Abstract
In the last two decades, a growing body of evidence has shown that lithium has several neuroprotective effects. Several neurobiological mechanisms have been proposed to underlie these clinical effects. Evidence from preclinical studies suggests that neuroprotection induced by lithium is mainly related to its potent inhibition of the enzyme glycogen synthase kinase-3β (GSK-3β) and its downstream effects, ie, reduction of both tau protein phosphorylation and amyloid-β42 production. Additional neuroprotective effects include increased neurotrophic support, reduced proinflammatory status, and decreased oxidative stress. More recently, neuroimaging studies in humans have demonstrated that chronic use is associated with cortical thickening, higher volume of the hippocampus and amygdala, and neuronal viability in bipolar patients on lithium treatment. In line with this evidence, observational and case registry studies have shown that chronic lithium intake is associated with a reduced risk of Alzheimer’s disease in subjects with bipolar disorder. Evidence from recent clinical trials in patients with mild cognitive impairment suggests that chronic lithium treatment at subtherapeutic doses can reduce cerebral spinal fluid phosphorylated tau protein. Overall, convergent lines of evidence point to the potential of lithium as an agent with disease modifying properties in Alzheimer’s disease. However, additional long-term studies are necessary to confirm its efficacy and safety for these patients, particularly as chronic intake is necessary to achieve the best therapeutic results.
Introduction
Lithium salts have been used in psychiatry since the end of the 1940s as a mood stabilizer for the treatment of affective disorders,Citation1 in particular bipolar disorder and as add-on therapy in treatment-resistant major depression. All major treatment guidelines recommend lithium, alone or in association, as a first-line agent for the treatment of acute mania and bipolar depression as well as for the prophylaxis of recurrent affective episodes in these patients (reviewed by Nivoli et al in 2011).Citation2 A growing body of evidence suggests that the benefits of lithium extend beyond mood stabilization. In particular, long-term lithium treatment has been associated with increased neuroprotection against neuronal injury not only in mood disorders but also in neurodegenerative diseases, such as Alzheimer’s disease (AD).Citation3,Citation4 This article aims to review the presumed mechanisms by which lithium may exert its neuroprotective effects and how such mechanisms may help to delay the progression of AD.
Lithium: pharmacological mechanisms
The specific pharmacological mechanisms of lithium are not clear, but current evidence suggests the direct involvement of classic pharmacologic targets, such as cell surface receptors or the direct modulation of neurotransmitters,Citation5 second messenger systems, and transcriptional factors. Lithium ion directly competes with magnesium (Mg2+) due to its similar ionic radii (0.60 Å and 0.65 Å, respectively) and its ability to bind to similar substrates’ sites. Therefore, lithium can inhibit Mg2+-dependent enzymatic activity.Citation6,Citation7 The competition between lithium and Mg2+ by substrate sites has a significant influence on the activity of several enzymes on intracellular pathways relevant to neuropsychiatric and neurodegenerative disorders, eg, glycogen synthase kinase-3β (GSK-3β), inositol monophosphatase (IMP), and Akt/β-arrestin-2.
Lithium inhibits GSK-3β activity by two distinct and interrelated mechanisms. GSK-3β is a constitutively active enzyme by the binding of Mg2+ to its catalytic core. By dislocating Mg2+ from the enzyme catalytic core, lithium directly inhibits the enzyme activity.Citation8,Citation9 In addition, lithium can also inhibit GSK-3β activity by inducing the phosphorylation of the serine-9 residue, leading to conformational changes and inactivation. This indirect mechanism is due to the lithium-induced activation of intracellular kinases (eg, Akt) or by inhibiting intracellular phosphatases (eg, protein phosphatase-2).Citation10–Citation12 In addition to the inhibition of GSK-3β activity, lithium can also reduce enzyme expression at the gene level.Citation13
The inhibition of IMP and inositol polyphosphate 1-phosphatase activity is another putative mechanism of action of lithium. Lithium also causes a direct inhibition of IMP activity by noncompetitive dislocation of Mg2+ from enzyme catalytic sites.Citation14 An important consequence of IMP and inositol polyphosphate 1-phosphatase inhibition is the significant reduction of inositol triphosphate formation, which leads to the modulation of many intracellular pathways relevant to neuropsychiatric disorders, in particular the stimulation of autophagy ().Citation15
Figure 1 Putative mechanisms of action of lithium. (A) Lithium inhibits GSK-3β activity by dislocating magnesium ions from the catalytic core (direct pathway). Lithium can also inhibit GSK-3β activity by increasing the phosphorylation of Ser9 residue. This is secondary to the lithium-induced activation of kinases (eg, Akt) and/or inhibition of phosphatases (eg, protein phosphatase-2) (indirect pathway). (B) Lithium increases autophagy by inhibiting the activity of IMP and IPP and, consequently, reducing IP3 levels. (C) Lithium can also directly stimulate the production of neurotrophic factors BDNF and VEGF by activation of gene expression in the nucleus.
