Abstract
Prosopagnosia, the inability to recognize faces, has a history going back to Charcot and Hughlings-Jackson, but was first named by Bodamer in 1947. Its anatomical loci are still unclear. However, progressive prosopagnosia is normally linked to right dominant temporal lobe atrophy, and diagnosed as part of frontotemporal lobar degeneration. Here we report a case of prosopagnosia linked to posterior cortical atrophy. Although case reports of posterior cortical atrophy-prosopagnosia do already exist, it is normally described as an accessory symptom. The interest of our own posterior cortical atrophy patient, possibly the first such case, is that he had a rare apperceptive type of prosopagnosia unrelated to the associative, frontotemporal lobar degeneration-type.
Introduction
Progressive prosopagnosia was first reported by Evans et alCitation1 following the work of Tyrrell et al.Citation2 These cases have right temporal lobe atrophy. As to left temporal lobe atrophy: it is known that progressive fluent aphasia, also known as semantic dementia, is a subtype of frontotemporal lobar degeneration.Citation3 Progressive prosopagnosia, on the other hand, may represent a right hemisphere type semantic dementia.Citation1
Posterior cortical atrophy (PCA) presents visual agnosia including prosopagnosia.Citation4,Citation5 But in most PCA cases the atrophic changes start with the occipitoparietal lobe where visuospatial information is processed.Citation4,Citation5 Thus prosopagnosia reports as an accessory symptom.Citation4,Citation5
The first ventral-type PCA report was by Beversdorf and Heilman,Citation6 and the patient presented alexia for music and words with left dominant brain atrophy. Here we report, what we believe is a first case of right dominant ventral PCA, with a primary prosopagnosia that is both progressive and apperceptive. Apperceptive type prosopagnosia involves disability for: unknown face matching, recognition of facial expression, sex, and age. The associative type patient does not have these problems but cannot name famous faces, because of an inability to match visual perception to memory.Citation7
We describe our case vis à vis established descriptions of prosopagnosia, and summarize the findings in a schematic diagram. Furthermore, we discuss the relationship of PCA’s neuropsychological symptoms to atypical dementia.
Case report
A 58-year-old, right-handed man developed difficulty in recognizing familiar people. The case came to our attention 3 years after appearance of the first symptoms. At first, the patient devised polite social strategies to get around his problem, but it then become too difficult for him to manage. Finally, when he failed to recognize his own family members, he visited the ophthalmologist. The evaluation was normal. He continued working as an engineer, although he needed assistance recognizing people. Furthermore, he devised an ingenious strategy for recognizing family members in a given situation. Each member of the family used his/her own teacup with a picture on it, and each had a particular seat at the dinner table. By allocating cups to sitting places he was able to establish to whom he was speaking at family meals. He was also able to drive around without getting lost. Finally he realized something was wrong and came to the hospital. His general physical condition was good, but a neurological examination revealed left lower quadrantanopia, but no signs of Gerstmann’s or Bálint’s syndromes. There was no rigidity or Parkinsonism.
His Mini-Mental State Examination (MMSE) score was 28/30 points with errors in subtraction and copying of a double pentagon. He had severe prosopagnosia and disability in famous face naming, famous face pointing, discriminating and matching unfamiliar faces, and recognizing facial expression, sex, and age. He also presented picture agnosia. Primary visual functions and recognition of symbols including characters and words were relatively preserved. He showed mild, left, hemispatial agnosia. shows his visual perception test for agnosia as used for Japanese patients.Citation8 He presented no obvious signs of aphasia, and there was no ideomotor, ideational, or constructional apraxia. He was able at that stage to manage ordinary life and his Barthel IndexCitation9 score was 100/100.
Table 1 Visual perception test for agnosiaCitation8
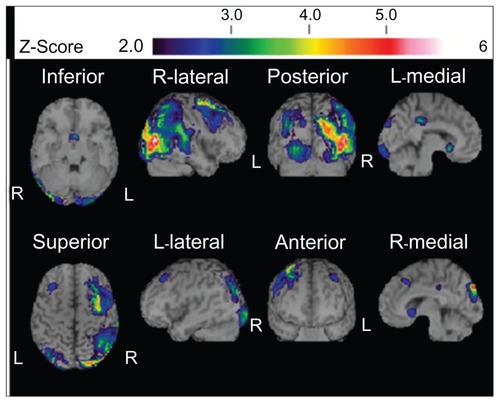
shows temporo-parieto-occipital lobe atrophy with right-side dominance on magnetic resonance imaging: 99mTc-ethyl cysteinate dimer SPECT using the easy Z-score imaging system (eZIS)Citation10 showed decreased blood flow in the temporo-parieto-occipital lobe ().
