Abstract
Introduction
It has recently been highlighted that patients affected by schizophrenia (SCZ) and those affected by bipolar disorder (BD) undergo gradual chronic worsening of cognitive and social functioning. The objective of the current study was to evaluate and compare (using the Facial Action Coding System [FACS]) the way by which patients with the two disorders experience and display emotions in relation to specific emotional stimuli.
Materials and methods
Forty-five individuals participated in the study: 15 SCZ patients, 15 BD patients, and 15 healthy controls. All participants watched emotion-eliciting video clips while their facial activity was videotaped. The congruent/incongruent feeling of emotions and the facial expression in reaction to emotions were evaluated.
Results
SCZ and BD patients presented similar incongruent emotive feelings and facial expressions (significantly worse than healthy participants); SCZ patients expressed the emotion of disgust significantly less appropriately than BD patients.
Discussion
BD and SCZ patients seem to present a similar relevant impairment in both experiencing and displaying emotions; this impairment may be seen as a behavioral indicator of the deficit of social cognition present in both the disorders. As the disgust emotion is mainly elaborated in the insular cortex, the incongruent expression of disgust of SCZ patients can be interpreted as a further evidence of a functional deficit of the insular cortex in this disease. Specific remediation training could be used to improve emotion and social cognition in SCZ and BD patients.
Introduction
Schizophrenia (SCZ) and bipolar disorder (BD) are severe and disabling diseases. The main clinical features of the acute phases of the two disorders are very different; however, both disorders are characterized by gradual chronic worsening of cognitive and social functioning.Citation1
Cognitive and social impairment of SCZ patients has been widely studied and described in the last decades.Citation2 On the other hand, the course of BD has traditionally been viewed as episodic, with symptomatic and functional recovery between mood episodes;Citation3 this view has recently been challenged by clinical and epidemiological studies that document how, despite symptomatic improvements, many individuals with BD experience difficulties in daily functioning also during recovery following mood episodes (for example, there are higher rates of unemployment and disability among individuals with BD than in the normal population).Citation4–Citation7 Cognitive and social impairments have nowadays been found in BD patients even during the euthymic phase of the disease, thus representing trait-associated, rather than state-associated, characteristics of the disorder.Citation8,Citation9
Social cognition (SC) is a multifaceted construct that focuses on how people process information within social contexts; it includes Theory of Mind (ToM), social knowledge/perception, attributional styles, and emotional processing.Citation10,Citation11 A large body of evidence indicates that both patients with SCZ and those with BD present deficits in some constructs of SC, including ToM and emotional processing, and that these deficits may underlie those areas of the disorders that usually persist despite pharmacological treatments, such as low social functioning, affective flattening, disability, and cognitive deficits.Citation1,Citation12–Citation15
It has been repeatedly reported that SCZ patients show characteristic facial activities, such as reduced levels of facial expressivity in reaction to emotional stimuli and during social interactions; this reduction can be seen as a behavioral indicator of emotional processing deficits.Citation12,Citation16,Citation17
As BD and SCZ seem to share complex chronic deficits in cognitive functioning and SC that are independent from acute psychopathological manifestations, the aim of the present study is to investigate if and/or to what extent BD patients present reduced levels of facial expressivity, as an index of emotional experiences, similarly to SCZ patients.
Materials and methods
Participants
The sample consisted of outpatients recruited at the Alfredo Fiorini Hospital of Terracina, Sapienza University of Rome, and 15 healthy controls. After having signed a written informed consent, patients underwent a structured clinical interview on the model of SCID-I (Structured Clinical Interview for DSM-IV Axis I Disorders) and, according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR), they were divided into two groups: patients with SCZ (n = 15) and patients with BD Type I without psychotic symptoms (n = 15; ). BD patients were in the euthymic phase of the disease while SCZ patients presented a chronic and stable clinical condition; healthy participants were selected to match demographics to the patient group.
Table 1 Study participants
Exclusion criteria were: significant concomitant neurological diseases, other Axis I diagnosis, tardive dyskinesia, history of abuse of alcohol or other drugs of abuse, hospitalization in the last 12 months, pharmacological treatment with typical antipsychotics, clinical onset within the previous 24 months, Hamilton Depression Rating Scale score >7 (for BD patients), young mania rating scale >11 (for BD patients), scores >4 for “delusions”, “conceptual disorganization”, and “hallucinations” items of the positive and negative syndrome scale (for SCZ patients).
