Abstract
Aging is the primary risk factor for dementia. With increasing life expectancy and aging populations worldwide, dementia is becoming one of the significant public health problems of the century. The most common pathology underlying dementia in older adults is Alzheimer’s disease. Proton magnetic resonance spectroscopy (MRS) may provide a window into the biochemical changes associated with the loss of neuronal integrity and other neurodegenerative pathology that involve the brain before the manifestations of cognitive impairment in patients who are at risk for Alzheimer’s disease. This review focuses on proton MRS studies in normal aging, mild cognitive impairment, and dementia, and how proton MRS metabolite levels may be potential biomarkers for early diagnosis of dementia-related pathologic changes in the brain.
Introduction
Biomarkers of Alzheimer’s disease (AD) are important for both early diagnoses and evaluating treatment effects. Three decades of research indicate that proton magnetic resonance spectroscopy (MRS) is a potential biochemical imaging marker in AD. The focus of this review is to discuss the role of proton MRS in Alzheimer’s disease. MRS allows regional measurement of metabolites including myo-Inositol (mI), choline (Cho), N-acetyl aspartate (NAA), and creatine (Cr). Cr is typically used as an internal reference to control for variability in measurement because it remains unchanged in AD.Citation1–Citation3 Other metabolites that can be measured with proton MRS with advanced MRS sequences and post-processing methods include gamma-Aminobutyric acid (GABA) and glutathione which may not be available in conventional clinical scanners and are not the focus of this review. MRS may serve to identify patients with AD before clinical symptom onset as well as help distinguish AD from other neurodegenerative disorders.
Development of metabolic biomarkers for AD
In 1992, Klunk et alCitation4 demonstrated a decrease in the neuronal metabolite NAA on MRS on autopsy brain samples of patients with AD compared to controls. The lower NAA level correlated with the amount of plaque and tangle pathology.Citation4 NAA is considered a neuronal marker and is synthesized in mitochondria.Citation5 Supporting the notion that NAA levels correspond to neuronal integrity, reduced NAA levels in cortical tissue from patients with AD demonstrated a correlation between NAA concentration and neuronal density.Citation6 Decreased NAA seen in head trauma, seizure, or coronary artery bypass surgery can resolve after recovery.Citation5,Citation7–Citation9 Further, NAA levels decreased in AD show transient improvement with acetylcholinesterase inhibitor treatment.Citation10 The neurofibrillary tangles pathology of AD follow a typical progression from limbic to neocortical areas as AD advances.Citation11 Similarly, changes in NAA follow a regional pattern as disease advances. For example, AD patients show a regional decrease in NAA/Cr ratio in the superior temporal lobe and posterior cingulate voxels compared to controls, but mild cognitive impairment (MCI) patients do not show a decline in NAA/Cr as AD-related neurodegeneration has not yet extended to these regions.Citation12 Medial occipital lobe AD pathology involvement typically occurs at the final stage of the disease.Citation11 Therefore, it is not surprising that there is no regional decrease in NAA/Cr in mild AD in the occipital lobe while more advanced cases demonstrate decrease NAA/Cr ratio.Citation12–Citation14 Eventually, NAA changes become widespread and have been shown to involve the parietal, temporal, and frontal lobe.Citation12,Citation15–Citation19 Decreased NAA/Cr ratios are non-specific and can be seen in other types of dementia including normal pressure hydrocephalus and have even been reported in cognitive decline associated with acquired immunodeficiency syndrome (AIDS).Citation20,Citation21
In 1993, it was demonstrated that in addition to decreased NAA levels, AD patients have elevated myo-Inositol to creatine (mI/Cr) levels.Citation13 While NAA is a neuronal marker, mI is associated with glia and elevated levels with glial proliferation.Citation22,Citation23 The finding of decreased NAA and elevated mI in AD has been confirmed in several studies.Citation12,Citation16,Citation24
The role of choline metabolite in AD is more controversial. A number of studies demonstrated increased choline in AD.Citation25–Citation27 Other studies have demonstrated no change in choline concentration in AD compared to controls.Citation28–Citation31 One study reported a decrease of choline/H20 in the medial temporal lobes of AD patients.Citation32 Brain choline is concentrated in phospholipids. The choline peak in MRS represents cytosolic glycerophosphocholine and phosphocholine which are breakdown products of phosphatidylcholine.Citation33 Therefore the larger choline peak may be due to increased membrane turnover. Also, it has been proposed that catabolism of the phospholipid membrane bilayer allows AD subjects to produce choline to compensate for declining acetylcholine.Citation34 Administration of xanomeline, an M1 selective muscarinic cholinergic agonist, to AD patients resulted in a significant decline in Cho/Cr ratios, perhaps representing a reduction in compensatory mechanisms to produce acetylcholine through phospholipid breakdown.Citation35 Cho/Cr increases in amnestic MCI if it progresses to AD but the ratio decreases if cognition remains stable.Citation36