Abbreviations: BDNF, brain-derived neurotrophic factor; GSK-3β, glycogen synthase kinase-3β; IMP, inositol monophosphatase; IP3, inositol triphosphate; IPP, inositol polyphosphate 1-phosphatase; Ser9, serine-9; VEGF, vascular endothelial growth factor.
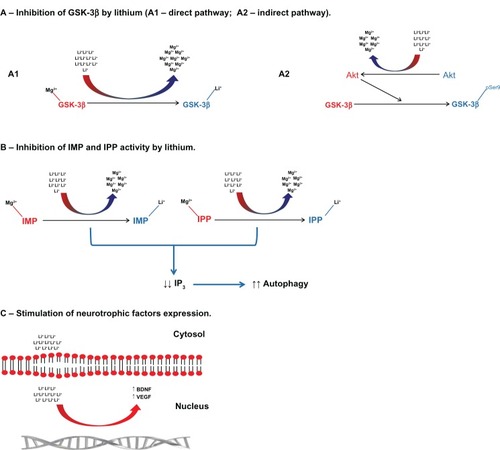
Another mechanism by which lithium can exert its action is by stimulating gene expression and the release of neurotrophic factors, eg, brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor.Citation16,Citation17 These effects are particularly interesting as (1) reduced neurotrophic factors play an important role in the physiopathology of AD and affective disorders, and (2) restoration of neurotrophic factors’ levels may be a therapeutic target for these disorders.Citation18
Evidence for the neuroprotective effects of lithium from preclinical studies
The neuroprotective effects of lithium are due to its modulation on several biologic cascades. In neuronal cultures, lithium significantly reduces tau phosphorylationCitation19,Citation20 and amyloid-β42,Citation21,Citation22 and protects neurons against toxic effects and cell death secondary to amyloid-β42 exposure.Citation23,Citation24 Lithium is also able to stimulate the proliferation of progenitor cells in cultured neuronal cellsCitation25,Citation26 and the expression of antiapoptotic proteins, eg, B-cell lymphoma-2.Citation27,Citation28 These neuroprotective effects of lithium are mediated, at least in part, by the inhibition of GSK-3β activity in neurons.Citation29
Animal studies provide additional evidence for the neuroprotective effects of lithium. The most consistent effect of chronic lithium treatment is the significant reduction of tau phosphorylation either in mice bearing amyloid precursor protein (APP)-related genetic mutations or wild-type animals.Citation30–Citation33 The effects of lithium on tau phosphorylation are mediated by the inhibition of GSK-3β activity. In transgenic mice bearing mutations on the APP gene, lithium reduced amyloid-β42 production by direct modulation of APP processing and also by inhibition of GSK-3β activity.Citation22,Citation34–Citation37 These effects of lithium on tau phosphorylation and amyloid-β42 are, in general, accompanied by a significant improvement in memory deficits.Citation36,Citation37
The neuroprotective effects of lithium in APP transgenic mice might be time dependent. In a recent study, lithium treatment when started earlier in 2-month-old mice had a significantly stronger effect in reducing AD-related neuropathology and memory impairment than when started later in 6-month-old mice.Citation38 On the other hand, lithium treatment can prevent amyloid-induced neurotoxicity, in particular tau phosphorylation and neuronal death.Citation37,Citation39 Finally, the inhibition of GSK-3β activity secondary to lithium treatment can increase synaptic plasticity, facilitate long-term potentiation, and consequently improve memory performance in animals.Citation40–Citation44
Autophagic processes are important in the degradation and clearance of amyloid-β and phosphorylated tau proteins in neurons. Impairment in autophagy is observed in AD and may contribute to the accumulation of extracellular deposits of amyloid-β42 in neuritic plaques and intracellular deposits of hyperphosphorylated tau proteins in neurofibrillary tangles.Citation45–Citation47 Lithium treatment inhibits IMP/inositol polyphosphate 1-phosphatase activity, which decreases inositol triphosphate formation and, in turn, stimulates the autophagic processes in neurons. The stimulation of autophagy by lithium leads to the more effective clearance of amyloid-β42 and hyperphosphorylated tau protein,Citation15,Citation48,Citation49 protecting neurons from their deleterious effects.