At the time of the assessment, all patients were on pharmacological treatment as requested by their clinical condition ().
Table 2 Number of patients treated with each medication
Facial Action Coding System and video clips
The Facial Action Coding System (FACS) by Ekman and FriesenCitation18 is one of the most widely used instruments for the analysis of facial expression. It is based on an anatomical analysis of facial action through the codification of facial expression in 44 “action units” (AUs; ). AUs are anatomically defined; they represent the basic repertory of human facial expressions. Using the FACS, the variety of facial movements can be observed objectively.
The FACS investigator’s guideCitation19 codes the exact combinations of AUs that should be observed in response to each emotional stimulus ().
Figure 2 Expected action units for each emotion.
Abbreviations: AU, action units; R, actions only on the right side of the face; L, actions only on the left side of the face.

The use of video clips to elicit specific emotions has a long history in clinical psychology and psychiatry;Citation20 Gross et al systematized this issue selecting and validating 16 films that successfully elicited amusement, anger, contentment, disgust, sadness, surprise, fear, and a neutral state.Citation21 Video clip details are given in .
Table 3 Video clips used to elicit emotions
Study design
All patients watched video clips designed to elicit significant and specific emotions. The facial expression of each patient in response to the vision of the films was video recorded. These video clips, all in Italian, were watched by the three groups of subjects in the same order, according to the protocol established by GrossCitation21 and according to the protocol of a previous study by our research team:Citation22 video 1 = neutral; video 2 = amusement; video 3 = fear; video 4 = surprise; video 5 = anger; video 6 = sadness; video 7 = disgust; video 8 = amusement.
Between single video clips, patients were asked to fill out a post film questionnaire to indicate what emotion they experienced in relation to the video ().Citation22 Each response to the questionnaire was then scored in relation to the expected emotion.
Table 4 Post film questionnaire
Two different reports were produced. In the report of concordant responses, a score of 1 was given for each emotional report concordant with the expected emotion and a score of 0 was given if the patient reported to not have felt any emotion (neutrality) or to have felt an emotion different from that expected. Scores could range between a minimum of 0 and a maximum of 8; for example, patients obtained an 8 when they reported concordant emotions in response to all the 8 video clips. In the report of discordant responses, a score of 1 was given for each emotional report discordant with the expected emotion associated with the video clip and 0 if the patient reported to not have felt any emotion (neutrality). Scores could range between a minimum of 0 and a maximum of 8; for example, patients obtained the score 8 when they reported wrong emotions in response to all the 8 video clips.
At the end of the film session, the mimic reactions to the video clips were coded following the indications of the FACS investigator’s guide.Citation19 The same three examiners (EP, G V, DL) evaluated all video registrations. The three examiners previously attended a specific 3-day workshop organized by the Centro Ricerche FACS Onlus (CRF; FACS research center) at the University of Trieste. For the scoring it was considered the specific expected emotion associated to the video and the combination of AUs observed in response. Since each video was evocative of only one specific emotion, specific combinations of AUs were expected in relation to each video (). If one of the expected combinations of AUs occurred, the examiners assigned the score 1; if the expected combinations did not occur the examiners assigned the score 0 (this scoring was repeated for each movie clip); for example, patients obtained the score 8 when they presented the expected facial expression ( and ) in response to all eight video clips.
Statistical analysis
The data were analyzed using SPSS software version 20 (IBM Corporation, Armonk, NY, USA). One-way univariate analysis of variance (ANOVA) was used to compare socio-demographic data of participants.
In addition to descriptive statistics, one-way univariate ANOVA was performed to compare the mean scores of participants divided into diagnosis groups. As the diagnosis factor had more than two levels, post-hoc tests were calculated using the Bonferroni test.
Chi-squared and Fisher’s exact test were used to compare the right or wrong facial expression in reaction to emotional stimuli of BD and SCZ patients.