Decreased glutamate (Glu) or glutamate + glutamine/Cr ratio has also been found in the grey matter of AD patients, but not the white matter.Citation37–Citation40 Furthermore, increased Glu and the ratio of Glu to Cr measured from the hippocampus by MRS after galantamine treatment were associated with increased cognitive performance.Citation41 MRS studies in transgenic mice with AD mutations have shed light on the pathological correlates of metabolite changes seen in AD. Transgenic mice with AD mutations demonstrate similar decreased NAA and increased mI as seen in human AD patients.Citation6,Citation42 In addition, lower NAA and glutamate levels correlate with amyloid beta (Aβ) plaque load in the frontal cortex of mice with PS2APP mutation.Citation43 Further, the MRS metabolite changes consistent with AD precede overt cognitive dysfunctions in early-stage AD.Citation44
The temporal progression of metabolite abnormalities in AD are characterized by an increased mI/Cr followed by a decrease in NAA/Cr and an increase in Cho/Cr.Citation12 A recent study of pathologic correlates of MRS metabolite changes in cases of varying AD pathology demonstrated that antemortem NAA/Cr and mI/Cr levels correlate with the pathologic severity of AD, and that the strongest predictor of AD pathology was a NAA/mI ratio.Citation45 Longitudinal studies have demonstrated that NAA/Cr and NAA/mI decrease over time compared to controls.Citation36,Citation46,Citation47
NAA/Cr and mI/Cr ratios correlate with cognitive testing in Alzheimer’s disease.Citation16,Citation17,Citation24,Citation48–Citation51 In one study, NAA/Cr in the medial temporal lobe, primary motor and sensory cortices correlated with Mini-Mental State Examination and the cognitive part of the Alzheimer Disease Assessment Scale scores.Citation16 NAA/Cr, mI/Cr, NAA/mI have also been shown to correlate with verbal memory testing (Auditory Verbal Learning Test) and general cognition (Dementia Rating Scale).Citation50
Several studies have investigated the ability of MRS to distinguish AD patients from controls with varying results depending on the anatomic area analyzed and acquisition parameters. The sensitivity was as high as 90% in the temporoparietal region and as low as 57% in the parietal lobe grey matter. The specificity was as a high as 95% in the medial occipital lobe and as low as 73% in the posterior cingulate.Citation14,Citation19,Citation52–Citation54 Furthermore, adding hippocampal volume to MRS, improves the ability to distinguish AD.Citation19,Citation53–Citation56
A few studies with relatively small sample sizes have investigated MRS as a biomarker for treatment response in AD. NAA/Cr improved after acetylcholinesterase inhibitor treatment in AD.Citation10,Citation28 Another trial showed decreases in Cho/Cr and mI/Cr in the hippocampus in absence of clinical improvement in AD subjects, however, this study showed continued decrease in NAA/Cr in contrast to the studies mentioned above.Citation39
MRS also correlates with psychiatric symptoms in AD patients. AD subjects with psychosis have significantly reduced cortical NAA compared to AD subjects without psychosis.Citation57 Psychiatric and behavioral symptoms in AD including delusional thinking correlated with a decrease in NAA/Cr and an increase in mI/Cr in the anterior cingulate.Citation58
MRS for MCI
NAA/Cr levels in MCI are mildly reduced but decline as patients with MCI progress to AD.Citation36 Further, lower NAA/Cr in MCI patients predicts progression to AD.Citation36,Citation59,Citation60 Cho/Cr and mI/Cr levels are also elevated in the posterior cingulate in MCI although higher levels of these metabolites are detected in AD.Citation12 The Cho/Cr ratio is also useful in determining progression from MCI to AD. In MCI patients, a decline in Cho/Cr predicted stability versus an increase, which predicted conversion to AD. The changes in metabolite concentration on MRS correlate as strongly as ventricular volume in predicting cognitive decline.Citation36
MRS is also useful in distinguishing subtypes of MCI. Amnestic MCI patients have smaller hippocampi with elevated mI/Cr ratios compared to patients with non-amnestic MCI in line with the observation that amnestic MCI patients are more likely to progress to AD than non-amnestic MCI.Citation61
MRS based identification of biomarkers for other neurological disorders, to distinguish from AD
The main differential diagnoses of Alzheimer’s dementia are other types of dementia including dementia with Lewy bodies (DLB), frontotemporal dementia (FTD), and vascular dementia (VaD). The MRS signature of AD is decreased NAA/Cr and elevated Cho/Cr and mI/Cr metabolites.Citation25 Several studies have investigated the metabolite patterns among different types of dementia in order to identify patterns of metabolite changes unique to each dementia.
