Abstract
Purpose
Epidemiology research has demonstrated that magnesium (Mg) deficiency is associated with a high incidence of Parkinson’s disease (PD). It is known that the systemic administration of MgSO4 is not able to elevate the Mg concentration in cerebrospinal fluid (CSF). This study aims to verify the protective effect of magnesium-L-threonate (MgT) in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) mouse model.
Methods
C57BL/6J mice were orally administered MgT or MgSO4 for 4 weeks, and received MPTP in the third week. After analysis of open-field and rotarod tests on the last day, tyrosine hydroxylase (TH) immunopositive cells and protein levels were quantified in the substantia nigra pars compacta (SNpc) and striatum. The expression of inducible nitric oxide synthase (iNOS) level was evaluated. Mg concentration in serum and CSF was measured after oral administration of MgSO4 or MgT in normal mice. Mg concentration in the CSF was increased in the mice treated with MgT but not MgSO4.
Results
The total distance and mean speed in open-field tests, and the time spent on rotarod in the MgT group were increased, compared with MPTP group. The MgT treatment but not MgSO4 dose-dependently attenuated the loss of TH-positive neurons, and the reduction of the TH expression in the SNpc. The MgT treatment also inhibited the expression of iNOS as measured by immunohistochemistry and Western blots. Double-immunofluorescence staining of TH and iNOS showed iNOS-positive cells were collocalized for TH-positive cells.
Conclusion
The treatment with MgT is associated with an increase of Mg in the CSF. MgT, rather than MgSO4, can significantly attenuate MPTP-induced motor deficits and dopamine (DA) neuron loss.
Introduction
Parkinson's disease (PD) is a neurodegenerative disease and its characterization includes muscular rigidity, bradykinesia, resting tremors, and postural instability, as well as several non-motor symptoms (Parkinson).Citation1 Pathological features of PD are the progressive loss of dopamine producing neurons in substantia nigra (SN), cytoplasmic inclusions occur in surviving neurons of SN, which are called Lewis bodies.Citation1–Citation3 The pathogenesis of PD may include a variety of factors, such as genetic factors or/and environmental factors. It is a relatively high incidence for agricultural workers when using herbicides and pesticides, particularly paraquat.
One previously epidemiological study has demonstrated that the function of low-Mg diet in elective neurodegeneration of dopaminergic pathway is associated with Parkinson-dementia syndrome (PDC).Citation4 The characterization of PDC involves progressive cognitive decline, parkinsonism and severe loss of neurons in the SN and widespread neurofibrillary tangles in the PDC brain. In addition, PDC is a deadly disease for the Chamorro people in Guam. High concentration of aluminum and low concentration of Mg and calcium in the water consumed by Chamorro natives have been reported for the high incidence of PD in Guam.Citation5
To further investigate the pathogenesis of PDC, a study was designed to limit the intake of Mg and calcium in rats over two generations. The intention of the study was to simulate the conditions for humans on Guam. Severe loss of dopaminergic neurons in SN were found exclusively in 1-year-old rats that had taken a continuous intake of low Mg over generations.Citation6 Another research evaluated the effect of MPTP in Mg-deficient mice, they found a low dose (like 10 mg/kg) MPTP treatment can reduce the content of dopamine (DA) and its metabolites in striatum of Mg-deficient mice. It indicates Mg-deficiency appears to enhance sensitivity in MPTP neurotoxicity.Citation7
Although the etiologic mechanism of PD related to Mg-deficiency is poorly understood, it can be assumed that hypermagnesemia can influence the development of experimental PD, because a low-Mg diet contributes to the high occurrence of PD. Hashimoto et al have proved that the toxicity of 1-methyl-4-phenylpyridinium (MPP+) could be significantly inhibited by increasing the concentration of Mg ions to 1.2 mM, and any reduction of dopaminergic neurons in in vitro MPP Parkinson’s model can be completely prevented by increasing the concentration to 4 mM.Citation8
Magnesium sulfate is a commonly used clinical medicine, and the first choice of clinical magnesium supplement (REF). Mainly intravenous magnesium sulfate has been used to investigate the neuroprotective effect of magnesium in preclinical and clinical studies.Citation9–Citation12 In a preclinical experiment, magnesium sulfate cannot play a neuroprotective role.Citation13 In some clinical experiments, magnesium sulfate cannot improve the prognosis of patients with cerebral ischemia or subarachnoid hemorrhage.Citation14–Citation16 We speculate that the difference in the efficacy of magnesium sulfate is due to its poor permeability in the blood–brain barrier. Our previous study demonstrated that the increasing of Mg concentration in serum had no effect on the concentration of Mg in CSF after intraperitoneal injection of MgSO4, even when the serum Mg level increased from 8 to 10-fold in normal mice.Citation17 Therefore, magnesium-L-threonate (MgT), a Mg compound that is very permeable through the blood–brain barrier (BBB),Citation18,Citation19 was used in the present study. There was no adverse effect of MgT on normal rats. Biochemical analysis showed that the level of Mg in plasma increased significantly after MgT administration. Compared with the control group, the behavioral evaluation of elevated plus maze (EPM) test and forced swimming test (FST) showed that MgT could significantly improve the memory of rats and reduce the depression-like symptoms of healthy rats.Citation20 We measured Mg concentration in the CSF following chronic administration of MgSO4 or MgT in normal mice, evaluated behavioral analysis, DA neuron loss and expression of iNOS after oral MgSO4 or MgT in MPTP treatment mice.