Results
Post film questionnaire – concordant responses
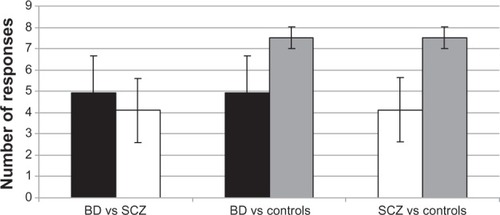
One-way ANOVA determined statistically significant differences between group means (F2 = 25.39, P < 0.01). Mean score ± standard deviation of the report of concordant responses was 4.13 ± 1.50 for SCZ patients, 4.93 ± 1.75 for BD patients, and 7.53 ± 0.51 for healthy controls. Comparing between groups with the post-hoc Bonferroni test revealed that healthy controls had significantly higher scores than both SCZ (difference of means = 3.40; P<0.01) and BD patients (difference of means = 2.60; P < 0.01). There were not significant differences between SCZ and BD patients (P = 0.34; ).
Post film questionnaire - discordant responses
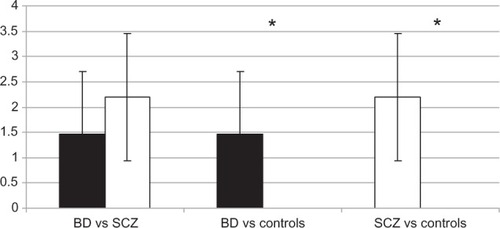
One-way ANOVA determined statistically significant differences between group means (F2 = 17.91, P < 0.01). Mean scores ± standard deviation of the report of discordant responses were 2.20 ± 1.26 for SCZ patients, 1.46 ± 1.24 for BD patients, and 0.00 ± 0.00 for healthy controls. Comparing between groups using the post-hoc Bonferroni test showed that healthy controls had significantly lower scores than both SCZ (difference of means = −2.20; P < 0.01) and BD patients (difference of means = −1.46; P < 0.01). There were no significant differences between SCZ and BD patients (P = 0.17; ).
Facial expressions
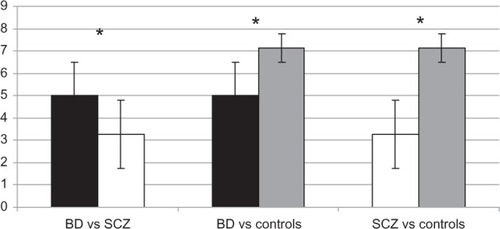
One-way ANOVA determined statistically significant differences between group means (F2 = 33.42, P < 0.01). Mean FACS score was 3.26 ± 1.53 for SCZ patients, 5.00 ± 1.51 for BD patients, and 7.13 ± 0.63 for healthy controls. Comparing between groups using the post-hoc Bonferroni test showed that the FACS score of SCZ patients was significantly lower than BD (difference of means = −1.73; P < 0.01) and healthy controls (difference of means = −3.86; P < 0.01). BD patients and healthy controls also had significantly different scores (different of means = 2.13; P < 0.01; ).
Facial expression in reaction to emotional stimuli in BD and SCZ patients
According to the FACS investigator’s guide, all BD and SCZ patients showed congruent facial expression in response to neutral stimuli, seven BD and two SCZ (P = 0.10) patients showed congruent facial expressions in response to fear stimuli, three BD and four SCZ (P = 0.45) patients showed congruent facial expressions in response to surprise stimuli, five BD and three SCZ (P = 0.68) patients showed congruent facial expression in response to anger stimuli, nine BD and four SCZ (P = 0.13) patients showed congruent facial expression in response to sadness stimuli, 14 BD and seven SCZ (P = 0.01) patients showed congruent facial expressions in response to disgust stimuli, 18 BD and 14 SCZ (P = 0.43) patients showed congruent facial expression in response to amusement stimuli (amusement stimuli were showed twice; ).
Table 5 Patients that expressed congruent facial expression in response to video clips stimuli
Discussion
To our knowledge, this is the first study investigating and comparing the facial expression of patients with SCZ and BD using the FACS system.
As expected, healthy controls showed more appropriate emotional experience and facial expression than patient groups (–). This finding partially confirms the hypothesis that BD patients, as well as SCZ patients, present reduced levels of facial expressivity presumably attributable to deficit of emotional processing and SC.