Many patients with dementia have significant overlap in underlying pathology.Citation62 In patients with VaD, the location of the metabolite change is important for distinguishing VaD from AD. For example, NAA levels in VaD are decreased in a similar way to those in AD patients and the decrease is greater than AD in the white matter.Citation63–Citation65 Unlike AD, cortical mI is normal in VaD.Citation66,Citation67 Further, mI/Cr is higher in AD compared to VaD.Citation25 Therefore, mI and grey matter NAA may serve as useful markers to distinguish AD from VaD.
Similar to AD, FTD is associated with elevated mI/Cr and decreased NAA/Cr.Citation68,Citation69 Despite the similarities, regional differences in metabolites may help distinguish the two dementias. Compared to early AD, patients with FTD have higher mI/Cr and lower NAA/Cr in the frontal cortex.Citation68,Citation70 However, others have noted no difference between FTD and AD with similar levels of metabolite abnormalities outside the frontal lobes.Citation25
Similar to the other dementias, significant overlap exists between DLB and AD. Compared to other dementia subtypes, DLB has a normal NAA/Cr ratio in the posterior cingulate.Citation25 The normal NAA/Cr is possibly related to the relative preservation of neurons in DLB compared to AD.Citation71 In the hippocampus of DLB patients, the NAA has been reported to be decreased although it is unclear if this decrease represents concomitant AD given the overlap in pathologies mentioned above.Citation72 Additionally, NAA is similarly decreased in the white matter of patients with DLB.Citation73 Therefore, NAA levels in the posterior cingulate may help distinguish AD from DLB. The relative preservation of the cingulate is in agreement with fluorodeoxyglucose (FDG) positron emission tomography (PET) which demonstrates a relative preservation of glucose metabolism in the cingulate of DLB when compared to AD.Citation74
Similar to AD, DLB patients have an elevated Cho/Cr compared to controls.Citation25 This finding is intriguing because both AD and DLB are characterized by a cholinergic deficit.Citation75 Since Cho/Cr levels decrease with cholinergic agonist treatment in AD,Citation35 the decrease raises the possibility that Cho/Cr can be used as a therapeutic biomarker in AD and DLB.
In summary, while significant overlap exists between dementias, AD has a unique metabolite pattern compared to other dementias when regional differences are taken into account.
Current place of MRS in the differential diagnosis of AD
The American Academy of Neurology practice parameter recommends against routine imaging with quantitative MRI techniques in the evaluation of dementia because of insufficient evidence.Citation76 Nonetheless, as a research tool, MRS can provide valuable information in the differential diagnoses of dementia. AD biomarkers, such as Amyloid PET imaging and cerebrospinal fluid Aβ, provide useful information about whether AD is the pathology underlying a given dementia. MRS can provide complementary predictive information. In addition, MRS allows for identification of a metabolite signature of different dementia subtypes which can provide complementary data to the clinical impression. To date, no MRS study has used metabolite signature in conjunction with regional differences to determine the underlying pathology.
Table 1 In vivo studies with MRS in AD and MCI
Utility of MRS along with other AD biomarkers
In 2011, the National Institute on Aging and Alzheimer’s Association revised criteria for preclinical, MCI, and Alzheimer’s disease.Citation77–Citation80 The pathology that contributes to AD begins to accumulate years before clinical symptoms. Therefore, identifying the population at high risk of developing symptoms has become important. Principal AD imaging biomarkers include Aβ imaging with PET, FDG-PET, and structural MRI. In cognitively normal older adults, mI/Cr and Cho/Cr correlate with Aβ load in amyloid PET imaging as demonstrated in two example cases with high and low Aβ load in their brains in .Citation81
Figure 1 Association between MRS metabolite ratios and cortical Pittsburgh compound-B (PiB) retention ratio on PET.
Abbreviations: Cho, choline; Cr, creatine; mi, myo-inositol; MRS, magnetic resonance spectroscopy; NAA, N-acetyl aspartate; PET, positron emission tomography.