The goal of this study was to demonstrate the neuroprotective effect of MgT on MPTP mice. It is well known that MPTP was the first agent to be distinguished for human PD.Citation21 By means of monoamine oxidase B in astrocytes, MPTP can be converted to MPP+ after systemic administration. MPP+ interferes with the flow of electrons and damages mitochondrial complex I, which cause a severe shortage in ATP formation of dopaminergic neurons transferred from the dopamine transporter.
Materials And Methods
For the care and use of animals in research, this study was implemented according to NIH guidelines.Citation22 The procedures of animal experiment in this study were approved by the Animal Care and Use Committee of Guilin Medical University.
Animals
Male C57BL/6J mice (n=87, Vital River Laboratory Animal Technology Co. Ltd, Beijing, People’s Republic of China), 8 weeks old with 20 to 22 g, were used. All mice were fed separately, and they were freely provided with water and food in the light and dark cycle of 12:12 hrs, and light was given at 8:00 pm.
Drugs
MPTP was purchased from Sigma (St. Louis, MO, USA) and then diluted with saline (0.9% NaCl) at a concentration of 4 mg/mL. Two Mg compounds:MgSO4•7H2O (Rong Sheng Pharmaceutical Co., Ltd, People’s Republic of China) and MgT (Magceutics Inc., USA) were used in the current study.
Experimental Protocols
In order to examine the effect of Mg compounds in the mice model, the behavioral difference and Mg concentration in CSF and serum were measured after the individual treatment of MgSO4 or MgT. Normal mice received 1.2 mM MgSO4 or MgT via drinking water throughout the experiment. Behavioral tests and Mg assays were conducted on day 0, 21, and 28 (n=6 in each subgroup).
To detect neuroprotective effects of MgSO4 or MgT, the mice were separated into four groups randomly: 1) control group, fed the basal diet, n=12; 2) MPTP group, mice were intraperitoneally injected 30 mg/kg/day MPTP-HCl with saline for successive 7 days after 2 weeks of the experiment start, n=12; 3) MgSO4-MPTP group, mice received MgSO4 (1.2 mM) via drinking water throughout the experiment, and were injected with MPTP-HCl, as the same as the MPTP group, n=12; 4) MgT-MPTP groups, mice received 3 different doses of MgT (0.8, 1.2, and 1.6 mM) by oral administration in three subgroups, and were injected with MPTP-HCl as the same as the MPTP group, n=12, in each subgroup. The dose of two Mg compounds was chosen by adjusting the minimum effective dose in mice, the description can be found in the previous study.Citation19
To observe the dose of MgSO4 and MgT (mm/kg/day), it is necessary to measure body weight and water intake daily (8:30 pm). Based on the amount of water consumption daily and body weight, the amount of MgSO4 and MgT was obtained to ensure the target doses, and then the daily drinking water for each mouse (about 6 mL/mouse/day) was used to dissolve the drug.
Measurement Of Mg Concentration
In order to collect the serum of the mice model, 1 to 1.5 cm was dissected from the tip of the tail, and polypropylene tips (catalog no. RS-200Y, Renover Science, Co., Ltd., Tokyo, Japan) was used to collect blood with a polypropylene tube (catalog No. 430791, Corning, NY, USA). CSF was achieved through the cisterna magna. The tubes were placed into the centrifuge at 1500 rpm. The muscle and skin in the neck were separated, and then the exposed arachnoid membrane and dura mater were penetrated with a butterfly needle (27 G×1/2, 0.4× 13 mm, TOP Co., Tokyo, Japan) to inhale CSF. The Milli-Q water was used to dilute the samples at 1000-fold.
Quadrupole ICP-MS (7500 Series, Agilent Technologies, Tokyo, Japan) was inductively coupled plasma mass spectrometry and used to measure the concentration of Mg. For internal standardization, the samples were mixed in 1% nitric acid with Yttrium (Kanto Kagaku, Co., Ltd., Tokyo, Japan), and the final concentration was 10 μgL−1. Clean the instrument with Milli-Q water before adding the sample or normalizing. The working conditions of ICP-MS were given: argon flow rate, carrier 0.35 L/min, plasma 0.7 L/min; cooling chamber temperature at 2°C; ICP RF power with 1500 W; scanning quality, y m/z=89mg, m/z=25; hydrogen flow rate, 0.4. Each standard solutions or sample solutions were measured with 3 times.
Behavioral Analysis
Open-field and rotarod tests were used to measure the locomotor activity and motor coordination dysfunction of mice for 7 days after the last MPTP injection. Before any treatment, mice were exposed to the adaptive training with 2–3 times.
The automatic open-field apparatus was performed to evaluate open-field test-spontaneous locomotor activity.Citation23,Citation24 An open-field box (60×60×50 cm) divided into 16 squares was conducted to assess the spontaneous activity of mice. Mice were placed in the open-field apparatus and kept for 5 mins subjected to normal lighting. ANY-maze software (Shanghai new soft information technology Co., Ltd, People’s Republic of China) was used to investigate the trajectories and movements of mice based on video-taped. To remove the body scent, water and 70% alcohol were used to clean the box after each testing. Mice were put in the open-field box for 1 min to adapt to the venue before any treatments.