Another important neuroprotective effect of lithium is stimulation of the synthesis and release of neurotrophic factors, in particular BDNF and vascular endothelial growth factor. Increased availability of neurotrophic factors protects neurons against amyloid-β42 neurotoxic effect, stimulates hippocampal neurogenesis, and positively regulates cell survival.Citation50–Citation53 These biological effects are accompanied by a significant reduction in amyloid-related pathology, memory improvement, and slow rates of age-related memory decline in animal models of AD.
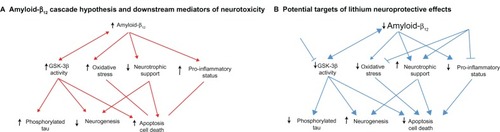
inflammation is another important component of AD physiopathology and can accelerate neurodegenerative changes in animal models of this disorder.Citation54 Lithium can regulate the inflammatory processes by lessening the proinfammatory response. Lithium can reduce the production of interleukin-1β and tumor necrosis factor-α induced by lipopolysaccharide-induced inflammation in glial cellsCitation55 and reduce microglia activation secondary to ischemic insults in mice.Citation56 Chronic lithium treatment can attenuate arachidonic acid production, an essential feature of unspecific inflammatory response ().Citation57
Figure 2 The mechanism and targets of lithium against Alzheimer’s disease-related pathology. (A) The main components of the amyloid-β cascade hypothesis of Alzheimer’s disease pathophysiology. (B) The possible targets and effects of lithium in the amyloid-β cascade.
Abbreviation: GSK-3β, glycogen synthase kinase-3β.
Evidence for the neuroprotective effects of lithium from clinical studies
In addition to the evidence for the neuroprotective properties of lithium in preclinical studies, a growing body of evidence corroborates its neuroprotective effects in human subjects as well. Most of the evidence derives from studies of subjects with bipolar disorder. Case registry studies found a lower risk for incident dementia, in particular of AD, in bipolar patients after long-term lithium use.Citation58,Citation59 In a retrospective study, Terao et al found that patients on chronic lithium treatment showed lower rates of cognitive decline as measured by the Mini-Mental State Examination.Citation60 A prospective observational study showed that older bipolar patients on chronic lithium treatment had a significantly lower incidence of AD compared to those with no lithium exposure.Citation61 In this study, the incidence rates of AD in the group treated with lithium was comparable to those observed in the general population,Citation62 suggesting that chronic lithium treatment can be protective against the development of AD in high-risk populations.
The exact mechanisms by which lithium may reduce the risk of AD in bipolar subjects are unclear, but may involve the modulation of multiple cascades that are abnormal in both disorders. Lithium treatment can significantly increase GSK-3β phosphorylation and, consequently, reduce enzymatic activity in the leukocytes of patients with bipolar disorder and recurrent major depression.Citation63,Citation64 The inhibition of GSK-3β in vivo can mediate the therapeutic effect of lithium as a mood stabilizer as well as its neuroprotective effect in humans.
Several studies showed that lithium treatment can significantly increase BDNF, which influences the response to treatment.Citation3,Citation65 In a clinical trial with patients in acute mania, de Sousa et al reported a significant increase in plasma BDNF levels after 4 weeks of treatment. However, increased BDNF levels were not associated with treatment response.Citation66 In addition, maintenance treatment with lithium was associated with a persistent high level of BDNF and reduced risk of affective episode relapse.Citation67
Studies have also evaluated the effect of lithium on inflammatory and oxidative stress markers. Lithium treatment of an acute mania episode was associated with a reduction in pro-oxidative stress markers, eg, thiobarbituric acid reactive substances.Citation68,Citation69 In addition, lithium treatment increased anti-oxidative stress markers and reduced pro-oxidative stress markers in healthy subjects.Citation70 A recent study demonstrated that patients with bipolar disorder who showed a good response to lithium also had a significant reduction in plasma tumor necrosis factor-α level; in contrast, the patients who did not respond well to lithium showed a significant increase in tumor necrosis factor-α levels.Citation71 Lithium can restore the balance in the production of interleukin-1β and interleukin-6 in monocytes of bipolar patients in vitro; this effect is similar to that observed in vivo.Citation72
Another line of evidence that demonstrates the potential for the neuroprotective effect of lithium comes from neuroimaging studies in subjects with bipolar disorder. Structural neuroimaging studies have demonstrated that short- and long-term lithium treatment was associated with increased hippocampal and amygdala volume, and cortical thickness.Citation73–Citation76 In addition, lithium treatment was associated with increased N-acetylaspartate and myo-inositol levels in magnetic resonance spectroscopy.Citation77,Citation78 These neuroimaging findings suggest that long-term lithium treatment may have a significant effect on synaptic density and neuronal vitality in bipolar patients.
