Abstract
Background
Bipolar disorder (BP) is often associated with a change in hypothalamus– pituitary–adrenal axis function change due to chronic stress. Salivary α-amylase (sAA) levels increase in response to psychosocial stress and thus function as a marker of sympathoadrenal medullary system activity. However, sAA has been studied less often than salivary cortisol in BP patients.
Method
We measured Profile of Mood States and State-Trait Anxiety Inventory scores, heart rate variability, and salivary cortisol levels during electrical stimulation stress in 25 BP patients and 22 healthy volunteers.
Results
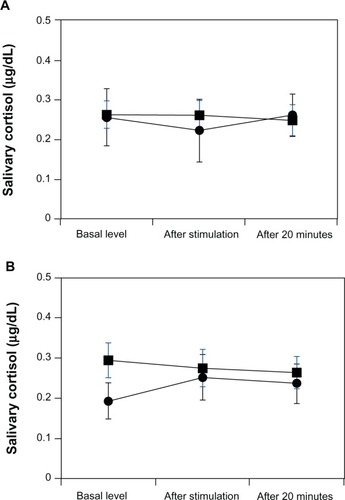
Tension–anxiety, depression–dejection, anger–hostility, fatigue, and confusion scores in BP patients significantly increased compared with those of the healthy controls. In contrast, the vigor scores of BP patients significantly decreased compared with those of the healthy controls. Significant difference in the sAA levels was observed between BP patients and healthy controls. sAA of female patients was significantly higher than that of female healthy controls, and sAA in male patients tended to be higher than that of male healthy controls. No difference in salivary cortisol was observed between BP patients and the healthy controls. Only three time points were measured before and after the electrical stimulation stress. Furthermore, sAA secretion by BP patients increased before and after electrical stimulation.
Conclusion
These preliminary results suggest that sAA may be a useful biological marker for BP patients.
Introduction
A number of studiesCitation1,Citation2 have focused on the specific interaction between bipolar disorder (BP) and stress in the field of psychiatry. Extensive research has been conducted on dysregulation of the hypothalamus–pituitary–adrenal (HPA) axis and its association with BP.Citation3 This dysregulation has become a promising target for drug therapy in BP patients.Citation4 However, the role of the HPA axis in BP is poorly understood. One possibility is that the HPA axis plays an important role in dysregulated systemic cortisol metabolism.
The HPA axis plays an important role in maintaining body homeostasis in response to stressCitation5,Citation6 and is involved in therapy for manic episodes and depression in adults and youth. The HPA axis is also associated with an increase in cortisol levels in saliva, urine, and serum during the daytime. Overactivation of cortisol is associated with lack of suppression of adrenocorticotropic hormone release by corticotrophin-releasing hormone (CRH) challenge, which is not inhibited by dexamethasone (DEX) challenge, and continues to occur during DEX pretreatment.Citation7 Enhancement of the HPA axis activity is due to hyperactivity of CRH in the hypothalamus of BP patients; these findings are based on the failure of suppression with cortisol in glucocorticoid receptors (GR) in the hypothalamus and pituitary gland.
Fluoxetine, amitriptyline, desipramine, and electric shock treatment have been used to normalize CRH levels.Citation8 However, the likelihood of relapse is high in BP patients with sustained dysregulation of the HPA axis.Citation9 HPA hyperactivity has been used as an indicator of BP recurrence.
The sympathoadrenal medullary (SAM) is related to anxiety and awakening.Citation10 It has been suggested that salivary α-amylase (sAA) is an index of SAM activity, because branches of the sympathetic and the parasympathetic nerves are distributed in the salivary glands. Stimulation of sympathetic nerves increases salivary protein secretion; however, stimulation of parasympathetic nerves increases the flow of saliva.Citation11 sAA activity is connected with the sympathetic nervous system stress response.Citation12 Unlike most salivary analytes, such as cortisol and testosterone, that are transported from the plasma, sAA is an enzyme produced locally in the salivary glands that line the mouth. A nerve is resonated and distributed over the salivary glands by the parasympathetic nerve, and salivary secretion from various glands (situated at the bottom of the jaw and the hypoglottis at the parotid) occurs according to neurotransmitter activation. Thus, sAA is a prime candidate for specifying autonomic activity.Citation13 Furthermore, Chatterton et alCitation14 linked sAA to the sympathetic nervous system component of stress response. In particular, Chatterton et al suggest that the plasma norepinephrine level is related to locus coeruleus nucleus/autonomic nervous system activity and that sAA concentration can estimate the stress response in humans. Patients with borderline personality disorder have low salivary cortisol and increased sAA activity in response to psychological stress.Citation15 Ishitobi et al recently reported the levels of sAA and cortisol in unremitted patients.Citation16 sAA levels were positively associated with Hamilton Rating Scale for DepressionCitation17 (HAM-D) scores in unremitted patients with major depressive disorder (MDD).Citation16 Tanaka et al also reported that sAA levels in females with MDD significantly increased compared with female controls before and after an electric stimulus, although no difference in salivary cortisol levels was observed between MDD patients and controls before or after an electric stimulus.Citation18
In this study, we electrically activated the SAM and HPA axis in BP patients and examined the results of composite neuroendocrinology. We hypothesized a difference in sAA and cortisol responsiveness between the patients and control groups. Changes in autonomic measures were expected to parallel changes in sAA and salivary cortisol levels in BP patients.