Rotarod test-Refer to test Rozas method,Citation24,Citation25 mice were placed on a rotarod 3 cm in diameter, which rotated along its longitudinal axis at four speeds (10, 12, 14, 16 rpm). The time spent on the rotarod before falling was recorded. The trial stopped when the mouse fell down or when 150 s were completed, and there was an interval of 5 mins between trials to reduce stress and fatigue. The average time on the rotarod from the five trials was used for analysis. The effects of drug treatments were assessed by testing the various groups of animals at the same time after injection.
Histological Preparation
Paraffin tissue and frozen tissue were prepared in two time points: the next day after the last MPTP (day 21) treatment to evaluate the expression of iNOS; the last day (day 28) after end of the behavioral experiments to assess the expression of TH. For paraffin tissue, Nembutal (50 mg/kg, Dainippon Pharmaceutical Co., Ltd.) was used to anesthetize mice, and then 4% phosphate buffer polyformaldehyde (PFA) solution was used to perfuse and fix through heart. The brain of mouse was coronally cut at 3 mm posterior to the bregma (superior colliculus level), 1.5 mm posterior to the bregma (infundibular level) and 0.5 mm anterior to the bregma (chiasmal level). The obtained blocks were embedded in paraffin and cut with 4 microns thickness. The resulting blocks were put in paraffin and cut into slice with 4 µm. For frozen tissue, mice were perfused with 4% PFA, the brains of mice were processed into blocks as mentioned above with dehydration using different concentrations of sucrose (low to high); they were then mounted in OCTA mold filling and sliced with 16 µm thickness.
Immunohistochemistry
The sections were immersed in H2O2 (3%) for 30 mins, and washed with PBS. The normal goat serum (10%) was used to treat the slices for 30 mins, and then the slices were incubated by a mouse IgG secondary antibody or biotinylated goat anti-rabbit for 60 mins. To observe the binding antibody, the biotin-biotinylated peroxidase complex (ABC) method was used. After 60 mins, the peroxidase reaction was carried out in diaminobenzidine (DAB). The numbers of immunopositive in SN were determined by a microscope attached with a drawing tube (Nikon, Tokyo, Japan) at 200× magnification. In this study, the experimental conditions of each histological specimen were blinded to the researchers.
The primary antibodies, including rabbit anti-iNOS (Chemicon, Temecula, CA, USA; 1:2000 dilution) and mouse anti-tyrosine hydroxylase (Chemicon, Temecula, CA, USA; 1:2000 dilution) were used in this study.
For double-immunofluorescence staining of TH and iNOS, the slices were put in PBS with 10% NGS at room temperature for 60 mins and incubated with mouse anti-tyrosine hydroxylase and rabbit anti-iNOS overnight at 4°C. Subsequently, slices of brains were washed by PBS, and incubated in Alexa 594-coupled anti-mouse IgG and Alexa 488-coupled anti-rabbit IgG for 2 hrs at room temperature. Double positive cells were examined with a Zeiss M510 laser scanning confocal imaging system.
Western Blotting Analysis
To assess the protein expression of iNOS (day 21) and TH (day 28), control-, MPTP-, and 1.2 mM MgT mice were prepared (n=6). The dissected tissues were well distributed in ice-cold Tris-HCl buffer with 25 mM, which included protease inhibitors (benzenesulfonyl fluoride, bestatin, leupeptin, and aprotinin) and 2 mM EDTA. The homogenate was centrifuged with 11 kg for 5 mins at 4°C, the pellet was resuspended in fresh buffer (sodium dodecyl sulfate, Tris, h-mercaptoethanol, and glycerol), and decentered with 20 kg for 30 mins. The source of proteins was obtained from the supernatant, and the concentration of protein was evaluated by Coomassie blue protein assay. The protein was electrophoresed on polyacrylamide gels, shifted to the membrane of nitrocellulose, and detected by polyclonal affinity purified anti-iNOS, anti-Th antibodies, and HRP coupled IgG, respectively. Subsequently, ECL Western blotting kit (Amersham Pharmacia Biotech, Piscataway, NJ) was used to observe the immunoreactive protein on the filter NIH image program was used to quantify the Western blot intensity by density scanning, and the recombinant beta-actin signal was normalized on the same Western blot.
Statistical Analysis
The data were examined using repeated measurement variance analysis (ANOVA), and the testing day was taken as an independent variable. Bonferroni postmortem multiple comparison test was used to analyze the groups. All results are illustrated with mean±SD. Statistical significance level was set at P<0.05.