Figure 1 Magnetic resonance imaging coronal sections of fluid-attenuated inversion recovery images on first examination. The calcarine sulcus (arrow) and collateral sulcus (arrow head) were broader on the right than the left. The finding was temporo-parieto-occipital lobe atrophy with right-side dominance.
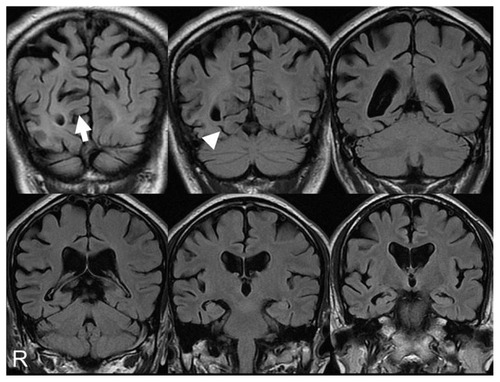
Figure 2 99mTc-ethyl cysteinate dimer SPECT using eZISCitation10 on first examination showed decreased blood flow in the temporo-parieto-occipital lobe with right-side dominance.
Figure 3 18F-fluorodeoxyglucose positron emission tomography, 7 months after first examination, showing hemihypometabolism, especially from the inferior lateral temporal lobe to the lateral occipital lobe.
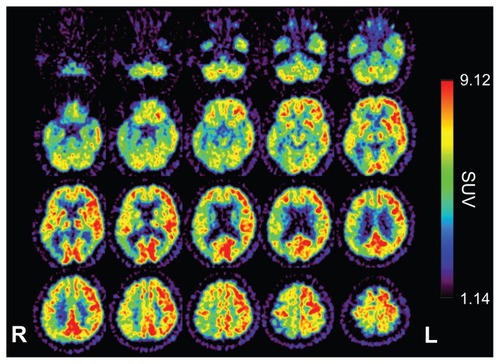
On follow-up examination, visual agnosia gradually progressed. Positron emission tomography (PET) was carried out 7 months after first examination: 18F-fluorodeoxyglucose PET revealed right hemihypometabolism, especially from the inferior lateral temporal lobe to the lateral occipital lobe (). 11C-labeled Pittsburgh Compound-B PET showed no accumulation of amyloid beta in the cerebral cortex. Eleven months after the first examination magnetic resonance imaging () and SPECT using eZISCitation10 () showed progression of temporo-parieto-occipital lobe atrophy with right-side dominance. The visual perception tests for agnosia and MMSE were retested 14 months after our first examination: the patient completed no task correctly with regard to face or object recognition. Previously preserved recognition of symbols was also lost. His ability on primary visual functions like perception of line length was also impaired. The MMSE score was 24/30 with errors in orientation to time (−1 point), recall (−1 point), reading (−1 point), complex commands (−3 points), and copying of a double pentagon. He also now showed Bálint’s syndrome, and failed to register the presence of members of medical staff until they spoke. On occasions he could distribute letters to family members by reading names on envelopes. All these observations were noted 14 months after our first meeting. We believe our patient met the criteria for dementia at the second evaluation, because he had cognitive dysfunctions in a number of regions as shown by MMSE, and was unable to continue working. The Barthel IndexCitation8 score was 60/100 because he needed help managing activities that involved visual ability. Furthermore, he gradually showed rigidity of the left wrist. Other Parkinsonism was not confirmed.
Figure 4 Magnetic resonance imaging coronal sections of fluid-attenuated inversion recovery images 11 months after first examination showed progression of temporo-parieto-occipital lobe atrophy with right-side dominance.
