table 3 Correlations between the four subdomains of the TALD scale and three subdomains of the PANSS
Discussion
To our knowledge, this is the first study to investigate the association between CRP and FTD. The findings of this study are as follows: (a) CRP levels are significantly associated with the total scores on the TALD and the TALD Objective Positive subscale, (b) CRP levels are significantly associated with the PANSS positive subscale, and (c) the total score on the TALD and some TALD subscale scores are significantly correlated with all scores on the PANSS.
In this study, the multivariate regression analysis indicated that CRP levels are significantly associated with the total score on the TALD (t=2.757, P=0.010) after sex, age, duration of illness (in years), and atypical antipsychotic use were adjusted for. Furthermore, in the analysis of the four TALD subscales, CRP was significantly associated with the TALD Objective Positive subscale (t=2.749, P=0.011). This is the first study to investigate the association between CRP and FTD in the four subscales. Kircher and his colleagues invented a comprehensive assessment tool, the TALD scale,Citation23 to evaluate four FTD subdomains: Objective Positive, Objective Negative, Subjective Positive, and Subjective Negative. Earlier FTD rating scales were in the descriptive psychopathological tradition.Citation25,Citation26 Furthermore, we observed a significantly positive correlation between the total score on the TALD and PANSS total score (ρ=0.751, P<0.001; ). In subscale analysis, the TALD Objective Positive subscale score was significantly correlated with three PANSS subscales (ρ=0.481, 0.540, and 0.585, all P<0.001; ). Our findings agree with the results of Kircher and his colleagues, who observed a correlation between the TALD Objective Positive subscale and the Scale for the Assessment of Positive Symptoms (SAPS; ρ=0.925, P<0.01).Citation23 A study that examined 51 patients with schizophrenia also reported a significant correlation between the TALD Objective Positive subscale score and executive dysfunctions.Citation27 Further investigation may be needed on the relationships between CRP, FTD, and cognition.
In this study, CRP was significantly associated with the PANSS positive subscale (t=2.102, P=0.045), although CRP’s associations with the PANSS total score and the two PANSS subscales of negative and general were nonsignificant. Our findings agree with a previous meta-analysisCitation2 in which meta-regression analyses suggested that the PANSS positive subscale was positively related to CRP levels (slope=0.12, 95% CI=0.03–0.23, P=0.013), but the association between the PANSS total score and PANSS negative subscale was nonsignificant. A study on 26 patients with either schizophrenia or schizoaffective disorder reported that patients with greater CRP levels had a significantly greater PANSS total score, negative subscale score, and general subscale score relative to patients with normal CRP levels (P=0.017, 0.016, and 0.014, respectively).Citation28 Another study whose sample comprised 48 patients with first-episode psychosis and 74 patients with schizophrenia in an acute exacerbation phase observed a positive correlation between high-sensitivity CRP levels and the severity of positive symptoms (ρ=0.494, P=0.004).Citation29 Two studies have observed that CRP is correlated with negative symptoms.Citation18,Citation30 However, some studies have observed no significant correlation between abnormal CRP levels and psychotic severity.Citation4,Citation6,Citation8 These discrepancies warrant further meta-analyses, trials with larger sample sizes, and comprehensive analyses of confounding factors to investigate the subgroup that is patients with severe psychosis.
We propose two reasons that CRP is associated with psychotic positive symptoms and impaired cognition: First, increased CRP may indirectly cause hypofunction of the N-methyl-D-aspartate (NMDA) receptor pathway that plays a crucial role in schizophrenia and cognition.Citation31,Citation32 Studies have observed that peripheral CRP can increase the permeability of the blood–brain barrier by influencing the tight junctionCitation33 that plays a role in psychiatric disorders.Citation34,Citation35 After entering the brain, CRP can induce an inflammatory state in the microglia,Citation36 which then releases interleukin (IL)-6 and transforming growth factor β.Citation36,Citation37 This induces the astrocytes to metabolize tryptophan into kynurenic acid, which may result in hypoglutamatergic function because it acts as an antagonist to NMDA receptors as well as dopaminergic hyperfunction in the limbic system. These functions are involved in the development of positive psychotic symptoms in schizophrenia.Citation38–Citation41 Second, CRP may act as a proxy for IL-6, correlated with positive psychotic symptoms.Citation37,Citation42
Limitations
This study has several limitations. First, we did not assess the cognitive functions using standard tools. Standard cognitive measurements should be used to evaluate the relationships between CRP, cognitive functions, and thought dysfunction in future studies. Second, we only evaluated CRP; other inflammatory biomarkers such as IL-6 were not evaluated. The results pertaining to CRP levels were based on only 33 patients. Further investigation should be conducted with a larger sample of patients in different psychotic phases. Third, additional factors, such as alcohol and substance abuse, were not included in our analysis. Furthermore, to confirm the diagnoses of the patients, we recommend further trials that use standardized clinical examination methods such as the Structured Clinical Interview for Mental Disorders.
Conclusion
CRP was significantly associated with formal thought and language dysfunction in the subgroup of patients that score highly on the TALD Objective Positive subscale and have positive psychotic symptoms.