Results
Treatment Of MgSO4 Or MgT In Normal Mice
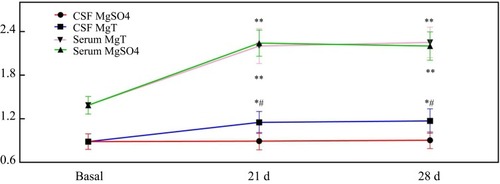
There were no significant changes in behavioral analysis when individual treatment of MgSO4 or MgT in normal mice. Mg concentrations in serum and CSF were 1.38±0.12 mM and 0.89±0.11 mM, respectively. It was found that there was no obvious change in the CSF that was treated with MgSO4, the concentration of Mg in the serum was remarkably promoted to 2.24±0.18 mM from 1.38±0.12 mM on day 21, and reached 2.21±0.19 mM on day 28. However, the concentration of Mg in the CSF increased significantly from 0.89±0.11 mM to 1.15±0.15 mM on day 21, and 1.17±0.16 mM on day 28 after the treatment with 1.2 mM MgT ().
MgT Improves Motor Coordination And Motor Dysfunction In MPTP-Injected Mice
Representative motor activity maps of movement of each treatment group mice (day 28) are shown in . Compared with the control group mice, the total distance and mean speed in MPTP group mice decreased 37.6% and 39.7%, respectively ( and ). However, compared with MPTP group, the total distance and mean speed in 1.2 mM MgT treated group were significantly increased 38.5% and 50.1%, respectively, respectively. The distance and time that mice travelled in the center decreased from 57.1% and 46.5% in MPTP group mice, however, there were no difference between all treatment groups and MPTP group ().
Figure 2 Open-field and rotarod tests. (A) Representative motor activity maps of mouse movement from each treatment group (day 28); (B) total distance; (C) average velocity and movement time; (D) rotarod time in 10, 12, 14, and 16 rpm/min, respectively. **P<0.01, compared with control. #P<0.05, compared with MPTP group.
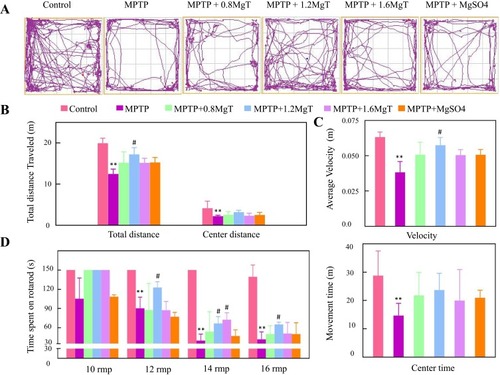
In the rotarod test, the rotarod time of MPTP group was significantly shorter in 12, 14, and 16 rpm/min than that of the control group but no change was found in 10 rpm/min test. The MgT treatment dose-dependently increased the rotarod time in 14 rpm/min test.
DA Neurons In SN
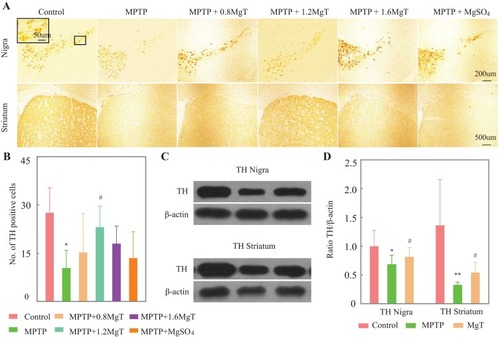
The value of TH-positive neurons (, inset) of SNpc was remarkably decreased for MPTP group (62.4% of the control group). As shown in and , the MgT treatment dose-dependently attenuated the loss of TH+ neurons compared to the MPTP group. TH-positive neurons were observed also in ventral tegmental area (VTA) in normal mice, and well preserved in MPTP group, and no significant change was found in MgT or MgSO4 treatment group. The analysis of Western blot demonstrated that the protein bands of TH deduced at 31.3% in SN and 76.1% in striatum ( and ) in MPTP treated mice, and the MgT treatment (1.2 mM) attenuated the reduction of TH, reaching 18.9% and 65.9% in SN and striatum of the control, respectively.
Figure 3 Immunohistochemical staining and Western blot analysis of TH. (A) Representative light micrographs of immuno-staining of TH in SN and striatum; (B) number of TH-positive cells in SNpc; (C) Western blot analysis of TH in SN and striatum. Upper row showed representative gel bands for TH protein, lower row showed β-actin signals from the same blot; (D) ration TH/β-actin. *P<0.05, **P<0.01, compared with control, #P<0.05, compared with MPTP group.
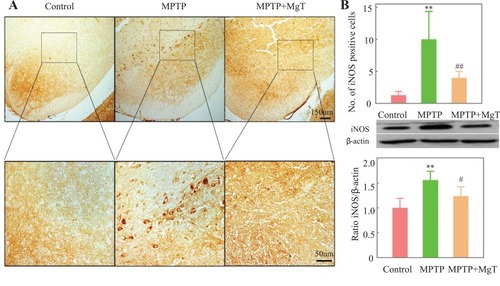
Expression Of iNOS In The SN
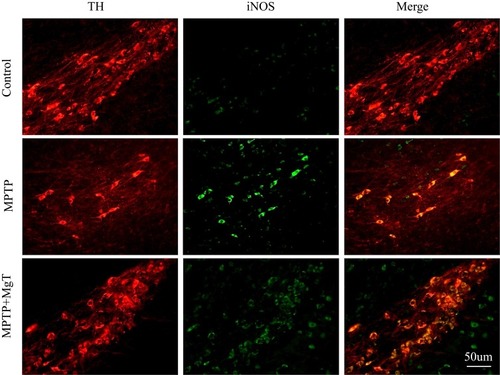
The expression of iNOS level was evaluated the next day (day 21) after MPTP treatment for 7 days. Compared with the control group, the number of iNOS-positive cells of the MPTP group was remarkably increased, and the number of iNOS-positive cells in the 1.2 mM MgT treatment significantly decreased (). The analysis of Western blot illustrated that the MPTP treatment enhanced the expression of iNOS in SN by 55.6% () and the MgT treatment at 1.2 mM significantly decreased iNOS expression. Double-immunofluorescence staining of TH and iNOS showed most of the iNOS-positive cells were TH-positive DA neurons ().