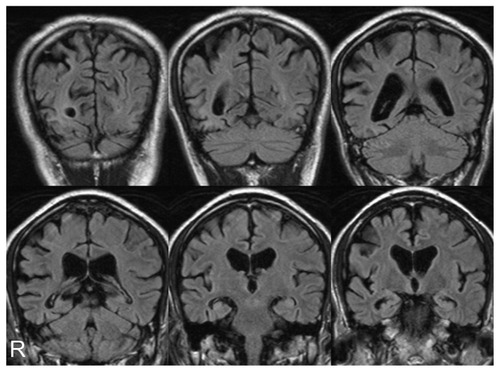
Figure 5 99mTc-ethyl cysteinate dimer SPECT using the eZISCitation10 11 months after first examination showed decreased blood flow in the temporo-parieto-occipital lobe with right-side dominance.
Discussion
We have described a patient with progressive prosopagnosia whose visual symptoms were identified using the visual perception test for agnosia,Citation8 a test standardized in 1995 using 335 Japanese subjects. The clinical diagnosis was PCA.
PCA was named by Benson et al.Citation11 It is an atypical dementia with lesion confined to the posterior association area. Visual dysfunction is a primary symptom, but there is no memory disturbance at an earlier stage. Primary visual dysfunction is now accepted as symptomatic of PCA.Citation4
BodamerCitation12 first named prosopagnosia on the basis of cerebrovascular disease, or trauma. More recently, activation studies with normal subjects have established the “fusiform face area” as important to face recognition.Citation13 We also confirmed the importance of the fusiform face area in an earlier paper on autopsy of cerebral infarction with prosopagnosia.Citation14
Progressive prosopagnosia has been linked to localized cortical degeneration similar to progressive aphasia: Tyrrell et alCitation2 and Evans et al.Citation1 Gentileschi et al,Citation15 Gainotti et al,Citation16 and Joubert et alCitation17 also reported cases of progressive prosopagnosia. These cases could not recognize familiar faces, but had almost intact primary face perception skills, so the symptoms were diagnosed as being part of associative prosopagnosia.Citation7 The reported lesions were also similar to right temporal lobe atrophy. These suggest that progressive associative prosopagnosia with right temporal atrophy is in contrast to progressive aphasia with left temporal atrophy in semantic dementia.Citation1
We believe our PCA case is an example of apperceptive prosopagnosia () due to the patient’s inability to: (1) discriminate and match unfamiliar faces, (2) recognize facial expressions, and (3) discriminate sex and age, while (4) retaining primary visual function. PCA is currently considered a degenerative disease with the prosopagnosia secondary to enlargement of the lesion from the dorsal to the ventral visual pathway.Citation4,Citation5 However, PCA may present apperceptive prosopagnosia with more primary visual dysfunctions linked to posterior cerebral atrophy. In the visual pathway, perception and recognition proceed from the occipital to the temporal (ventral pathway), or to the parietal lobe (dorsal pathway). Simultaneously they integrate with other modalities, or match to memory. There is functional evidence, therefore, for the progressive prosopagnosia of frontotemporal lobar degeneration to be regarded as associative,Citation1,Citation2,Citation15–Citation17 while that of PCA is apperceptive.
PCA presents mainly dorsal visual stream dysfunction.Citation4 While most PCA cases show Alzheimer disease pathology, the anatomical evidence is parietal-occipito-temporal.Citation5 The patient we describe here had ventral type PCA with prosopagnosia with no evidence of amyloid beta accumulation as indicated by Pittsburgh Compound-B-PET. Furthermore, our patient showed symptoms of atrophy that were right dominant. He also had gradual rigidity of the left wrist. And while it is possible he had a tauopathy, such as corticobasal degeneration, he did not have Alzheimer’s disease.Citation5
We believe this may be a first case of primary progressive prosopagnosia of ventral PCA with right dominancy. If so, it adds to the evidence for ventral PCA with left dominancy reported by Beversdorf and Heilman.Citation6 The Beversdorf case presented alexia, and had visual agnosia for music and words with left dominancy.