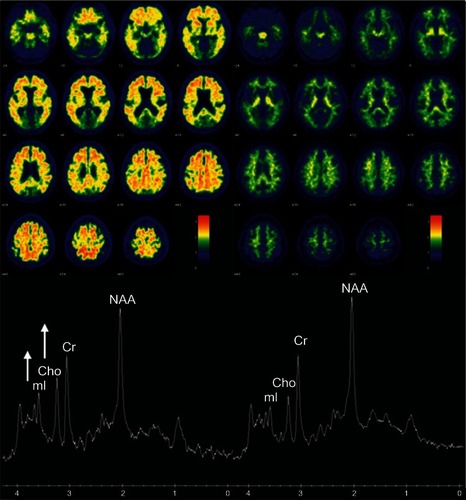
According to one model of biomarkers in AD, amyloid pathology accumulates before evidence of neurodegeneration.Citation82 Amyloid PET imaging serves as a surrogate for brain amyloid load and FDG-PET and Structural MRI serve as markers of neurodegeneration. While these imaging markers are well validated measures of amyloid and neurofibrillary tangle pathology, they do not measure microglial activation. MRS mI is a potential surrogate marker for glial activity. A recent 13-carbon MRS and 1H MRS linked glial and microglial activity to mI elevation in AD.Citation83 This raises the possibility that elevated mI represents inflammation which is an early event in the evolution of AD pathology.Citation2
While hippocampal atrophy predicts AD pathology and is an imaging marker of neurodegeneration in the National Institute on Aging – Alzheimer’s Association (criteria for preclinical AD),Citation84,Citation85 up to 11% of AD cases are hippocampal sparing, with corresponding preserved hippocampal volumes on MRI.Citation86,Citation87 Subjects with at least one type of hippocampal sparing AD (posterior cortical atrophy), have been demonstrated to have decreased NAA/Cr in the posterior cingulate.Citation88 Therefore, MRS may serve a role as a critical marker of AD pathology in a significant minority of AD cases where the hippocampus is relatively preserved. Other imaging markers that may provide important information in atypical AD cases include resting state functional MRI, and diffusion tensor imaging (DTI). Cognitive testing may provide additional information.
MRS can serve as a predictor of the degree of AD pathology in clinical trial design. For example, mI/Cr is elevated in MCI and mild AD even with normal NAA/Cr.Citation12,Citation24,Citation89 In addition, isolated mI/Cr elevation is associated with earlier stage AD pathology compared to elevated mI and decrease in NAA/Cr which is associated with a later stage AD pathology. Isolated elevation in mI/Cr can be seen prior to structural MRI changes in individuals with familial dementia prior to symptom onset.Citation90 Therefore, MRS could potentially serve as an adjunct to help select patients for intervention trials based on degree of AD pathology.
In addition to serving as a potential marker of glial activity, MRS can be used along with other biomarkers as a tool to predict cognitive decline. In a group of cognitively normal individuals, elevated Cho/Cr in the white matter predicts progression to dementia.Citation91 The metabolite formula changes in preclinical familial AD families with amyloid precursor protein, presenilin 1 or 2 mutations. Asymptomatic mutation carriers demonstrated elevated mI/Cr and decreased NAA/Cr with reduction in NAA/mI correlating with nearness to age of onset.Citation92 In addition to serving as a marker of preclinical disease, MRS has utility in monitoring disease progression. NAA/Cr levels predict cognitive decline in individuals with AD.Citation36,Citation46,Citation93
Future predictions for the use of MRS in the differential diagnosis of AD
While much progress has been made in understanding the role of MRS in Alzheimer’s disease, MRS is still not routinely used clinically in the assessment of dementia. Reasons for ineffective translation of technology to clinical practice or patient-oriented research are twofold: (1) Lack of standardization for multi-site applications and normative data; and (2) insufficient understanding of the pathologic basis of 1H MRS metabolite changes.Citation94 Although metabolite abnormalities in AD have been demonstrated in different samples and in pathologically confirmed cases, the pathological substrates for these metabolite abnormalities are not fully understood. Future studies are needed to elucidate the pathological significance of these metabolite changes in AD. As we learn more about the pathophysiologic underpinnings of the metabolite abnormalities, the routine use of MRS as a biomarker will become more prevalent.
Acknowledgment
Grant support: Dr Kantarci’s research program is supported by the National Institutes of Health: R01 AG40042, P50 AG16574/Project1, P50AG044170/Project 2, and R21 NS066147. The authors would like to acknowledge Samantha Wille for manuscript preparation.