Figure 4 Immunohistochemical staining (A) and Western blot analysis of iNOS in SN; (B) representative light micrographs of immuno-staining of iNOS in SN were given in (A) quantitative analysis of iNOS-positive cells was presented in (B) **P<0.01, compared with control; #P<0.05, ##P<0.01, compared with MPTP group.
Discussion
Mg has a basic role in the function of cell and its deficiency is associated with neurological disorders. The administration of intravenous MgSO4 has a neuroprotective influence on cerebral ischemia, subarachnoid hemorrhage, seizure and traumatic brain injury in both animals and humans based on clinical and experimental studies.Citation24,Citation26 Although many reports have investigated that serum Mg can enter CSF, the ionic composition in the CSF keeps significantly stable when blood ionic composition with variation in animals Citation27–Citation29 or humansCitation30 subjected to the normal pathologic conditions. The serum Mg level increased 2–4 times in dogs under the normal conditions, while Mg level in the CSF enhanced slightly in patients with brain injury.Citation31 However, Mg level in CSF in preeclampsia patients and hippocampal epilepsy rats increased remarkably, indicating the CSF Mg level is actively regulated, possible by Mg transporter located in BBB.Citation32 Our previous study also demonstrated that concentration of Mg in the CSF had no any variation with the raise of the Mg concentration in serum, even when the serum Mg level increased 8- to 10-fold in normal mice.Citation17 Here, our results showed that MgT, but not MgSO4, increased the CSF Mg level. We also showed that MgT, but not MgSO4 can significantly attenuate MPTP-induced motor deficits and DA neuronal degeneration in mice. These results indicated that the positive influence of MgT can be associated with the elevation of Mg concentration in the CSF after treatment with MgT.Citation33 The Mg concentration increased about 21.6% after treatment of MgT on day 28 in CSF, which were concordant with the previous data that Mg concentration was increased to 15% in rat CSF and increased to 30% in APPswe/PS1dE9 mice brain tissue after oral MgT.Citation19,Citation34
Mg-deficiency may be related with the pathogenesis of PDC that occurs in the Chamorro people of Guam, and rats with Mg deficiency for generations with severe loss of DA neurons,Citation35 and the sensitivity of mice to MPTP neurotoxicity seems to enhance.Citation7 However, the mechanism of PD related to Mg-deficiency is poorly understood. The deficiency of Mg is related to decreased antioxidant defense and increased oxidative stress in different tissues based on animal and human models.Citation18,Citation31,Citation36 In animals, Mg-deficiency resulted in a marked promotion of some pro-inflammatory molecules, such as VCAM, I IL-6, L-1b, PAI-1, and TNF-a.Citation37,Citation38 Inflammatory cytokines can induce free radical generation and promote neuronal degeneration by causing oxidative stress. In SN, the microglia is densest and the number is 4–5 times higher than in other regions in the central nervous system, therefore, it is conceivable that DA neurons in the SN are selective vulnerable when exposed continuously to low Mg intake.
MPTP was a neurotoxin and recognized early in 1982 as the first human parkinsonian agent was identified. MPTP generates a severe and irreversible Parkinson’s syndrome, which almost duplicates the characteristics of PD.Citation39 After systemic administration of MPTP, it is converted to MPP+ in astrocytes, released into extracellular space and freely entered into DA neurons by dopamine transporter. MPP+ damages mitochondrial complex I, interrupts the flow of electrons, and causes a severe shortage in the formation of ATP. The other result of mitochondrial complex I blocked from MPP+ is an enhanced result of reactive oxygen species (ROS), which produce toxic effects with other active substances like nitric oxide synthase (NO) generated from inducible NO synthase (iNOS) and neuronal NO synthase (nNOS) in the brain.Citation39,Citation40
The neurotoxic of NO may affect its function in excitotoxity, neuroinflammation, DNA damage, oxidative stress, and mitochondrial dysfunction. Mice knocked out of nNOSCitation41 or iNOS gene illustrated a stronger resistance to MPTP than wild-type mice. Administration of MPTP to mice induces upregulation of iNOS, and increases immunoreactivity for nNOS in the SN.Citation42 Pretreatment inhibitor of microglial activation inhibits nigrostriatal dopaminergic neuronal degeneration and formation of nitrotyrosine evoked from MPTP.Citation43–Citation46 In this study, we demonstrated the treatment of MgT attenuated the loss of TH-positive neurons and the reduction of TH protein in SNpc. The mechanism underlying the Mg effect remains unclear. One previous study suggested that Mg in enough content inhibits the formation of oxygen radical from scavenging free radicals and preventing oxidase of both NADPH and xanthine.Citation47 Moreover, Mg is required for normal functioning of the nervous system. It is a cofactor in hundreds of enzymatic reactions, such as activation of phosphotransferase and hydrolases (like ATPase), which are of central importance in the biochemistry of the cell, particularly energy metabolism. Mg concentrates ribosomes and is involved in the attachment of mRNA to them. Thus, Mg is required for protein and nucleic acid synthesis, cell cycle activity, cytoskeletal and mitochondrial integrity, and for binding of substances to the plasma membrane. In addition, iNOS activated by proinflammatory cytokines and Gram-negative endotoxins was increased in glial cells in MPTP model.Citation37,Citation48,Citation49 Our study also observed active microglia and astrocytes in SN, and the iNOS upregulation and co-localization with TH-positive cells in SNpc by laser scanning microscopy (), which were inhibited from MgT.