Taken together, the findings from clinical studies, in particular with bipolar patients, support preclinical evidence that lithium can modulate several biologic cascades related to the physiopathology and progression of AD. Patients with mild cognitive impairment (MCI) and AD show higher GSK-3β activity.Citation79 Inhibition of this enzyme by lithium may help reduce amyloidogenesis and tau phosphorylation, core features of AD pathology. In addition, lower neurotrophic support is a common feature of AD and stimulation of the synthesis and release of neurotrophic factors can confer resilience against amyloid neurotoxicity and stimulate synaptogenesis and neurogenesis. Finally, increased proinflammatory and pro-oxidative status is common in AD and amplifies the secondary downstream damage due to amyloid-β deposition and tau hyperphosphorylation. The modulation of these cascades can help lessen amyloid and tau-induced neurotoxicity and cell death and, as a consequence, reduce the risk of progression from AD-related pathology.
Evidence of disease modification in AD
Despite the wealth of evidence from preclinical and clinical studies that lithium modulates biologic cascades related to AD and may have disease modifying properties against this disorder, few studies have actually addressed such potential in patients with AD or MCI. A small open-label trial, including 25 patients with mild to moderate AD, found no significant effects of lithium treatment on cognitive function over a 1-year treatment period.Citation80 Despite the high dropout rate of this study (only eight patients completed the 1-year treatment protocol), Macdonald et al suggested that treatment with lithium was relatively safe, with most dropouts due to mild and reversible side effects at therapeutic levels. A more recent clinical trial using a microdose of lithium (300 μg daily) over 18 months demonstrated a significant improvement in cognitive performance starting after 6 months of treatment, which persisted until the trial endpoint.Citation81
A single-blind clinical trial including 71 patients with mild to moderate AD found no significant benefit of a 10-week treatment of lithium at therapeutic levels (0.5–0.8 mmol/L) on cognitive performance.Citation82 In this study, Hampel et al also evaluated the impact of lithium on biomarkers related to AD and found no significant changes in cerebrospinal fluid concentrations of amyloid-β and phosphorylated tau and leukocyte phosphorylated GSK-3β levels. Nonetheless, the short treatment period may not have been sufficient for lithium to exert its neuroprotective effects in these patients. Secondary analysis of this trial showed that lithium treatment was associated with increased serum levels of BDNF and that in a subset of patients who had increased BDNF levels it showed significant improvement in cognitive performance.Citation83 The effect of lithium was selective to BDNF as there was no significant change in the levels of glial-derived neurotrophic factor either on cerebrospinal fluid or serum of AD patients after 10 weeks of lithium treatment.Citation84
Recently, a double-blind, placebo-controlled clinical trial was carried out to evaluate whether lithium at subtherapeutic levels (serum levels of 0.2–0.4 mmol/L) could delay the progression of amnestic MCI subjects to AD. It also evaluated the disease modifying properties in cascades related to the core physiopathologic features of AD in MCI subjects.Citation85 This study recruited 45 amnestic MCI subjects and preliminary analysis of the 1-year follow-up showed that amnestic MCI subjects on the lithium regimen presented stable cognitive performance and lower conversion rates to AD compared to subjects on placebo, although the difference was not statistically significant. Despite the lack of clear clinical benefit, amnestic MCI subjects on lithium showed a significant reduction in phosphorylated tau levels compared to subjects on placebo. Additional analyses revealed that the effect size of lithium on phosphorylated tau levels was even greater in MCI subjects who did not progress to AD on follow-up. Overall, these results suggest that long-term lithium has disease modifying properties on core physiopathologic features of AD and a marginal clinical benefit, mostly if started at the earlier stages of clinical and pathological disease processes.
Are we ready to use lithium in AD?