Disclosure
Dr Kantarci serves on the data safety monitoring board for Pfizer Inc, and Janssen Amyloid Immunotherapy and Takeda Global Research and Development Center, Inc; she is funded by the National Institutes of Health (R01AG040042 [PI], R21 NS066147 [PI], P50 AG44170/Project 2 [PI], P50 AG16574/Project 1 [PI], and R01 AG11378 [Co-I]).
Dr Graff-Radford reports no conflicts of interest in this work.
References
- KantarciK1H magnetic resonance spectroscopy in dementiaBr J Radiol200780Spec No 2:S146S15218445744
- RossBDBlumlSCowanRDanielsenEFarrowNTanJIn vivo MR spectroscopy of human dementiaNeuroimaging Clin N Am1998848098229769343
- ValenzuelaMJSachdevPMagnetic resonance spectroscopy in ADNeurology200156559259811261442
- KlunkWEPanchalingamKMoossyJMcClureRJPettegrewJWN-acetyl-L-aspartate and other amino acid metabolites in Alzheimer’s disease brain: a preliminary proton nuclear magnetic resonance studyNeurology1992428157815851353623
- BatesTEStrangwardMKeelanJDaveyGPMunroPMClarkJBInhibition of N-acetylaspartate production: implications for 1H MRS studies in vivoNeuroreport199678139714008856684
- ChengLLNewellKMalloryAEHymanBTGonzalezRGQuantification of neurons in Alzheimer and control brains with ex vivo high resolution magic angle spinning proton magnetic resonance spectroscopy and stereologyMagn Reson Imaging200220752753312413598
- BrooksWMStidleyCAPetropoulosHMetabolic and cognitive response to human traumatic brain injury: a quantitative proton magnetic resonance studyJ Neurotrauma200017862964010972240
- BendszusMReentsWFrankeDBrain damage after coronary artery bypass graftingArch Neurol20025971090109512117356
- HuggJWKuznieckyRIGilliamFGMorawetzRBFraughtREHetheringtonHPNormalization of contralateral metabolic function following temporal lobectomy demonstrated by 1H magnetic resonance spectroscopic imagingAnn Neurol19964022362398773605
- ModregoPJPinaMAFayedNDiazMChanges in metabolite ratios after treatment with rivastigmine in Alzheimer’s disease: a nonrandomised controlled trial with magnetic resonance spectroscopyCNS Drugs2006201086787716999455
- BraakHBraakENeuropathological stageing of Alzheimer-related changesActa Neuropathologica19918242392591759558
- KantarciKJackCRJrXuYCRegional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: A 1H MRS studyNeurology200055221021710908893
- MillerBLMoatsRAShonkTErnstTWoolleySRossBDAlzheimer disease: depiction of increased cerebral myo-inositol with proton MR spectroscopyRadiology199318724334378475286
- ShonkTKMoatsRAGiffordPProbable Alzheimer disease: diagnosis with proton MR spectroscopyRadiology1995195165727892497
- FrederickBBSatlinAYurgelun-ToddDARenshawPFIn vivo proton magnetic resonance spectroscopy of Alzheimer’s disease in the parietal and temporal lobesBiol Psychiatry19974221471509209733
- JessenFBlockWTraberFProton MR spectroscopy detects a relative decrease of N-acetylaspartate in the medial temporal lobe of patients with ADNeurology200055568468810980734
- SchuffNAmendDLMeyerhoffDJAlzheimer disease: quantitative H-1 MR spectroscopic imaging of frontoparietal brainRadiology19982071911029530304
- TedeschiGBertolinoALundbomNCortical and subcortical chemical pathology in Alzheimer’s disease as assessed by multislice proton magnetic resonance spectroscopic imagingNeurology19964736967048797467
- ZhuXSchuffNKornakJEffects of Alzheimer disease on fronto-parietal brain N-acetyl aspartate and myo-inositol using magnetic resonance spectroscopic imagingAlzheimer Dis Assoc Disord2006202778516772742
- ShiinoAMatsudaMMorikawaSInubushiTAkiguchiIHandaJProton magnetic resonance spectroscopy with dementiaSurg Neurol19933921431478394604
- BarkerPBLeeRRMcArthurJCAIDS dementia complex: evaluation with proton MR spectroscopic imagingRadiology1995195158647892496
- BitschABruhnHVougioukasVInfammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopyAJNR Am J Neuroradiol19992091619162710543631
- GlanvilleNTByersDMCookHWSpenceMWPalmerFBDifferences in the metabolism of inositol and phosphoinositides by cultured cells of neuronal and glial originBiochimica et Biophysica Acta1989100421691792546591