Our results also showed the administration of MgSO4 had a marginal effect on mean speed induced in open-field test, but had no effect on neurodegeneration. Center time and center distance were generally regarded as an indicator of anxiety. A previous study has evaluated the effect of chronic administration of MgSO4 in MPTP mice, and shows that the administration of MgSO4 with low dose (like 2.5 g/l) generated a raise in latency and motor activity under thermal stimuli.Citation50 However, the exhaustion of dopamine in striatum induced by MPTP treatment was not improved, and even exacerbated by high doses of MgSO4. A number of factors are involved in anxiety disorders, such as serotonin, dopamine, and other neurotransmitter system perturbations,Citation51 and more than 40% of patients with PDcould have the clinical anxiety caused by the above factors.Citation52 There was no difference in light-dark preference test in mice with 7 days after MPTP injury,Citation53 and DA depletion was imperative but not enough to influence anxiety disorder.Citation23
In the current study, the data suggest that the beneficial effect of MgT on neuroprotection is likely due to its effect on the Mg permeation into the brain, as the administration of MgT increased the concentration of Mg in both the CSF and serum. On the other hand, MgSO4 had no effect on the neurodegeneration as it only increased the serum Mg level but did not affect the CSF Mg level. These data also suggest that the only supplementation of Mg in the periphery does not help to protect the brain and the combination of Mg with an agent that promotes its transportation to the brain is essential for the neuroprotection of this element.
Conclusion
In conclusion, our results indicate MgT can significantly attenuate MPTP-induced motor deficits and DA neuron injury, which may be related to its ability of increasing the Mg concentration in the CSF.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81260205) and Guangxi Natural Science Foundation (2018GXNSFAA281246).
Disclosure
The authors report no conflicts of interest in this work.
References
- De Virgilio A, Greco A, Fabbrini G, et al. Parkinson’s disease: autoimmunity and neuroinflammation. Autoimmun Rev. 2016;15(10):1005–1011. doi:10.1016/j.autrev.2016.07.02227497913
- González-Casacuberta I, Juárez-Flores DL, Morén C, Garrabou G. Bioenergetics and autophagic imbalance in patients-derived cell models of parkinson disease supports systemic dysfunction in neurodegeneration. Front Neurosci. 2019;13:894. doi:10.3389/fnins.2019.0089431551675
- Vila M, Przedborski S. Genetic clues to the pathogenesis of parkinson’s disease. Nat Med. 2004;10(Suppl):S58–S62. doi:10.1038/nm106815272270
- Taniguchi R, Tan-no K. Combined low calcium and lack magnesium is a risk factor for motor deficit in mice. Biosci Biotechnol Biochem. 2013;77(2):4. doi:10.1271/bbb.120671
- Li Y, Jiao Q, Xu H. Biometal dyshomeostasis and toxic metal accumulations in the development of alzheimer’s disease. Front Mol Neurosci. 2017. doi:10.3389/fnmol.2017.00339
- Oyanagi K, Hashimoto T. Magnesium in Parkinson’s Disease: An Update in Clinical and Basic Aspects. South Australia: University of Adelaid Press; 2011.
