Abstract
The ABCB1 gene encodes the P-glycoprotein (Pgp) protein, which is thought to transport various antiepileptic drugs. The single nucleotide polymorphism (SNP) (C3435T) in exon 26 of this gene correlates with the altered expression levels of P-glycoprotein, range of drug response and clinical conditions. In order to investigate the influence of this polymorphism on the susceptibility to and efficacy of carbamazepine therapy, we evaluated the allelic frequency and genotype distribution of this variant in 162 epilepsy patients from the Republic of Macedonia. Statistically significant differences were detected neither in the allelic frequency and genotype distribution between carbamazepine-resistant and carbamazepine-responsive epilepsy patients nor between the subgroups of carbamazepine (CBZ)-responsive patients treated with different CBZ doses. However, the T-allele was enriched in CBZ-responsive patients who required higher maintenance CBZ doses, This observation was substantiated by the findings that the median total plasma levels were the lowest in patients with CC (20 μmol/L) followed by CT (23 μmol/L) and TT (29 μmol/L) genotypes. Patients with a CC genotype also had a higher likelihood of response compared to patients with CT or TT genotypes over a wide range (400–1000 mg/day) of initial doses of CBZ. The T allele showed a reduced expression of ~5% compared to the C allele in peripheral blood mononuclear cells in heterozygotes for the variant. This difference might be translated into ~10% difference in homozygotes for the variant, which would explain the trend towards a dose-dependent efficacy of the CBZ treatment in patients with different genotypes. A larger prospective study is warranted to clarify the clinical utility of a genotypespecific individualized CBZ therapy.
Introduction
The ABCB1 gene (also known as multidrug resistance protein 1-MDR1), is potentially an important candidate gene influencing the response to antiepileptic drugs (AEDs). This gene encodes P-glycoprotein (Pgp), which is thought to transport various antiepileptic drugs.Citation1 Carbamazepine (CBZ) is a lipophilic agent and thus a theoretical substrate for Pgp, but the scientific evidence is still equivocal.Citation2–Citation5 Pgp takes part in the energy-dependent export of substances from the inside of cells, as well as from membranes, to the outside. Pgp is expressed in tissues with excretory functions (eg, the small intestine, liver, and kidney), and at blood–tissue barriers (eg, the blood–brain barrier [BBB], the blood–testis barrier and the placenta), thus limiting drug entry into body tissues after oral administration, promoting drug elimination into bile and urine, and limiting drug penetration into sensitive tissues such as the brain.Citation6
The single nucleotide polymorphism (SNP) C3435T in exon 26 is one of more than 100 polymorphic variants of this gene that have been discovered to date.Citation7 This polymorphism correlates with altered expression levels of P-glycoprotein, range of drug response and clinical conditions.Citation8 However, the nature of this relationship is still unclear.
Some of the published studies have documented an association of pharmaceutical resistance with the 3435CC genotype of the MDR1/ABCB1 geneCitation9–Citation11 whereas other studies were unable to identify any association.Citation12,Citation13 In addition, several studies have shown that the CC genotype is associated with increased expression of Pgp in the brain, liver, and duodenum.
The present study investigates the influence of the ABCB1 C3435CT SNP on the efficacy of carbamazepine therapy in patients with epilepsy from the Republic of Macedonia.
Materials and methods
In this study, 162 adult patients of both sexes (76 males and 86 females, average age 53 ± 15.5) from the Republic of Macedonia, all of them with normal renal and hepatic function, were treated with a carbamazepine monotherapy for at least 2 years. The type of seizures and epileptic syndrome were classified according to the ILAE classification (). The participation of each subject was voluntary and could be cancelled by any individual at any time during this study (according to the Helsinki II declaration). The Ethics Committees of the Faculty of Pharmacy and Faculty of Medicine, St Cyril and Methodius University, Skopje, approved the research protocol for this study and all volunteers signed the informed consent form.
Table 1 Characteristics of patients
Genomic DNA was extracted from whole blood, using a QIAGEN DNA extraction kit and the procedure recommended by the manufacturer (QIAGEN AS, Oslo, Norway). The presence of the ABCB1 C3435T polymorphism (rs 1045642) was analyzed by the allelic discrimination TaqMan assay (MxPro 3005P, Strategene, La Jolla, CA) according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA).