SCZ patients presented a more compromised facial expression than BD patients (). This issue is consistent with existing data: previous studies already found that deficits in expressing emotions are often present in SCZ patients and correlate with neuroleptics treatmentCitation23 and the intensity of negative symptoms (in particular with the affective flattening).Citation24
The only significant difference between patients’ facial expression in reaction to video clips was found for the expression of disgust (): one BD and eight SCZ patients (P = 0.01) showed incongruent facial expression in response to disgust stimuli represented by scenes from the “Pink Flamingos” film. This issue is consistent with a previous study of Kholer et alCitation25 where it was found that SCZ patients had more severe impairment in relation to specific emotions such as amusement, fear, and disgust.
The deficit in the expression of disgust highlights some possible new evidence with respect to the relationship between neuroanatomy of SCZ. Functional neuroimaging research demonstrated that facial expressions of disgust engage the insular cortex more consistently than other facial expressions;Citation26 furthermore, several studies found that lesions in the insular cortex compromise the capacity of recognizing and expressing the disgust emotion.Citation27–Citation29
The insular cortex is believed to be involved in consciousness and plays a role in diverse functions including perception, motor control, self-awareness, cognitive functioning, and interpersonal experience.Citation30–Citation33 There is an increasingly amount of data suggesting possible hypofunctioning of the insular cortex in SCZ patients,Citation34,Citation35 while there is no data about its possible involvement in BD. As the disgust emotion is mainly elaborated in the insular cortex, the incongruent expression of disgust of SCZ patients could be seen as further evidence of a deficit of the functionality of the insular cortex in SCZ.
The results of the post film questionnaires indicated that BD and SCZ patients seem to present similar relevant impairment in recognizing and experiencing emotions: there was no significant difference between the results of the two groups of patients (both of them having significantly worse results than healthy controls; and ). Difficulty in recognizing and experiencing emotions observed through the facial expression in patients could reflect the impairment in SC recently described in both SCZ and BD.Citation1,Citation14 Frommann et al, Marsh et al, and Lewandowski et al all found that emotional processing and social cognition in SCZ can be improved through targeted remediation training.Citation36–Citation38 This training could potentially be used to improve the global functioning of both SCZ and BD patients in addition to medications and psychotherapies.
Limitations
The small number of participants is a major limitation of the study; for this reason, the results should be considered preliminary. Moreover, the coders were not blinded about the status of patients.
The choice to reduce the power of FACS to a binary number (expression prototype present or absent) makes it a less sensitive measure and as such different degrees of more refined and subtle facial movements could not be evaluated. This step was deemed necessary, however, as it reduced the possibility of codification bias.
Further studies are needed to further assess differences and similarities in emotional responses in BD, SCZ, and other psychiatric diseases.
Disclosure
The authors report no conflicts of interest in this work.
References
- DonohoeGDuignanAHargreavesASocial cognition in bipolar disorder versus schizophrenia: comparability in mental state decoding deficitsBipolar Disord201214774374823020773
- GreenMFCognitive impairment and functional outcome in schizophrenia and bipolar disorderJ Clin Psychiatry20066710e1217107235
- TredeKSalvatorePBaethgeCGerhardAMagginiCBaldessariniRJManic-depressive illness: evolution in Kraepelin’s Textbook, 1883–1926Harv Rev Psychiatry200513315517816020028
- KoganJNOttoM WBauerMSSTEP-BD InvestigatorsDemographic and diagnostic characteristics of the first 1000 patients enrolled in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD)Bipolar Disord20046646046915541061
- TohenMZarateCAHennenJThe McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrenceAm J Psychiatry2003160122099210714638578
- KesslerRCAkiskalHSAmesMPrevalence and effects of mood disorders on work performance in a nationally representative sample of US workersAm J Psychiatry200616391561156816946181