- Muroyama A, Inaka M, Matsushima H, Sugino H, Marunaka Y, Mitsumoto Y. Enhanced susceptibility to MPTP neurotoxicity in magnesium-deficient C57BL/6N mice. Neurosci Res. 2009;63(1):72–75. doi:10.1016/j.neures.2008.09.00918977253
- Hashimoto T, Nishi K, Nagasao J, et al. Magnesium exerts both preventive and ameliorating effects in an in vitro rat parkinson disease model involving 1-methyl-4-phenylpyridinium (MPP+) toxicity in dopaminergic neurons. Brain Res. 2008;1197(p):9. doi:10.1016/j.brainres.2007.12.033
- Shindo Y, Yamanaka R, Suzuki K, Hotta K, Oka K. Intracellular magnesium level determines cell viability in the MPP+ model of parkinson’s disease. Biochim Biophys Acta Mol Cell Res. 2015;1853(12):3182–3191. doi:10.1016/j.bbamcr.2015.08.013
- Bennet L, Galinsky R, Draghi V, et al. Time and sex dependent effects of magnesium sulphate on post‐asphyxial seizures in preterm fetal sheep. J Physiol. 2018;596(23):6079–6092. doi:10.1113/tjp.2018.596.issue-2329572829
- Saver JL, Starkman S, Eckstein M, et al. Methodology of the Field Administration of Stroke Therapy–Magnesium (FAST-MAG) phase 3 trial: part 2–prehospital study methods. Int J Stroke. 2014;9(2):220–225. doi:10.1111/ijs.1224224444117
- Huang Y, Huang X, Zhang L, et al. Magnesium boosts the memory restorative effect of environmental enrichment in alzheimer’s disease mice. CNS Neurosci Ther. 2018;24(1):70–79. doi:10.1111/cns.2018.24.issue-129125684
- Zhu H-D, Martin R, Meloni B, et al. Magnesium sulfate fails to reduce infarct volume following transient focal cerebral ischemia in rats. Neurosci Res. 2004;49(3):347–353. doi:10.1016/j.neures.2004.04.00115196783
- Reddy D, Fallah A, Petropoulos J-A, Farrokhyar F, Macdonald RL, Jichici D. Prophylactic magnesium sulfate for aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Neurocrit Care. 2014;21(2):356–364. doi:10.1007/s12028-014-9964-024619389
- Saver JL, Starkman S, Eckstein M, et al. Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N Engl J Med. 2015;372(6):528–536. doi:10.1056/NEJMoa140882725651247
- Huenges Wajer I, Dorhout Mees S, van den Bergh W, et al. Effect of magnesium on cognition after aneurysmal subarachnoid haemorrhage in a randomized trial. Eur J Neurol. 2018;25(12):1486–1489. doi:10.1111/ene.2018.25.issue-1230048027
- Sun L, Kosugi Y, Kawakami E, Piao YS, Hashimoto T, Oyanagi K. Magnesium concentration in the cerebrospinal fluid of mice and its response to changes in serum magnesium concentration. Magnes Res. 2009;22(4):266–272. doi:10.1684/mrh.2009.018620228005
- Slutsky I, Abumaria N, Wu LJ, et al. Enhancement of learning and memory by elevating brain magnesium. Neuron. 2010;65(2):165–177. doi:10.1016/j.neuron.2009.12.02620152124
- Li W, Yu J, Liu Y, et al. Elevation of brain magnesium prevents synaptic loss and reverses cognitive deficits in Alzheimer’s disease mouse model. Mol Brain. 2014;7:65. doi:10.1186/s13041-014-0065-y25213836
- Sadir S, Tabassum S, Emad S, et al. Neurobehavioral and biochemical effects of magnesium chloride (MgCl 2), magnesium sulphate (MgSO 4) and magnesium-L-threonate (MgT) supplementation in rats: a dose dependent comparative study. Pak J Pharm Sci. 2019;32.
- Gil-Martinez AL, Cuenca L, Sanchez C, Estrada C, Fernandez-Villalba E, Herrero MT. Effect of NAC treatment and physical activity on neuroinflammation in subchronic Parkinsonism; is physical activity essential? J Neuroinflammation. 2018;15(1):328. doi:10.1186/s12974-018-1357-430477535
- Council NR. Guide for the Care and Use of Laboratory Animals. National Academies Press; 2010.
- Li Y, Jiao Q, Du X, et al. Investigation of behavioral dysfunctions induced by monoamine depletions in a mouse model of parkinson’s disease. Front Cell Neurosci. 2018;12:241. doi:10.3389/fncel.2018.0024130135645
- Miyanishi KC, Mohammed E, Watanabe M, Kubo M. Behavioral tests predicting striatal dopamine level in a rat hemi-Parkinson’s disease model. Neurochem Int. 2019;122:8. doi:10.1016/j.neuint.2018.11.00530336179
- Rozas G. The overall rod performance test in the MPTP-treated-mouse model of parkinsonism. J Neurosci Methods. 1998;83(2):10. doi:10.1016/S0165-0270(98)00078-8
- Westermaier T, Stetter C, Kunze E, et al. Magnesium treatment for neuroprotection in ischemic diseases of the brain. Exp Transl Stroke Med. 2013;5(1):6. doi:10.1186/2040-7378-5-623618347
- Lingam I, Meehan C, Avdic-Belltheus A, et al. Short-term effects of early initiation of magnesium infusion combined with cooling after hypoxia-ischemia in term piglets. Pediatr Res. 2019.
- Gee JN. Hypermagnesemia does not increase brain intracellular magnesium in newborn swine. Pediatr Neurol. 2001;25(4):4. doi:10.1016/S0887-8994(01)00317-4
- Pamphlett R. Magnesium supplementation does not delay disease onset or increase survival in a mouse model of familial ALS. J Neurol Sci. 2003;216(1):3. doi:10.1016/S0022-510X(03)00216-8
- Sen AP, Gulati A. Use of magnesium in traumatic brain injury. Neurotherapeutics. 2010;7(1):91–99. doi:10.1016/j.nurt.2009.10.01420129501
- McKee JA. Magnesium neuroprotection is limited in humans with acute brain injury. Neurocrit Care. 2005;2(3):9. doi:10.1385/NCC:2:3:342
- Euser AG, Cipolla MJ. Magnesium sulfate for the treatment of eclampsia: a brief review. Stroke. 2009;40(4):1169–1175. doi:10.1161/STROKEAHA.108.52778819211496
- Wenwen X, Jing Y, Yingchao S, Qinglu W. The effect of magnesium deficiency on neurological disorders: a narrative review article. Iran J Public Health. 2019;48(3):379.31223564
- Li W, Yu J, Liu Y, et al. Elevation of brain magnesium prevents and reverses cognitive deficits and synaptic loss in Alzheimer’s disease mouse model. Soc Neurosci. 2013;7:65.
