Abstract
Until now there has been no information available on drug interaction between paliperidone and TS-1®, an oral anticancer drug containing a 5-fluorouracil derivative. The patient in the case presented here was a 39-year-old man with a 15-year history of schizophrenia. The patient’s usual treatment of 2 mg/day of risperidone was changed to 3 mg/day of paliperidone extended release. He experienced worsening psychotic symptoms after switching from risperidone to paliperidone while he was also receiving TS-1. Retrospective analyses showed plasma concentration of paliperidone was consistently lower during the treatment with TS-1 than without TS-1. This case suggests there is drug interaction between paliperidone extended-release tablets and TS-1.
Introduction
Paliperidone is the primary active metabolite of the older antipsychotic risperidone.Citation1 A previous study of paliperidone showed that no metabolites were detected in plasma and that renal excretion was the major route of elimination, with 59% of the nonmetabolized dose excreted in urine.Citation2 Therefore, it is unlikely that paliperidone is metabolized in the body and that paliperidone distribution is influenced by other drugs. In the case presented in this report, the authors observed a patient who experienced worsening psychotic symptoms after switching from risperidone to paliperidone extended-release tablets while he was also receiving TS-1® (Taiho Pharmaceutical Co, Ltd, Tokyo, Japan), an oral anticancer drug containing a 5-fluorouracil derivative.Citation3
Case report
The patient in this case was a 39-year-old man with a 15-year history of schizophrenia. His primary symptoms were auditory hallucinations, persecution mania, and self-talking. His mental condition was maintained for several years with a dose of 2–4 mg/day of risperidone. He was working as an office clerk. His Positive and Negative Syndrome Scale (PANSS) scores ranged from 42 to 50. Additionally, he suffered from colon cancer and had received a sigmoid colon resection at age 34, as well as undergoing treatment with the anticancer agent TS-1 (100 mg daily over 6-week intervals – that is, TS-1 treatment for 4 weeks and cessation of medication for 2 weeks). TS-1 is a novel oral anticancer agent comprising tegafur, a prodrug of 5-fluorouracil (5-FU), and two modulators.Citation3 One observed side effect of the TS-1 medication was mild to moderate diarrhea (grade 2–3), which disappeared during the cessation of TS-1. The patient gave his written informed consent to participate in this switching study, and the patient’s usual treatment of 2 mg/day of risperidone was changed to 3 mg/day of paliperidone extended-release tablets. The authors assumed that the equivalent dose ratio of paliperidone should be 1:2 (4 mg/day), but the only available paliperidone extended-release tablet was in a 3 mg dose. Three months after switching to paliperidone extended-release tablets, the patient’s mental condition gradually deteriorated with the development of auditory hallucinations, persecution mania, and self-talking, and his PANSS score was 94. The patient’s dose was increased to 6 mg/day but his mental condition remained unchanged (PANSS score of 82). The dose was then increased to 12 mg/day and after this there was no evidence of psychotic symptoms. The patient’s mental condition was maintained over 12 months with 12 mg/day without any adverse effects, and his PANSS scores ranged from 44 to 52 ().
Figure 1 Clinical course of Positive and Negative Syndrome Scale (PANSS) scores and plasma concentrations of paliperidone (PAL) after switching from risperidone (RIS) to PAL.
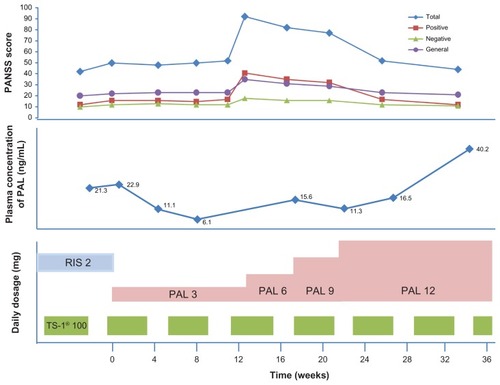
An analysis of the plasma drug concentrations showed that with 2 mg/day of risperidone, the patient’s risperidone plasma levels were less than 0.5 ng/mL, and his paliperidone plasma levels ranged between 21.3 and 22.9 ng/mL. and show the patient’s paliperidone plasma concentrations at various doses both with and without TS-1 after beginning the paliperidone extended-release tablet regimen. The concentration-to-dose ratio of paliperidone during the TS-1 treatment was 1.55 ± 0.34, and the concentration-to-dose ratio of paliperidone without TS-1 treatment was 3.21 ± 0.46 ().