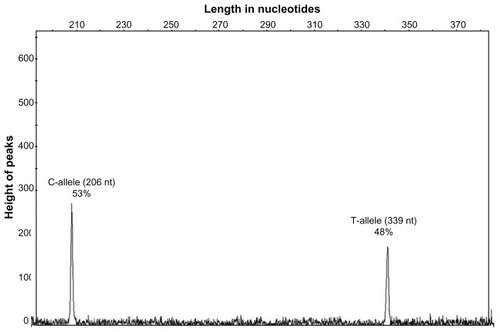
The relative level of expression of C versus T allele of the variant in peripheral blood mononuclear cells (PBMC) was performed using a RFLP based quantitative PCR method, as described in details previously.Citation14 Briefly, total RNA was isolated from peripheral blood mononuclear cells of 25 heterozygotes (15 patients and 10 controls), 5 homozygotes, each for the C and the T alleles, using the QIAGEN RNA blood extraction kit (QIAGEN AS, Oslo, Norway). Three microliters of these RNA samples was reversely transcribed into cDNA and subsequently amplified for 35 cycles (95°C for 1 minute, 60°C for 1 minute, and 72°C for 1.5 minutes) using two primers (ABCB1 Eg25 Dir 5′-AAG AGC ACA GTG GTC CAG CTC C-3′ and ABCB1 Eg27 Rev 5′-ACG AGC TAT GGC AAt GCS TTG-3′) located in the flanking exon 26 where the ABCB1 3435 C-T polymorphism resides. Five microliters of this reaction was subjected to one cycle of primer extension using 100 pM of 5′ FAM labeled primer ABCB1 Eg25 Dir/FAM 5′-AAG AGC ACA GTG GTC CAG CTC C-3′ (95°C for 15 minutes, 60°C for 2 minutes, and 72°C for 10 minutes). The resulting labeled 339 bp fragment was digested with 0.5 μL MboI restriction enzyme at 37°C for 12 hours. MboI cuts the PCR fragment from the C-allele into two fragments of 206 and 133 bp, whereas the PCR fragments from the T-allele remain undigested. The digestion products were separated with capillary electrophoresis on an ABI 310 Genetic Analyzer (Applied Biosystems, Carlsbad, CA) and the relative quantities of the 339 bp fragment (T-allele) and 206 bp fragment (C-allele) in heterozygotes were determined using the GeneMapper® software 4.0 (Applied Biosystems). The samples from homozygotes for the T and C alleles were used as controls for specificity and completeness of the enzymatic digestion, respectively.
Plasma CBZ concentrations were measured using the fluorescence polarization immunoassay (TDx/FLx system, Abbott Laboratories, Abbott Park, IL) and HPLC method. For the HPLC method, the separation was carried out on a Waters HPLC system (Waters Corporation, Milford, MA) with a reversed-phase column (Zorbax Extend C18, 150 × 4.6 mm, 5 μm; Agilent Technologies, Colorado Springs, CO) using isocratic elution with acetonitrile and water (35:65) as a mobile phase. The temperature was 30°C and UV detection was set at 220 nm. Method validation was developed following the recommendations for validation of bioanalytical methods of EMA guideline. The preparation of samples was based on the solid-phase extraction. The plasma from epileptic patients was spiked with 100 μL IS, while the blank plasma was previously spiked with CBZ standard for analyses. The mixture was vortex-mixed for 30 seconds and loaded into Oasis HLB cartridges (Waters Corporation, Milford, MA) previously conditioned with 1 mL methanol/water. This was followed by washing with 1 mL 5% methanol and elution with 1 mL of absolute methanol.
For the purpose of our investigation, we proposed the following working definitions:
individual therapeutic dose: a dose of a given drug which has not been changed for two or more consecutive visits in the history of the patient’s treatment.
drug responsiveness: a completely seizure-free condition for at least 1 year during treatment with CBZ.
drug resistance: the occurrence of at least four seizures over a period of 1 year during treatment with CBZ.
The Hardy–Weinberg equilibrium (HWE) for ABCB1 C3435T SNP was determined using an online calculator (http://ihg2.helmholtz-muenchen.de/cgi-bin/hw/hwa1.pl). The descriptive statistical analysis was done using the GraphPad Prism version 5.00 for Windows (GraphPad Software Inc, La Jolla, CA). Kruskal–Wallis and Mann–Whitney tests (P < 0.05) were used to compare the CBZ responsiveness doses between patients with different genotypes.
Results
The CC, CT, and TT genotypes were identified in 162 patients. No statistically significant differences were found in the allelic frequency and genotype distribution between CBZ-resistant and CBZ-responsive epilepsy patients. The distribution of all genotype frequencies did not deviate significantly from Hardy–Weinberg expectations ().