- Oyanagi K, Kawakami E, Kikuchi-Horie K, et al. Magnesium deficiency over generations in rats with special references to the pathogenesis of the parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Neuropathology. 2006;26(2):115–128. doi:10.1111/neu.2006.26.issue-216708544
- Mazur A, Maier JA, Rock E, Gueux E, Nowacki W, Rayssiguier Y. Magnesium and the inflammatory response: potential physiopathological implications. Arch Biochem Biophys. 2007;458(1):48–56. doi:10.1016/j.abb.2006.03.03116712775
- Kharitonova M, Iezhitsa I, Zheltova A, Ozerov A, Spasov A, Skalny A. Comparative angioprotective effects of magnesium compounds. J Trace Elem Med Biol. 2015;29:227–234. doi:10.1016/j.jtemb.2014.06.02625127069
- Wolf FI, Trapani V, Simonacci M, Ferré S, Maier JA. Magnesium deficiency and endothelial dysfunction: is oxidative stress involved? Magnesium Res. 2008;21(1):58–64.
- López A. Mitochondrial impairment and melatonin protection in parkinsonian mice do not depend of inducible or neuronal nitric oxide synthases. PLoS One. 2017;12(8):e0183090. doi:10.1371/journal.pone.018309028800639
- Dzamko N, Geczy CL, Halliday GM. Inflammation is genetically implicated in Parkinson’s disease. Neuroscience. 2015;302:89–102.25450953
- Kurosaki H, Yamaguchi K, Man-yoshi K, Muramatsu S-I, Hara S, Ichinose H. Administration of tetrahydrobiopterin restored the decline of dopamine in the striatum induced by an acute action of MPTP. Neurochem Int. 2019;125:16–24. doi:10.1016/j.neuint.2019.02.00530739038
- Aras S, Tanriover G, Aslan M, Yargicoglu P, Agar A. The role of nitric oxide on visual-evoked potentials in MPTP-induced parkinsonism in mice. Neurochem Int. 2014;72:48–57. doi:10.1016/j.neuint.2014.04.01424795109
- Wu DC. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of parkinson disease. J Neurosci Methods. 2002;22(5):8.
- Kim HG, Ju MS, Ha SK, et al. Acacetin protects dopaminergic cells against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neuroinflammation in vitro and in vivo. Biol Pharm Bull. 2012;35(8):1287–1294. doi:10.1248/bpb.b12-0012722863927
- Hammond SL. The Nurr1 Ligand,1,1-bis(3ʹ-Indolyl)-1-(p-Chlorophenyl)methane, modulates glial reactivity and is neuroprotective in MPTP-induced parkinsonism. J Pharmacol Exp Ther. 2018;365(3):15. doi:10.1124/jpet.117.246389
- Jiang L. Clozapine metabolites protect dopaminergic neurons through inhibition of microglial NADPH oxidase. J Neuroinflammation. 2016;13(1). doi:10.1186/s12974-016-0573-z
- Barbagallo M, Belvedere M, Sprini D, Dominguez LJ. Magnesium and alzheimer’s disease: implications for diet and nutrition In: Martin CR, Preedy VR, editors. Diet and Nutrition in Dementia and Cognitive Decline. Elsevier; 2015:585–592.
- Wang Q, Zhang H, Liu M, et al. [P38 MAPK signaling pathway regulates nuclear factor-kappaB and inducible nitric oxide synthase expressions in the substantia nigra in a mouse model of parkinson’s disease]. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34(8):1176–1180.25176090
- Ghosh A, Kanthasamy A, Joseph J, et al. Anti-inflammatory and neuroprotective effects of an orally active apocynin derivative in pre-clinical models of parkinson’s disease. J Neuroinflammation. 2012;9:241. doi:10.1186/1742-2094-9-24123092448
- Tariq M, Khan HA, al Moutaery K, al Deeb SM. Effect of chronic administration of magnesium sulfate on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in mice. Pharmacol Toxicol. 1998;82(5):218–222. doi:10.1111/j.1600-0773.1998.tb01428.x9646326
- Erro R, Pappatà S, Amboni M, et al. Anxiety is associated with striatal dopamine transporter availability in newly diagnosed untreated Parkinson’s disease patients. Parkinsonism Relat Disord. 2012;18(9):1034–1038. doi:10.1016/j.parkreldis.2012.05.02222789824
- Wood SJ, Toth M. Molecular pathways of anxiety revealed by knockout mice. Mol Neurobiol. 2001;23(2–3):101–119. doi:10.1385/MN:23:2-3:10111817214
- Vuckovic MG, Wood RI, Holschneider DP, et al. Memory, mood, dopamine, and serotonin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. Neurobiol Dis. 2008;32(2):319–327. doi:10.1016/j.nbd.2008.07.01518718537