Table 1 Daily drug doses and drug concentrations with (+) and without (−) TS-1®
Discussion
This case suggests there is a drug–drug interaction between paliperidone extended-release tablets and TS-1; this is based on the observed clinical symptoms and the fluctuation of the paliperidone concentration-to-dose ratio (). This case report is the first to indicate a drug–drug interaction between paliperidone extended-release tablets and TS-1. The use of a standard drug interaction tool such as Hansten and HornCitation4 is strongly recommended when identifying drug interactions; however, there is no information available about this particular interaction in the Hansen/Horn tool because this tool has a slight bias against newly reported drug interactions.
The bioavailability of paliperidone (28%) is lower than that of risperidone (70%).Citation1 The time to peak concentration of paliperidone and risperidone is 24 and 1.5 hours, respectively.Citation1 These differences likely explain why decreased plasma levels occurred in this case with paliperidone and not risperidone. Because TS-1 induced diarrhea in this patient, it is considered that the concomitant administration of paliperidone extended-release tablets with TS-1 decreased this patient’s oral bioavailability of paliperidone, which resulted in more severe psychotic symptoms.
A preliminary study reported that unchanged drug represented 51.4%–67.5% (average, 59.4%) of the administered dose, indicating that paliperidone was metabolized to only a limited extent.Citation2 The absolute bioavailability of the instant-release formulation of paliperidone is 106%.Citation5 These data also suggest that the metabolism of paliperidone is limited. However, minor metabolism was observed in incubates of paliperidone with heterologous organisms expressing human CYP3A4 and CYP2D6, suggesting the possible involvement of these two cytochrome P450 (CYP) forms in the metabolism of paliperidone.Citation2 On the other hand, 5-FU is metabolized by CYP2A6 and 5-FU showed little or no inhibitory effect on CYP-catalyzed reactions such as CYP1A2, CYP2C9, CYP2C19, CYP2C8, CYP2E1, CYP2D6, and CYP3A in human liver microsomal preparations.Citation6 Therefore, it is less likely that drug–drug interaction between 5-FU and paliperidone extended release is attributable to CYP-catalyzed reactions.
Paliperidone extended-release tablets use OROS® (Osmotic-controlled Release Oral delivery System) technology. OROS is a controlled-release oral drug delivery system in the form of a tablet.Citation1 The tablet has a rigid, water-permeable jacket with one or more small, laser-drilled holes. As the tablet passes through the body, the osmotic pressure of water entering the tablet pushes the active drug through the opening in the tablet. The result is that the time to maximum plasma concentration of paliperidone extended release is approximately 24 hours, with no detection of the unchanged drug in the feces of healthy volunteers.Citation1 Several drugs such as nifedipine, hydromorphone, venlafaxine, and methylphenidate use OROS technology, with the system having spread all over the world.
Conclusion
In conclusion, this case indicates the existence of a drug–drug interaction between paliperidone extended release and TS-1. Although there is no known prevalence rate, physicians and psychiatrists should be aware of the effects that might result from administering these two drugs together.
Disclosure
The authors report no conflicts of interest in this work.
References
- YangLPPloskerGLPaliperidone extended releaseCNS Drugs2007215417425 discussion 426–42717447829
- VermeirMNaessensIRemmerieBAbsorption, metabolism, and excretion of paliperidone, a new monoaminergic antagonist, in humansDrug Metab Dispos200836476977918227146
- ShirasakaTDevelopment history and concept of an oral anticancer agent S-1 (TS-1): its clinical usefulness and future vistasJpn J Clin Oncol200939121519052037
- HanstenPDHornJRThe Top 100 Drug Interactions: A Guide to Patient Management2013 editionEdmonds, WAH & H Publications2013
- CletonATalluriKLeempoelsJNo pharmacokinetic interaction between trimethorpim and paliperidone in healthy subjects [abstract]Clin Pharmacol Ther20067922
- ParkJYKimKAInhibitory effect of 5-fluorouracil on human cytochrome P(450) isoforms in human liver microsomesEur J Clin Pharmacol2003595–640740912904931