Despite the robust evidence for disease modifying properties of lithium on AD, derived from preclinical and clinical studies, its use is still not recommended. Larger, multicenter, long-term clinical trials are needed to assess the benefits of lithium on cognitive and functional performance as well as its power to delay the progression from preclinical to clinical states of AD. To evaluate the impact of chronic lithium treatment on the core physiopathologic processes in AD, these studies must include, as a primary and/or secondary outcome, biomarkers related to the core features of AD (eg, cerebrospinal fluid amyloid-β42 and phosphorylated tau proteins in structural neuroimaging, and/or amyloid imaging). Also, it is of the utmost importance to evaluate the optimum serum level to combine potential clinical benefit and patient safety.
Another important issue relates to patient safety and long-term lithium use. Older patients are particularly vulnerable to the side effects of lithium, with gastrointestinal disturbances and tremor the most common side effects reported. In general, they are mild and reversible but can be troublesome to patients and are common reasons for drug discontinuation. Also, renal dysfunction (including asymptomatic elevation of creatinine and renal insufficiency) and hypothyroidism can emerge during long-term treatment. They are, in general, manageable medical conditions but often lead to lithium discontinuation. In a safety analysis from a trial in older subjects with MCI, subtherapeutic doses of lithium (0.2–0.4 mmol/L) were safe and there were no significant changes in laboratorial parameters related to renal and thyroid function, hematologic parameters, and energetic metabolism.Citation86
Drug interaction is another major concern. Concomitant use of lithium with some drugs can potentiate the adverse events related to lithium either by increasing serum drug levels (eg, thiazide diuretics) or by potentiating renal dysfunction (eg, nonsteroidal antiinflammatory drugs). However, the use of subtherapeutic levels can minimize such risks.
Conclusion
Current evidence points to a potential role of lithium as a drug with disease modifying properties in AD. Nonetheless, it is very important to emphasize that the risk/benefit ratio of using lithium for neuroprotection is still very unclear and lithium should not yet be used for neuroprotection in older adults. Additional clinical trials are necessary to establish its efficacy from the clinical and biological perspective and also to establish the optimal dose regimen/plasma levels and length of drug use to attain the best clinical benefit.
Disclosure
The authors report no conflicts of interest in this work.
References
- CadeJFLithium salts in the treatment of psychotic excitementMed J Aust194921034935218142718
- NivoliAMColomFMurruANew treatment guidelines for acute bipolar depression: a systematic reviewJ Affect Disord20111291–3142620538341
- Machado-VieiraRManjiHKZarateCAJrThe role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesisBipolar Disord200911Suppl 29210919538689
- ForlenzaOVde PaulaVJMachado-VieiraRDinizBSGattazWFDoes lithium prevent Alzheimer’s disease?Drugs Aging201229533534222500970
- PasqualiLBuscetiCLFulceriFPaparelliAFornaiFIntracellular pathways underlying the effects of lithiumBehav Pharmacol20102156473492
- AmariLLaydenBRongQGeraldesCFMota de FreitasDComparison of fluorescence, 31P NMR, and 7Li NMR spectroscopic methods for investigating Li+/Mg2+ competition for biomoleculesAnal Biochem199927211710405286
- BirchNJLithium and magnesium-dependent enzymes [letter]Lancet1974278869659664138334
- KleinPSMeltonDAA molecular mechanism for the effect of lithium on developmentProc Natl Acad Sci USA19969316845584598710892
- RyvesWJHarwoodAJLithium inhibits glycogen synthase kinase-3 by competition for magnesiumBiochem Biophys Res Commun2001280372072511162580
- Chalecka-FranaszekEChuangDMLithium activates the serine/ threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neuronsProc Natl Acad Sci USA199996158745875010411946
- O’BrienWTHuangJBuccafuscaRGlycogen synthase kinase-3 is essential for β-arrestin-2 complex formation and lithium-sensitive behaviors in miceJ Clin Invest201112193756376221821916