- HuangWAlexanderGEChangLBrain metabolite concentration and dementia severity in Alzheimer’s disease: a (1)H MRS studyNeurology200157462663211524470
- KantarciKPetersenRCBoeveBF1H MR spectroscopy in common dementiasNeurology20046381393139815505154
- MeyerhoffDJMacKaySConstansJMAxonal injury and membrane alterations in Alzheimer’s disease suggested by in vivo proton magnetic resonance spectroscopic imagingAnn Neurol199436140478024260
- PfefferbaumAAdalsteinssonESpielmanDSullivanE VLimKOIn vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: effects of normal agingMagn Reson Med199941227628410080274
- KrishnanKRCharlesHCDoraiswamyPMRandomized, placebo-controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer’s diseaseAm J Psychiatry2003160112003201114594748
- MoatsRAErnstTShonkTKRossBDAbnormal cerebral metabolite concentrations in patients with probable Alzheimer diseaseMagn Reson Med19943211101158084225
- RoseSEde ZubicarayGIWangDA 1H MRS study of probable Alzheimer’s disease and normal aging: implications for longitudinal monitoring of dementia progressionMagn Reson Imaging199917229129910215485
- SchuffNAmendDEzekielFChanges of hippocampal N-acetyl aspartate and volume in Alzheimer’s disease. A proton MR spectroscopic imaging and MRI studyNeurology1997496151315219409338
- ChantalSLabelleMBouchardRWBraunCMBoulangerYCorrelation of regional proton magnetic resonance spectroscopic metabolic changes with cognitive deficits in mild Alzheimer diseaseArch Neurol200259695596212056931
- KleinJMembrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipidsJ Neural Transm20001078–91027106311041281
- WurtmanRJBJMarieJC“Autocannibalism” of choline-containing membrane phospholipids in the pathogenesis of Alzheimer’s diseaseNeurochem Int1985736937220492936
- SatlinABodickNOffenWWRenshawPFBrain proton magnetic resonance spectroscopy (1H-MRS) in Alzheimer’s disease: changes after treatment with xanomeline, an M1 selective cholinergic agonistAm J Psychiatry199715410145914619326834
- KantarciKWeigandSDPetersenRCLongitudinal 1H MRS changes in mild cognitive impairment and Alzheimer’s diseaseNeurobiol Aging20072891330133916860440
- AntuonoPGJonesJLWangYLiSJDecreased glutamate + glutamine in Alzheimer’s disease detected in vivo with (1)H-MRS at 0.5 TNeurology200156673774211274307
- HattoriNAbeKSakodaSSawadaTProton MR spectroscopic study at 3 Tesla on glutamate/glutamine in Alzheimer’s diseaseNeuroreport200213118318611924885
- BarthaRSmithMRupsinghRRylettJWellsJLBorrieMJHigh field (1)H MRS of the hippocampus after donepezil treatment in Alzheimer diseaseProg Neuropsychopharmacol Biol Psychiatry200832378679318252268
- RupsinghRBorrieMSmithMWellsJLBarthaRReduced hippocampal glutamate in Alzheimer diseaseNeurobiol Aging201132580281019501936
- PennerJRupsinghRSmithMWellsJLBorrieMJBarthaRIncreased glutamate in the hippocampus after galantamine treatment for Alzheimer diseaseProg Neuro-Psychopharmacol Biol Psychiatry2010341104110
- ChenSQWangPJTenGJZhanWLiMHZangFCRole of myo-inositol by magnetic resonance spectroscopy in early diagnosis of Alzheimer’s disease in APP/PS1 transgenic miceDement Geriatr Cogn Disord200928655856620093832
- von KienlinMKunneckeBMetzgerFAltered metabolic profile in the frontal cortex of PS2 APP transgenic mice, monitored throughout their life spanNeurobiol Dis2005181323915649694
- ChenSQCaiQShenYYAge-related changes in brain metabolites and cognitive function in APP/PS1 transgenic miceBehav Brain Res201223511622828014
- KantarciKKnopmanDSDicksonDWAlzheimer disease: postmortem neuropathologic correlates of antemortem 1H MR spectroscopy metabolite measurementsRadiology2008248121022018566174
- JessenFBlockWTraberFDecrease of N-acetylaspartate in the MTL correlates with cognitive decline of AD patientsNeurology200157593093211552037
- SchottJMFrostCMacManusDGIbrahimFWaldmanADFoxNCShort echo time proton magnetic resonance spectroscopy in Alzheimer’s disease: a longitudinal multiple time point studyBrain2010133113315332220739347