Table 2 Allele and genotype distribution in epilepsy patients from the Republic of Macedonia
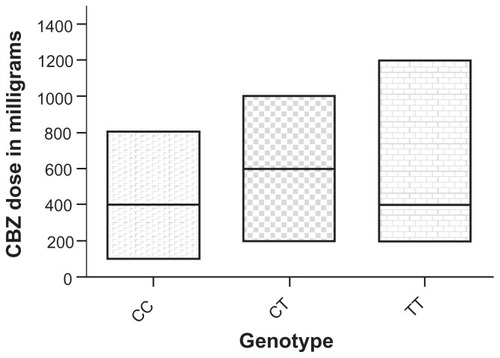
The results showed that CBZ-responsive patients with a TT genotype need a higher average maintenance dose of carbamazepine as a monotherapy (650 ± 343 mg/day) in comparison with patients with CC and CT genotypes (387 ± 217 mg/day and 548 ± 204 mg/day respectively). The values for median of CBZ doses were: 400 mg CBZ/day for TT, 600 mg CBZ/day for CT and 400 mg CBZ/day for CC genotypes (). The differences in doses among patients with different genotypes were not statistically significant ().
Table 3 Distribution of CBZ dose among CBZ-responsive patients with different genotypes from the Republic of Macedonia
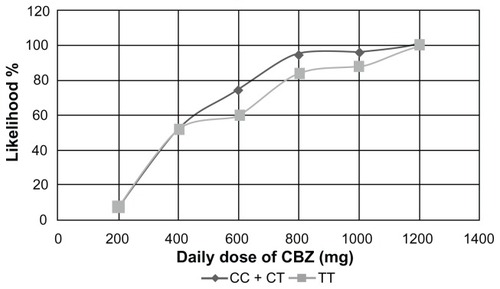
Our results indicate that the likelihood of a positive response to a CBZ monotherapy at an initial dose of 400 mg is 68%, 44.4%, and 52% in patients with CC, CT, and TT genotypes respectively (). Patients treated with 600 mg, 800 mg, 1000 mg, and 1200 mg had the highest likelihood of response if they had the CC genotype, followed by the CT and TT genotypes. The positive response in the group of patients with CC + CT genotypes in comparison with the TT genotype was 53% versus 52%, 74% versus 60%, 94% versus 84%, 96% versus 88%, and 100% versus 100%, when treated with 400 mg, 600 mg, 800 mg, 1000 mg, and 1200 mg daily doses respectively ().
Figure 2 Likelihood of a positive response to CBZ therapy between patients with different genotypes.
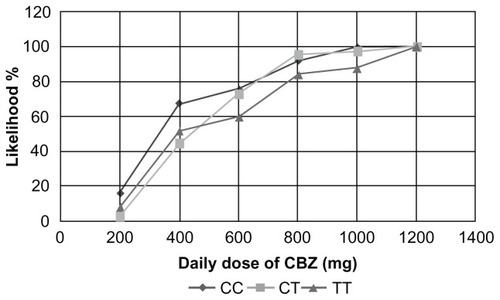
Figure 3 Likelihood of a positive response to CBZ therapy between patients with CC + CT genotypes vs TT genotype.
The in vivo analysis of expression of the ABCB1 C3435T variant showed a slightly higher expression of the C versus T allele in PBMC, (C/T allele = 48% ± 5%) ().
Figure 4 Likelihood of a positive response to carbamazepine therapy in patients with CC+CT genotypes vs TT genotypes.
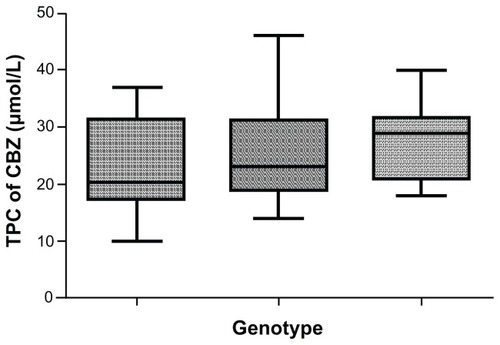
The CC genotype was also associated with lower average total plasma CBZ levels (23 ± 7.850 μmol/L) in comparison with the TT genotype (27.29 ± 6.326 μmol/L) (). The values for median of total plasma CBZ levels were 20 μmol/L for the CC, 23 μmol/L for the CT, and 29 μmol/L for the TT genotype.
Discussion
We evaluated the influence of the C3435T as a predictive factor for the efficacy of the CBZ therapy in patients with epilepsy. Our study demonstrated no differences in allele frequency and genotype distribution between patients with CBZ-resistant and CBZ-responsive epilepsy, suggesting that the variant is not a major factor which influences the responsiveness of patients to this drug. Although conflicting results were published in several smaller studies,Citation2–Citation5 a recently published meta-analysis of 23 studies (7067 patients) indicated no significant association of ABCB1 alleles, genotypes and haplotypes with the response to treatment in the overall population or in each ethnicity,Citation15 which is in line with our findings.
In this study we detected a trend indicating that patients with the TT genotype require a higher dose of CBZ in comparison with patients with the CT and CC genotypes, which was also followed by corresponding differences in plasma CBZ levels. These differences did not reach statistical significance, most probably due to the small number of patients in each group. However, the assumption that the genotype of the ABCB1 C3435T SNP might be clinically relevant was supported by the findings of a higher probability of response of CC genotype patients in comparison with patients with CT and TT genotypes across a wide range of doses.