- PanJQLewisMCKettermanJKAkt kinase activity is required for lithium to modulate mood-related behaviors in miceNeuropsychopharmacology20113671397141121389981
- MendesCTMuryFBde Sa MoreiraELithium reduces Gsk3b mRNA levels: implications for Alzheimer diseaseEur Arch Psychiatry Clin Neurosci20092591162218932008
- PatelSYenushLRodriguezPLSerranoRBlundellTLCrystal structure of an enzyme displaying both inositol-polyphosphate-1 -phosphatase and 3′-phosphoadenosine-5′-phosphate phosphatase activities: a novel target of lithium therapyJ Mol Biol2002315467768511812139
- SarkarSFlotoRABergerZLithium induces autophagy by inhibiting inositol monophosphataseJ Cell Biol200517071101111116186256
- SugawaraHIwamotoKBundoMEffect of mood stabilizers on gene expression in lymphoblastoid cellsJ Neural Transm2010117215516419949822
- YasudaSLiangMHMarinovaZYahyaviAChuangDMThe mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neuronsMol Psychiatry2009141515917925795
- DinizBSTeixeiraALBrain-derived neurotrophic factor and Alzheimer’s disease: physiopathology and beyondNeuromolecular Med201113421722221898045
- FuZQYangYSongJLiCl attenuates thapsigargin-induced tau hyperphosphorylation by inhibiting GSK-3β in vivo and in vitroJ Alzheimers Dis20102141107111721504119
- TakahashiMYasutakeKTomizawaKLithium inhibits neurite growth and tau protein kinase I/glycogen synthase kinase-3β-dependent phosphorylation of juvenile tau in cultured hippocampal neuronsJ Neurochem19997352073208310537067
- EsselmannHMalerJMKunzNLithium decreases secretion of Aβ1-42 and C-truncated species Aβ1-37/38/39/40 in chicken telencephalic cultures but specifcally increases intracellular Aβ1-38Neurodegener Dis200414–523624116908996
- PhielCJWilsonCALeeVMKleinPSGSK-3α regulates production of Alzheimer’s disease amyloid-β peptidesNature2003423693843543912761548
- AlvarezGMunoz-MontanoJRSatrusteguiJAvilaJBogonezEDiaz-NidoJLithium protects cultured neurons against β-amyloid-induced neurodegenerationFEBS Lett1999453326026410405156
- AlvarezGMunoz-MontanoJRSatrusteguiJAvilaJBogonezEDiaz-NidoJRegulation of tau phosphorylation and protection against β-amyloid-induced neurodegeneration by lithium. Possible implications for Alzheimer’s diseaseBipolar Disord20024315316512180271
- HashimotoRSenatorovVKanaiHLeedsPChuangDMLithium stimulates progenitor proliferation in cultured brain neuronsNeuroscience20031171556112605892
- KimJSChangMYYuITLithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivoJ Neurochem200489232433615056276
- ChenCLLinCFChiangCWJanMSLinYSLithium inhibits ceramide- and etoposide-induced protein phosphatase 2A methylation, Bcl-2 dephosphorylation, caspase-2 activation, and apoptosisMol Pharmacol200670251051716682503
- GhribiOHermanMMSpauldingNKSavoryJLithium inhibits aluminum-induced apoptosis in rabbit hippocampus, by preventing cytochrome c translocation, Bcl-2 decrease, Bax elevation and caspase-3 activationJ Neurochem200282113714512091474
- HooperCKillickRLovestoneSThe GSK3 hypothesis of Alzheimer’s diseaseJ Neurochem200810461433143918088381
- EngelTGoni-OliverPLucasJJAvilaJHernandezFChronic lithium administration to FTDP-17 tau and GSK-3β overexpressing mice prevents tau hyperphosphorylation and neurofibrillary tangle formation, but pre-formed neurofibrillary tangles do not revertJ Neurochem20069961445145517059563
- LeroyKAndoKHeraudCLithium treatment arrests the development of neurofibrillary tangles in mutant tau transgenic mice with advanced neurofibrillary pathologyJ Alzheimers Dis201019270571920110614
- LovestoneSDavisDRWebsterMTLithium reduces tau phosphorylation: effects in living cells and in neurons at therapeutic concentrationsBiol Psychiatry1999458995100310386182
- NobleWPlanelEZehrCInhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivoProc Natl Acad Sci USA2005102196990699515867159
- RockensteinETorranceMAdameANeuroprotective effects of regulators of the glycogen synthase kinase-3β signaling pathway in a transgenic model of Alzheimer’s disease are associated with reduced amyloid precursor protein phosphorylationJ Neurosci20072781981199117314294
- SuYRyderJLiBLithium, a common drug for bipolar disorder treatment, regulates amyloid-β precursor protein processingBiochemistry200443226899690815170327