- AcklNIsingMSchreiberYAAtiyaMSonntagAAuerDPHippocampal metabolic abnormalities in mild cognitive impairment and Alzheimer’s diseaseNeurosci Lett20053841–2232815905028
- DoraiswamyPMCharlesHCKrishnanKRPrediction of cognitive decline in early Alzheimer’s diseaseLancet1998352914116789853445
- KantarciKSmithGEIvnikRJ1H magnetic resonance spectroscopy, cognitive function, and apolipoprotein E genotype in normal aging, mild cognitive impairment and Alzheimer’s diseaseJ Int Neuropsychol Soc20028793494212405545
- Kwo-On-YuenPFNewmarkRDBudingerTFKayeJABallMJJagustWJBrain N-acetyl-L-aspartic acid in Alzheimer’s disease: a proton magnetic resonance spectroscopy studyBrain Res199466721671747697354
- FernandezAGarcia-SeguraJMOrtizTProton magnetic resonance spectroscopy and magnetoencephalographic estimation of delta dipole density: a combination of techniques that may contribute to the diagnosis of Alzheimer’s diseaseDement Geriatr Cogn Disord2005202–316917716020946
- KantarciKXuYShiungMMComparative diagnostic utility of different MR modalities in mild cognitive impairment and Alzheimer’s diseaseDement Geriatr Cogn Disord200214419820712411762
- Martinez-BisbalMCAranaEMarti-BonmatiLMollaECeldaBCognitive impairment: classification by 1H magnetic resonance spectroscopyEur J Neurol200411318719315009164
- MacKaySEzekielFDi SclafaniVAlzheimer disease and subcortical ischemic vascular dementia: evaluation by combining MR imaging segmentation and H-1 MR spectroscopic imagingRadiology199619825375458596863
- SchuffNCapizzanoAADuATSelective reduction of N- acetylaspartate in medial temporal and parietal lobes in ADNeurology200258692893511914410
- SweetRAPanchalingamKPettegrewJWPsychosis in Alzheimer disease: postmortem magnetic resonance spectroscopy evidence of excess neuronal and membrane phospholipid pathologyNeurobiol Aging200223454755312009504
- ShinnoHInagakiTMiyaokaTA decrease in N-acetylaspartate and an increase in myoinositol in the anterior cingulate gyrus are associated with behavioral and psychological symptoms in Alzheimer’s diseaseJ Neurol Sci20072601–213213817540407
- ChaoLLSchuffNKramerJHReduced medial temporal lobe N-acetylaspartate in cognitively impaired but nondemented patientsNeurology200564228228915668426
- MetastasioARinaldiPTarducciRConversion of MCI to dementia: role of proton magnetic resonance spectroscopyNeurobiol Aging200627792693215936850
- KantarciKWeigandSDPrzybelskiSARisk of dementia in MCI: combined effect of cerebrovascular disease, volumetric MRI, and 1H MRSNeurology200972171519152519398707
- SchneiderJAArvanitakisZBangWBennettDAMixed brain pathologies account for most dementia cases in community-dwelling older personsNeurology200769242197220417568013
- KattapongVJBrooksWMWesleyMHKodituwakkuPWRosenbergGAProton magnetic resonance spectroscopy of vascular-and Alzheimer-type dementiaArch Neurol19965376786808929176
- MacKaySMeyerhoffDJConstansJMNormanDFeinGWeinerMWRegional gray and white matter metabolite differences in subjects with AD, with subcortical ischemic vascular dementia, and elderly controls with 1H magnetic resonance spectroscopic imagingArch Neurol19965321671748639067
- SchuffNCapizzanoAADuATDifferent patterns of N- acetylaspartate loss in subcortical ischemic vascular dementia and ADNeurology200361335836412913198
- ShiinoAWatanabeTShirakashiYThe profile of hippocampal metabolites differs between Alzheimer’s disease and subcortical ischemic vascular dementia, as measured by proton magnetic resonance spectroscopyJ Cereb Blood Flow Metab201232580581522314267
- WaldmanADRaiGSMcConnellJRChaudryMGrantDClinical brain proton magnetic resonance spectroscopy for management of Alzheimer’s and sub-cortical ischemic vascular dementia in older peopleArch Gerontol Geriatr200235213714214764351
- MiharaMHattoriNAbeKSakodaSSawadaTMagnetic resonance spectroscopic study of Alzheimer’s disease and frontotemporal dementia/Pick complexNeuroreport200617441341616514368