- MorrisCDMiklowitzDJWisniewskiSRGieseAAThomasMRAllenMHCare satisfaction, hope, and life functioning among adults with bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement ProgramCompr Psychiatry20054629810415723025
- Martínez-AránAVietaEColomFCognitive impairment in euthymic bipolar patients: implications for clinical and functional outcomeBipolar Disord20046322423215117401
- de Almeida RoccaCCde Macedo-SoaresMBGorensteinCSocial dysfunction in bipolar disorder: pilot studyAust N Z J Psychiatry200842868669218622776
- PennDLSannaLJRobertsDLSocial cognition in schizophrenia: an overviewSchizophr Bull200834340841118375928
- GreenMFHoranW PSocial Cognition in schizophreniaCurr Dir Psychol Sci2010194243248
- Shamay-TsoorySGShurSHarariHLevkovitzYNeurocognitive basis of impaired empathy in schizophreniaNeuropsychology200721443143817605576
- SparksAMcDonaldSLinoBO’DonnellMGreenMJSocial cognition, empathy and functional outcome in schizophreniaSchizophr Res20101221–317217820609567
- BaezSHerreraEVillarinLContextual social cognition impairments in schizophrenia and bipolar disorderPLoS ONE201383e5766423520477
- BersaniFSCapraEMinichinoAFactors affecting interindividual differences in clozapine response: a review and case reportHum Psychopharmacol201126317718721455971
- AghevliMABlanchardJJHoranW PThe expression and experience of emotion in schizophrenia: a study of social interactionsPsychiatry Res2003119326127012914897
- PolliEBersaniFSDe RoseCFacial Action Coding System (FACS): an instrument for the objective evaluation of facial expression and its potential applications to the study of schizophreniaRiv Psichiatr2012472126138 Italian22622249
- EkmanPFriesenWVFacial Action Coding System: a technique for the measurement of facial movementPalo AltoConsulting Psychologists Press1978
- EkmanPFriesenWHagerJCFacial Action Coding System Investigator’s GuidePalo AltoConsulting Psychologists Press1978
- PhilippotPInducing and assessing differentiated emotion-feeling states in the laboratoryCogn Emot199372171193
- GrossJJLevensonRWEmotion elicitation using filmsCogn Emot19959187108
- BersaniGBersaniFSValerianiGComparison of facial expression in patients with obsessive-compulsive disorder and schizophrenia using the Facial Action Coding System: a preliminary studyNeuropsychiatr Dis Treat2012853754723269872
- SchneiderFEllgringHFriedrichJThe effects of neuroleptics on facial action in schizophrenic patientsPharmacopsychiatry19922552332391357682
- BlanchardJJSayersSLCollinsLMBellackASAffectivity in the problem-solving interactions of schizophrenia patients and their family membersSchizophr Res200469110511715145476
- KohlerCGMartinEAStolarNStatic posed and evoked facial expressions of emotions in schizophreniaSchizophr Res20081051–3496018789845
- PhillipsMLYoungAWScottSKNeural responses to facial and vocal expressions of fear and disgustProc Biol Sci19982651408180918179802236
- SprengelmeyerRYoungAWCalderAJLoss of disgust. Perception of faces and emotions in Huntington’s diseaseBrain1996119Pt 5164716658931587
- SprengelmeyerRYoungAWPundtIDisgust implicated in obsessive-compulsive disorderProc Biol Sci19972641389176717739447734
- CalderAJKeaneJManesFAntounNYoungAWImpaired recognition and experience of disgust following brain injuryNat Neurosci20003111077107811036262
- BamiouDEMusiekFELuxonLMThe insula (Island of Reil) and its role in auditory processing. Literature reviewBrain Res Brain Res Rev200342214315412738055
- CraigADHow do you feel – now? The anterior insula and human awarenessNat Rev Neurosci2009101597019096369
- BalikiMNGehaPYApkarianAVParsing pain perception between nociceptive representation and magnitude estimationJ Neurophysiol2009101287588719073802
- OrtigueSGraftonSTBianchi-DemicheliFCorrelation between insula activation and self-reported quality of orgasm in womenNeuroimage200737255156017601749
- ShepherdAMMathesonSLLaurensKRCarrVJGreenMJSystematic meta-analysis of insula volume in schizophreniaBiol Psychiatry201272977578422621997
- LinnmanCCoombsGGoffDCHoltDJLack of insula reactivity to aversive stimuli in schizophreniaSchizophr Res2013143115015723201307
- FrommannNStreitMWölwerWRemediation of facial affect recognition impairments in patients with schizophrenia: a new training programPsychiatry Res2003117328128412686371
- MarshPJGreenMJRussellTAMcGuireJHarrisAColtheartMRemediation of facial emotion recognition in schizophrenia: functional predictors, generalisability and durabilityAm J Psych Rehab2010132143170
- LewandowskiKEEackSMHogartySSGreenwaldD PKeshavanMSIs cognitive enhancement therapy equally effective for patients with schizophrenia and schizoaffective disorder?Schizophr Res20111252–329129421167689