- YuFZhangYChuangDMLithium reduces BACE1 overexpression, β amyloid accumulation, and spatial learning deficits in mice with traumatic brain injuryJ Neurotrauma201229132342235122583494
- ZhangXHengXLiTLong-term treatment with lithium alleviates memory deficits and reduces amyloid-β production in an aged Alzheimer’s disease transgenic mouse modelJ Alzheimers Dis201124473974921321394
- FiorentiniARosiMCGrossiCLuccariniICasamentiFLithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant micePLoS One2010512e1438221187954
- RamettiAEsclaireFYardinCCogneNTerroFLithium down-regulates tau in cultured cortical neurons: a possible mechanism of neuroprotectionNeurosci Lett20084341939818289787
- ContestabileAGrecoBGhezziDTucciVBenfenatiFGaspariniLLithium rescues synaptic plasticity and memory in Down syndrome miceJ Clin Invest2013123134836123202733
- HooperCMarkevichVPlattnerFGlycogen synthase kinase-3 inhibition is integral to long-term potentiationEur J Neurosci2007251818617241269
- NocjarCHammondsMDShimSSChronic lithium treatment magnifies learning in ratsNeuroscience2007150477478817996377
- ShimSSHammondsMDGanocySJCalabreseJREffects of subchronic lithium treatment on synaptic plasticity in the dentate gyrus of rat hippocampal slicesProg Neuropsychopharmacol Biol Psychiatry200731234334717097205
- VoytovychHKrivanekovaLZiemannULithium: a switch from LTD- to LTP-like plasticity in human cortexNeuropharmacology201263227427922507665
- CheungZHIpNYAutophagy deregulation in neurodegenerative diseases – recent advances and future perspectivesJ Neurochem2011118331732521599666
- RavikumarBRubinszteinDCCan autophagy protect against neurodegeneration caused by aggregate-prone proteins?Neuroreport200415162443244515538170
- RubinszteinDCThe roles of intracellular protein-degradation pathways in neurodegenerationNature2006443711378078617051204
- LiQLiHRoughtonKLithium reduces apoptosis and autophagy after neonatal hypoxia-ischemiaCell Death Dis20101e5621364661
- ShimadaKMotoiYIshiguroKLong-term oral lithium treatment attenuates motor disturbance in tauopathy model mice: implications of autophagy promotionNeurobiol Dis201246110110822249108
- BurgerSNoackMKirazovLPVascular endothelial growth factor (VEGF) affects processing of amyloid precursor protein and β-amyloidogenesis in brain slice cultures derived from transgenic Tg2576 mouse brainInt J Dev Neurosci200927651752319589380
- NagaharaAHMerrillDACoppolaGNeuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s diseaseNat Med200915333133719198615
- PatelNSMathuraVSBachmeierCAlzheimer’s β-amyloid peptide blocks vascular endothelial growth factor mediated signaling via direct interaction with VEGFR-2J Neurochem20101121667619818105
- TongLBalazsRThorntonPLCotmanCWβ-amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neuronsJ Neurosci200424306799680915282285
- SyMKitazawaMMedeirosRInflammation induced by infection potentiates tau pathological features in transgenic miceAm J Pathol201117862811282221531375
- NahmanSBelmakerRHAzabANEffects of lithium on lipopolysaccharide-induced inflammation in rat primary glia cellsInnate Immun201218344745821994254
- LiHLiQDuXLithium-mediated long-term neuroprotection in neonatal rat hypoxia-ischemia is associated with antiinflammatory effects and enhanced proliferation and survival of neural stem/ progenitor cellsJ Cereb Blood Flow Metab201131102106211521587270
- BasselinMVillacresesNELeeHJBellJMRapoportSIChronic lithium administration attenuates up-regulated brain arachidonic acid metabolism in a rat model of neuroinflammationJ Neurochem2007102376177217488274
- KessingLVSondergardLFormanJLAndersenPKLithium treatment and risk of dementiaArch Gen Psychiatry200865111331133518981345
- KessingLVFormanJLAndersenPKDoes lithium protect against dementia?Bipolar Disord2010121879420148870
- TeraoTNakanoHInoueYOkamotoTNakamuraJIwataNLithium and dementia: a preliminary studyProg Neuropsychopharmacol Biol Psychiatry20063061125112816753246
- NunesPVForlenzaOVGattazWFLithium and risk for Alzheimer’s disease in elderly patients with bipolar disorderBr J Psychiatry200719035936017401045
- NitriniRCaramelliPHerreraEJrIncidence of dementia in a community-dwelling Brazilian populationAlzheimer Dis Assoc Disord200418424124615592138
- PolterABeurelEYangSdeficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbancesNeuropsychopharmacology20103581761177420357757
- LiXFriedmanABZhuWLithium regulates glycogen synthase kinase-3β in human peripheral blood mononuclear cells: implication in the treatment of bipolar disorderBiol Psychiatry200761221622216806104
- RybakowskiJKSuwalskaAExcellent lithium responders have normal cognitive functions and plasma BDNF levelsInt J Neuropsychopharmacol201013561762220392298
- de SousaRTvan de BiltMTDinizBSLithium increases plasma brain-derived neurotrophic factor in acute bipolar mania: a preliminary 4-week studyNeurosci Lett20114941545621362460
- SuwalskaASobieskaMRybakowskiJKSerum brain-derived neurotrophic factor in euthymic bipolar patients on prophylactic lithium therapyNeuropsychobiolog y2010624229234
- AliyaziciogluRKuralBColakMKarahanSCAyvazSDegerOTreatment with lithium, alone or in combination with olanzapine, relieves oxidative stress but increases atherogenic lipids in bipolar disorderTohoku J Exp Med20072131798717785956
- Machado-VieiraRAndreazzaACVialeCIOxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: a possible role for lithium antioxidant effectsNeurosci Lett20074211333617548157
- KhairovaRPawarRSalvadoreGEffects of lithium on oxidative stress parameters in healthy subjectsMol Med Rep20125368068222200861
- GuloksuzSAltinbasKAktas CetinEEvidence for an association between tumor necrosis factor-α levels and lithium responseJ Affect Disord20121431–314815222749155
- KnijffEMBreunisMNKupkaRWAn imbalance in the production of IL-1β and IL-6 by monocytes of bipolar patients: restoration by lithium treatmentBipolar Disord20079774375317988365
- FolandLCAltshulerLLSugarCAIncreased volume of the amygdala and hippocampus in bipolar patients treated with lithiumNeuroreport200819222122418185112
- GermanaCKemptonMJSarnicolaAThe effects of lithium and anticonvulsants on brain structure in bipolar disorderActa Psychiatr Scand2010122648148720560901
- MooreGJCorteseBMGlitzDAA longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patientsJ Clin Psychiatry200970569970519389332
- YucelKMcKinnonMCTaylorVHBilateral hippocampal volume increases after long-term lithium treatment in patients with bipolar disorder: a longitudinal MRI studyPsychopharmacology (Berl)2007195335736717705060
- ForesterBPFinnCTBerlowYAWardropMRenshawPFMooreCMBrain lithium, N-acetyl aspartate and myo-inositol levels in older adults with bipolar disorder treated with lithium: a lithium-7 and proton magnetic resonance spectroscopy studyBipolar Disord200810669170018837863
- SilverstonePHWuRHO’DonnellTUlrichMAsgharSJHanstockCCChronic treatment with lithium, but not sodium valproate, increases cortical N-acetyl-aspartate concentrations in euthymic bipolar patientsInt Clin Psychopharmacol2003182737912598817
- ForlenzaOVTorresCATalibLLIncreased platelet GSK3β activity in patients with mild cognitive impairment and Alzheimer’s diseaseJ Psychiatr Res201145222022420576277
- MacdonaldABriggsKPoppeMHigginsAVelayudhanLLovestoneSA feasibility and tolerability study of lithium in Alzheimer’s diseaseInt J Geriatr Psychiatry200823770471118181229
- NunesMAVielTABuckHSMicrodose lithium treatment stabilized cognitive impairment in patients with Alzheimer’s diseaseCurr Alzheimer Res201310110410722746245
- HampelHEwersMBurgerKLithium trial in Alzheimer’s disease: a randomized, single-blind, placebo-controlled, multicenter 10-week studyJ Clin Psychiatry200970692293119573486
- LeyheTEschweilerGWStranskyEIncrease of BDNF serum concentration in lithium treated patients with early Alzheimer’s diseaseJ Alzheimers Dis200916364965619276559
- StratenGSaurRLaskeCinfluence of lithium treatment on GDNF serum and CSF concentrations in patients with early Alzheimer’s diseaseCurr Alzheimer Res20118885385921875410
- ForlenzaOVDinizBSRadanovicMSantosFSTalibLLGattazWFDisease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trialBr J Psychiatry2011198535135621525519
- AprahamianISantosFSSantosBLong-term, low-dose lithium treatment does not impair renal function in the elderly: a two-year placebo-controlled trial followed by single-blind extensionBr J Psychiatry2013