- ShonkTKMoatsRAGiffordPProbable Alzheimer disease: diagnosis with proton MR spectroscopy. [see comment]Radiology1995195165727892497
- ErnstTChangLMelchorRMehringerCMFrontotemporal dementia and early Alzheimer disease: differentiation with frontal lobe H-1 MR spectroscopyRadiology199720338298369169712
- Gomez-IslaTGrowdonWBMcNamaraMClinicopathologic correlates in temporal cortex in dementia with Lewy bodiesNeurology19995392003200910599772
- XuanXDingMGongXProton magnetic resonance spectroscopy detects a relative decrease of N-acetylaspartate in the hippocampus of patients with dementia with Lewy bodiesJ Neuroimaging200818213714118333837
- MolinaJAGarcia-SeguraJMBenito-LeonJProton magnetic resonance spectroscopy in dementia with Lewy bodiesEur Neurol200248315816312373033
- LimSMKatsifsAVillemagneVLThe 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodiesJ Nucl Med200950101638164519759102
- TiraboschiPHansenLAAlfordMEarly and widespread cholinergic losses differentiate dementia with Lewy bodies from Alzheimer diseaseArch Gen Psychiatry2002591094695112365882
- KnopmanDSDeKoskySTCummingsJLPractice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. [see comment]Neurology20015691143115311342678
- AlbertMSDeKoskySTDicksonDThe diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s diseaseAlzheimers Dement20117327027921514249
- JackCRJrAlbertMSKnopmanDSIntroduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s diseaseAlzheimers Dement20117325726221514247
- McKhannGMKnopmanDSChertkowHThe diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s diseaseAlzheimers Dement20117326326921514250
- RossAJSachdevPSWenWValenzuelaMJBrodatyH1H MRS in stroke patients with and without cognitive impairmentNeurobiol Aging200526687388215718046
- KantarciKLoweVPrzybelskiSAMagnetic resonance spectroscopy, beta-amyloid load, and cognition in a population-based sample of cognitively normal older adultsNeurology2011771095195821865577
- JackCRJrKnopmanDSJagustWJHypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascadeLancet Neurol20109111912820083042
- SailasutaNHarrisKTranTRossBMinimally invasive biomarker confirms glial activation present in Alzheimer’s disease: a preliminary studyNeuropsychiatr Dis Treat2011749549921931491
- JackCRJrDicksonDWParisiJEAntemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementiaNeurology200258575075711889239
- SperlingRAAisenPSBeckettLAToward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s diseaseAlzheimers Dement20117328029221514248
- MurrayMEGraff-RadfordNRRossOAPetersenRCDuaraRDicksonDWNeuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective studyLancet Neurol201110978579621802369
- WhitwellJLDicksonDWMurrayMENeuroimaging correlates of pathologically defined subtypes of Alzheimer’s disease: a case-control studyLancet Neurol2012111086887722951070
- WhitwellJLJackCRJrKantarciKImaging correlates of posterior cortical atrophyNeurobiol Aging20072871051106116797786
- CataniMCherubiniAHowardR(1)H-MR spectroscopy differentiates mild cognitive impairment from normal brain agingNeuroreport200112112315231711496102
- KantarciKBoeveBFWszolekZKMRS in presymptomatic MAPT mutation carriers: a potential biomarker for tau-mediated pathologyNeurology201075977177820805522
- den HeijerTSijensPEPrinsNDMR spectroscopy of brain white matter in the prediction of dementiaNeurology200666454054416505309
- GodboltAKWaldmanADMacManusDGMRS shows abnormalities before symptoms in familial Alzheimer diseaseNeurology200666571872216534109
- AdalsteinssonESullivanEVKleinhansNSpielmanDMPfefferbaumALongitudinal decline of the neuronal marker N-acetyl aspartate in Alzheimer’s diseaseLancet200035592161696169710905250
- KantarciKProton MRS in mild cognitive impairmentJ Magn Reson Imaging201337477077723526756
- ChantalSBraunCMBouchardRWLabelleMBoulangerYSimilar 1H magnetic resonance spectroscopic metabolic pattern in the medial temporal lobes of patients with mild cognitive impairment and Alzheimer diseaseBrain Res200410031–2263515019560