Acknowledgements
This work was supported by grants from Ministry of Science and Technology, Taiwan (MOST 108-2314-B-039-002), National Health Research Institutes (NHRI-EX108-1073NI), China Medical University, Taiwan (CMU107-BC-4), and Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW108-TDU-B-212-133004).
Disclosure
The authors report no conflicts of interest in this work.
References
- Lopresti AL, Maker GL, Hood SD, Drummond PD. A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:102–111. doi:10.1016/j.pnpbp.2013.09.01724104186
- Fernandes BS, Steiner J, Bernstein HG, et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry. 2016;21(4):554–564. doi:10.1038/mp.2015.8726169974
- Fond G, d’Albis MA, Jamain S, et al. The promise of biological markers for treatment response in first-episode psychosis: a systematic review. Schizophr Bull. 2015;41(3):559–573. doi:10.1093/schbul/sbv00225759473
- Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93(1–3):261–265. doi:10.1016/j.schres.2007.03.02217490859
- Faugere M, Micoulaud-Franchi JA, Alessandrini M, et al. Quality of life is associated with chronic inflammation in schizophrenia: a cross-sectional study. Sci Rep. 2015;5:10793. doi:10.1038/srep1079326041435
- Fond G, Godin O, Brunel L, et al. Peripheral sub-inflammation is associated with antidepressant consumption in schizophrenia. Results from the multi-center FACE-SZ data set. J Affect Disord. 2016;191:209–215. doi:10.1016/j.jad.2015.11.01726674214
- Frydecka D, Misiak B, Pawlak-Adamska E, et al. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2015;265(6):449–459. doi:10.1007/s00406-014-0533-525214388
- Micoulaud-Franchi JA, Faugere M, Boyer L, et al. Elevated C-reactive protein is associated with sensory gating deficit in schizophrenia. Schizophr Res. 2015;165(1):94–96. doi:10.1016/j.schres.2015.03.01825864954
- Fond G, Lancon C, Auquier P, Boyer L. C-Reactive protein as a peripheral biomarker in schizophrenia. An updated systematic review. Front Psychiatry. 2018;9:392. doi:10.3389/fpsyt.2018.0039230190688
- Dickerson F, Stallings C, Origoni A, et al. C-reactive protein is elevated in schizophrenia. Schizophr Res. 2013;143(1):198–202. doi:10.1016/j.schres.2012.10.04123218564
- Fond G, Resseguier N, Schurhoff F, et al. Relationships between low-grade peripheral inflammation and psychotropic drugs in schizophrenia: results from the national FACE-SZ cohort. Eur Arch Psychiatry Clin Neurosci. 2018;268(6):541–553. doi:10.1007/s00406-017-0847-129127503
- Misiak B, Stanczykiewicz B, Kotowicz K, Rybakowski JK, Samochowiec J, Frydecka D. Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: a systematic review. Schizophr Res. 2018;192:16–29. doi:10.1016/j.schres.2017.04.01528416092
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Additive effects of elevated C-reactive protein and exposure to herpes simplex virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr Res. 2012;134(1):83–88. doi:10.1016/j.schres.2011.10.00322048011
- Bulzacka E, Boyer L, Schurhoff F, et al. Chronic peripheral inflammation is associated with cognitive impairment in schizophrenia: results from the multicentric FACE-SZ dataset. Schizophr Bull. 2016;42(5):1290–1302. doi:10.1093/schbul/sbw02927143795
- Dorofeikova M, Neznanov N, Petrova N. Cognitive deficit in patients with paranoid schizophrenia: its clinical and laboratory correlates. Psychiatry Res. 2018;262:542–548. doi:10.1016/j.psychres.2017.09.04128951142
- Hope S, Hoseth E, Dieset I, et al. Inflammatory markers are associated with general cognitive abilities in schizophrenia and bipolar disorder patients and healthy controls. Schizophr Res. 2015;165(2–3):188–194. doi:10.1016/j.schres.2015.04.00425956633
- Joseph J, Depp C, Martin AS, et al. Associations of high sensitivity C-reactive protein levels in schizophrenia and comparison groups. Schizophr Res. 2015;168(1–2):456–460. doi:10.1016/j.schres.2015.08.01926341579
- Boozalis T, Teixeira AL, Cho RY, Okusaga O. C-Reactive protein correlates with negative symptoms in patients with schizophrenia. Front Public Health. 2017;5:360. doi:10.3389/fpubh.2017.0008129404313
- Roche E, Creed L, MacMahon D, Brennan D, Clarke M. The epidemiology and associated phenomenology of formal thought disorder: a systematic review. Schizophr Bull. 2015;41(4):951–962. doi:10.1093/schbul/sbu12925180313
- Roche E, Lyne JP, O’Donoghue B, et al. The factor structure and clinical utility of formal thought disorder in first episode psychosis. Schizophr Res. 2015;168(1–2):92–98. doi:10.1016/j.schres.2015.07.04926260080