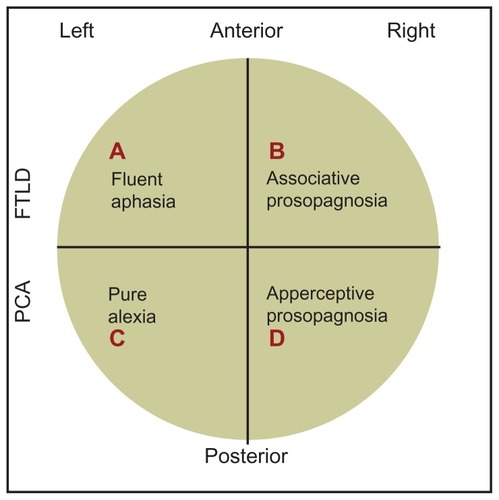
To summarize these findings and our hypothesis, we provide a simple schematic diagram showing the relative loci of associative and apperceptive prosopagnosia, fluent aphasia, and pure alexia (). If our own findings are accurate, then our case history of PCA/apperceptive prosopagnosia will relate to area D in the model: that is to the ventral region of the right posterior area of the brain.
Figure 6 A simple, schematic diagram of the relative loci of associative and apperceptive prosopagnosia, fluent aphasia, and pure alexia. Our own case history of PCA/apperceptive prosopagnosia relates to area D in the ventral part of the right posterior area of the brain.
Acknowledgments
Supported by the Tamagawa University Center of Excellence under the Ministry of Education, Culture, Sports, Science and Technology (MK); Core Research for Evolutionary Science and Technology (CREST 17022035); a Grant-in-Aid for Scientific Research on Innovative Areas, “Face perception and recognition,” from MEXT (21119518); a Grant-in-Aid for Scientific Research on Priority Areas – System Study on High-Order Brain Functions from Showa University Grant-in-Aid for Innovative Collaborative Research Projects; a Special Research Grant-in-Aid for Development of Characteristic Education from MEXT; a Grant-in-Aid for Comprehensive Research on Dementia (No 1103404) from the Ministry of Health, Labor, and Welfare of Japan (KeI).
Disclosure
The authors report no conflicts of interest in this work.
References
- EvansJJHeggsAJAntounNHodgesJRProgressive prosopagnosia associated with selective right temporal lobe atrophy. A new syndrome?Brain1995118Pt 11137894996
- TyrrellPJWarringtonEKFrackowiakRSRossorMNProgressive degeneration of the right temporal lobe studied with positron emission tomographyJ Neurol Neurosurg Psychiatry19905312104610502292695
- HodgesJRPattersonKOxburySFunnellESemantic dementia. Progressive fluent aphasia with temporal lobe atrophyBrain1992115Pt 6178318061486461
- McMonaglePDeeringFBerlinerYKerteszAThe cognitive profile of posterior cortical atrophyNeurology200666333133816476930
- Tang-WaiDFGraff-RadfordNRBoeveBFClinical, genetic, and neuropathologic characteristics of posterior cortical atrophyNeurology20046371168117415477533
- BeversdorfDQHeilmanKMProgressive ventral posterior cortical degeneration presenting as alexia for music and wordsNeurology19985036576599521252
- De RenziEFaglioniPGrossiDNichelliPApperceptive and associative forms of prosopagnosiaCortex19912722132211879150
- MahoneyFIBarthelDWFunctional evaluation: the Barthel indexMd State Med J196514616514258950
- Japan Society for Higher Brain DysfunctionVisual Perception Test for AgnosiaTokyo, JapanShinkoh Igaku Shuppan1998 (in Japanese)
- MizumuraSKumitaSStereotactic statistical imaging analysis of the brain using the easy Z-score imaging system for sharing a normal databaseRadiat Med200624754555217058152
- BensonDFDavisRJSnyderBDPosterior cortical atrophyArch Neurol19884577897933390033
- BodamerJDie prosopagnosiaArch Psychiatr Nervenkr1947179653
- KanwisherNMcDermottJChunMMThe fusiform face area: a module in human extrastriate cortex specialized for face perceptionJ Neurosci19971711430243119151747
- SugimotoAMillerMWKawaiYShiotaJKawamuraMAnother piece in the jigsaw: a case report of prosopagnosia with symptomatological, imaging and post mortem anatomical evidenceCortex2011 [Epub ahead of print.]
- GentileschiVSperberSSpinnlerHCrossmodal agnosia for familiar people as a consequence of right infero polar temporal atrophyCogn Neuropsychol200118543946320945224
- GainottiGBarbierAMarraCSlowly progressive defect in recognition of familiar people in a patient with right anterior temporal atrophyBrain2003126Pt 479280312615639
- JoubertSFelicianOBarbeauEProgressive prosopagnosia: clinical and neuroimaging resultsNeurology200463101962196515557526