The influence of the ABCB1 C3435T polymorphism on Pgp expression and function is still not clear. We detected a minor difference (C/T = 48%) in the expression levels between the two alleles in peripheral blood mononuclear cells of heterozygotes for the variant using a highly sensitive assay that is able to detect differences in expression of less than 10% between allelles.Citation16 This difference of ~5% in expression levels in heterozygotes might be translated into ~10% difference in homozygotes for the variant, which would explain the trend towards a dose-dependent efficacy of a CBZ treatment in patients with different genotypes. Whether the differences detected in the expression level of ABCB1 in PBMC are accentuated in therapeutically more relevant tissues, such as enterocytes, blood–brain barrier or nerve cells, is a subject of further evaluation. It is also possible, as suggested by Kimchi-Sarfaty et al,Citation17 that synonymous SNPs have functional effect by affecting the timing of cotranslational folding and insertion of Pgp into the membrane, thus altering the structure of substrate and/or inhibitor interaction sites. However, we cannot exclude the possibility that these minor differences are not functionally relevant and that there might be another linked nonsynonymous SNP in our population that primarily influences the activity levels of P-glycoprotein. Further studies for complete sequencing of the ABCB1 gene as well as determination of both mRNA and protein expression levels in patients with different variants from our population should clarify this issue.
However, our data suggest that even minor differences in expression of a certain variant might result in clinically relevant differences in the efficacy of the therapy in patients treated with different initial doses of CBZ. A larger prospective study designed to address and clarify this issue is warranted.
Disclosure
The authors report no conflicts of interest in this work.
References
- LöscherWPotschkaHRole of multidrug transporters in pharmaco-resistance to antiepileptic drugsJ Pharmacol Exp Ther2002301171411907151
- OwenAPirmohamedMTetteyJNMorganPChadwickDParkBKCarbamazepine is not a substrate for P-glycoproteinBr J Clin Pharmacol200151434534911318771
- PotschkaHFedrowitzMLoscherWP-glycoprotein and multidrug resistance-associated protein are involved in the regulation of extracellular levels of the major antiepileptic drug carbamazepine in the brainNeuroreport200112163557356011733711
- MainesLWAntonettiDAWolpertEBSmithCDEvaluation of the role of P-glycoprotein in the uptake of paroxetine, clozapine, phenytoin and carbamazepine by bovine retinal endothelial cellsNeuropharmacology200549561061715961125
- RiversFO’BrienTJCallaghanRExploring the possible interaction between anti-epilepsy drugs and multidrug efflux pumps; in vitro observationsEur J Pharmacol20085981–31818835265
- FrommMFImportance of P-glycoprotein at blood-tissue barriersTrends Pharmacol Sci200425842342915276711
- FungKLGottesmanMMA synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein functionBiochim Biophys Acta20091794586087119285158
- TateSKDepondtCSisodiyaSMGenetic predictors of the maximum doses patients receive during clinical use of antiepileptic drugs carbamazepine and phenytoinProc Natl Acad Sci U S A2005102155507501215805193
- SiddiquiAKerbRWealeMEAssociation of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1N Engl J Med2003348151442144812686700
- ZimprichFSunder-PlassmannRStogmannEAssociation of an ABCB1 gene haplotype with pharmacoresistance in temporal lobe epilepsyNeurology20046361087108915452305
- HungCCTaiJJLinCJLeeMJLiouHHComplex haplotypic effects of the ABCB1 gene on epilepsy treatment responsePharmacogenomics20056441141716004559
- TanNCHeronSESchefferIEFailure to confirm association of a polymorphism in ABCB1 with multidrug-resistant epilepsyNeurology20046361090109215452306
- SillsGJMohanrajRButlerELack of association between the C3435T polymorphism in the human multidrug resistance (MDR1) gene and response to antiepileptic drug treatmentEpilepsia200546564364715857428
- EfremovDGDimovskiAJHuismanTHThe -158(C→T) promoter mutation is responsible for the increased transcription of the 3’γ gene in the Atlanta type of hereditary persistence of fetal hemoglobinBlood19948311335033557514907
- HaerianBSLimKSTanCTRaymondAAMohamedZAssociation of ABCB1 gene polymorphisms and their haplotypes with response to antiepileptic drugs: a systematic review and meta-analysisPharmacogenomics201112571372521391884
- EfremovDGDimovskiAJSukarovaEGamma-mRNA and Hb levels in beta–thalassaemiaBr J Haematol19948823113177528532
- Kimchi-SarfatyCOhJMKimIWA “silent” polymorphism in the MDR1 gene changes substrate specificityScience2007315511852552817185560