- Kerns JG, Berenbaum H. Cognitive impairments associated with formal thought disorder in people with schizophrenia. J Abnorm Psychol. 2002;111(2):211–224.12003444
- Bora E, Yalincetin B, Akdede BB, Alptekin K. Neurocognitive and linguistic correlates of positive and negative formal thought disorder: a meta-analysis. Schizophr Res. 2019. doi:10.1016/j.schres.2019.05.025
- Kircher T, Krug A, Stratmann M, et al. A rating scale for the assessment of objective and subjective formal Thought and Language Disorder (TALD). Schizophr Res. 2014;160(1–3):216–221. doi:10.1016/j.schres.2014.10.02425458572
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi:10.1093/schbul/13.2.2613616518
- Andreasen NC. Scale for the assessment of thought, language, and communication (TLC). Schizophr Bull. 1986;12(3):473–482. doi:10.1093/schbul/12.3.4733764363
- Andreasen NC, Grove WM. Thought, language, and communication in schizophrenia: diagnosis and prognosis. Schizophr Bull. 1986;12(3):348–359. doi:10.1093/schbul/12.3.3483764356
- Nagels A, Fahrmann P, Stratmann M, et al. Distinct neuropsychological correlates in positive and negative formal thought disorder syndromes: the thought and language disorder scale in endogenous psychoses. Neuropsychobiology. 2016;73(3):139–147. doi:10.1159/00044165727058747
- Fan X, Pristach C, Liu EY, Freudenreich O, Henderson DC, Goff DC. Elevated serum levels of C-reactive protein are associated with more severe psychopathology in a subgroup of patients with schizophrenia. Psychiatry Res. 2007;149(1–3):267–271. doi:10.1016/j.psychres.2006.07.01117112596
- Bolu A, Aydin MS, Akgun A, et al. Serum levels of high sensitivity C-reactive protein in drug-naive first-episode psychosis and acute exacerbation of schizophrenia. Clin Psychopharmacol Neurosci. 2019;17(2):244–249. doi:10.9758/cpn.2019.17.2.24430905124
- Fawzi MH, Fawzi MM, Fawzi MM, Said NS. C-reactive protein serum level in drug-free male Egyptian patients with schizophrenia. Psychiatry Res. 2011;190(1):91–97. doi:10.1016/j.psychres.2011.05.01021621854
- Lin CH, Lane HY, Tsai GE. Glutamate signaling in the pathophysiology and therapy of schizophrenia. Pharmacol Biochem Behav. 2012;100(4):665–677. doi:10.1016/j.pbb.2011.03.02321463651
- Li F, Tsien JZ. Memory and the NMDA receptors. N Engl J Med. 2009;361(3):302–303. doi:10.1056/NEJMcibr090205219605837
- Campbell BM, Charych E, Lee AW, Moller T. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci. 2014;8:12. doi:10.3389/fnins.2014.0001224567701
- Najjar S, Pearlman DM, Devinsky O, Najjar A, Zagzag D. Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: a review of clinical and experimental evidence. J Neuroinflammation. 2013;10:142. doi:10.1186/1742-2094-10-15124289502
- Uranova NA, Zimina IS, Vikhreva OV, Krukov NO, Rachmanova VI, Orlovskaya DD. Ultrastructural damage of capillaries in the neocortex in schizophrenia. World J Biol Psychiatry. 2010;11(3):567–578. doi:10.3109/1562297090341418820109113
- Adami C, Sorci G, Blasi E, Agneletti AL, Bistoni F, Donato R. S100B expression in and effects on microglia. Glia. 2001;33(2):131–142.11180510
- Schwieler L, Larsson MK, Skogh E, et al. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia–significance for activation of the kynurenine pathway. J Psychiatry Neurosci. 2015;40(2):126–133.25455350
- Kegel ME, Bhat M, Skogh E, et al. Imbalanced kynurenine pathway in schizophrenia. Int J Tryptophan Res. 2014;7:15–22. doi:10.4137/IJTR.S1680025288889
- Koshy Cherian A, Gritton H, Johnson DE, Young D, Kozak R, Sarter M. A systemically-available kynurenine aminotransferase II (KAT II) inhibitor restores nicotine-evoked glutamatergic activity in the cortex of rats. Neuropharmacology. 2014;82:41–48. doi:10.1016/j.neuropharm.2014.03.00424647121
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13(7):465–477. doi:10.1038/nrn325722678511
- Wu HQ, Okuyama M, Kajii Y, Pocivavsek A, Bruno JP, Schwarcz R. Targeting kynurenine aminotransferase II in psychiatric diseases: promising effects of an orally active enzyme inhibitor. Schizophr Bull. 2014;40(Suppl 2):S152–S158. doi:10.1093/schbul/sbt15724562494
- Magalhaes CA, Ferreira CN, Loures CMG, et al. Leptin, hsCRP, TNF-alpha and IL-6 levels from normal aging to dementia: relationship with cognitive and functional status. J Clin Neurosci. 2018;56:150–155. doi:10.1016/j.jocn.2018.08.02730150062