Materials and methods
Participants
Twenty-eight BP patients and 22 healthy controls participated in this study. Patients were interviewed by a psychiatrist using the semistructured interview in the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV (the Mini-International Neuropsychiatric Interview [MINI]).Citation19 The control group comprised healthy volunteer staff members of the Oita University Hospital, Oita, Japan. Exclusion criteria for controls included the use of any medicine, present tobacco use, a history of BP, body mass index ≥32, or steroid use within the last 3 years. All subjects were requested to refrain from eating within 3 hours of arrival; BP patients were also requested to refrain from taking medications within 5 hours of arrival. All subjects provided informed consent following description of the procedures and had an opportunity to ask questions about the research.
Patients with a main diagnosis of BP were enrolled. Diagnostic exclusion criteria included any acute and/or chronic medical illness as assessed by physical examination and routine laboratory testing. Patients were carefully matched with healthy control subjects for sex and age (except for the use of pharmaceutical drug treatments). A total of 22 patients were eligible; four patients did not meet the inclusion criteria. Moreover, two patients refused the procedures.
The final study sample comprised 25 patients with a main diagnosis of BP; nine of these patients had a second diagnosis of panic disorder and 12 patients suffered from social anxiety disorder. The average age of BP onset was 43.6 years (standard deviation [SD] 12.7 years). The patients were under medication at the time of testing, and these medications included selective serotonin reuptake inhibitors (n=19) and selective norepinephrine reuptake inhibitors (n=3).
Healthy sex- and age-matched control subjects (n=22) were enrolled at Oita University, Oita, Japan. Of 184 volunteers, eleven were excluded due to a present or whole-life major mental disorder according to the MINI. Of the 173 remaining volunteers, 22 were appropriate age- and sex-matched controls for the 25 patients. shows the characteristics of the patients and their matched controls. The research protocol was approved by the ethics committee of the medical department of Oita University.
Table 1 Demographic and medical characteristics by group
Stimulation
Subjects wore a stimulator coil on the wrist connected to a stimulator. This equipment supplied current to the motor and sensory fibers of the median nerve of the right wrist. The subject was stimulated in increasing steps until a threshold value stimulus was reached. We determined the threshold stimulus during the experiment. We gave the following instruction to the subject to determine their strongest tolerable stimulus: “If you cannot take the pain, please tell us.” The strongest stimulus continued 40 seconds. The thresholds of the electric stimulus were between 16 and 27 mA (). Subjects were told that the level of the electric stimulus was sufficient to cause pain but would not cause any other injury. All participants were tested between 1 and 5 pm. Participants were instructed not to smoke, exercise, eat, or consume any caffeine-containing drinks at least 1 hour before testing and 1 hour after the electric stimulus was administered.
Table 2 Characteristics of bipolar disorder patients and controls
Statistical analysis
We adjusted all analyses in agreement with sex and age. Data are shown as the mean ± SD. We analyzed the data using SPSS software (version 19; IBM Corporation, Armonk, NY, USA). We used the χ2 test and t-test for the descriptive characteristics. A two-way analysis of variance followed by Dunnett’s least significant difference test was used to compare sAA and cortisol responses. P<0.05 was considered significant.
Procedures
We measured sAA and salivary cortisol levels three times (before stimulation, immediately after stimulation and 20 minutes after stimulation), similar to previous reports.Citation17,Citation18,Citation20 Exposure to the physical stress and saliva collection were performed between 1 and 5 pm to control for circadian variations in sAA and cortisol levels. sAA was measured using the Dry Chemistry System (Nipro Corporation, Tokyo, Japan) according to the manufacturer’s instructions. Saliva was sampled by directly dipping a saliva-sampling strip under the tongue for 30 seconds.Citation21,Citation22 The strip was immediately placed in an automatic saliva transfer system, and the saliva was transferred by compression to the α-amylase test paper on the reverse side of the strip sleeve. The α-amylase test paper contained the substrate 2-chloro-4-nitrophenyl-4-O-β-D-galactopyranosylmaltoside (Gal-G2-CNP). The enzyme reaction was begun by transfer and compression, and free Gal-G2-CNP levels were optically measured within 20 seconds. The α-amylase activity that reduced sugars equivalent to 1 μmol/minute of maltose was defined as one unit. The concentration of salivary cortisol was analyzed by enzyme-linked immunosorbent assay,Citation23 with intra- and inter-assay coefficients of variation of 3% and 10%, respectively. Samples were stored at −20°C until analysis.
We recorded the high- and low-frequency heart rate variability (HRV) and HRV low-/high-frequency ratio immediately after electrical stimulation by use of the APG Heart-Rater SA-3000P (Tokyo Iken Co, Ltd, Tokyo, Japan).
We administered the State-Trait Anxiety Inventory (STAI)Citation24 and Profile of Mood States (POMS)Citation25 tests before conducting the electrical stimulation protocol. Furthermore, we determined the HAM-D scores before electrical stimulation. BP patients scored ≥8 points on HAM-D.
Results
Tension–anxiety, depression–dejection, anger–hostility, fatigue, and confusion POMS scores in BP patients were significantly increased compared with those in healthy controls (). In contrast, vigor scores in BP patients were significantly decreased compared with those in healthy controls. The STAI state and trait scores significantly increased in BP patients compared with those in healthy controls. No differences were observed in any HRV measure between BP patients and healthy controls. No difference was observed in the threshold of electrical stimulation between BP patients and healthy controls (). Significant differences in sAA levels were observed between BP patients and healthy controls (F[1, 45] =6.96, P<0.01). Significantly higher sAA levels were observed in female BP patients versus female controls (F[1, 20] =4.38, P<0.05) (). A trend toward higher sAA levels was observed in male BP patients versus male controls (F[1, 23] =2.65, P=0.12) (). Finally, no differences in salivary cortisol levels were observed between BP patients and controls (F[1, 45] =0.54, P=0.48) ().
Figure 1 Salivary α-amylase (sAA) responses to electrical stimulation stress in patients with bipolar disorder (BP) and healthy matched control subjects.
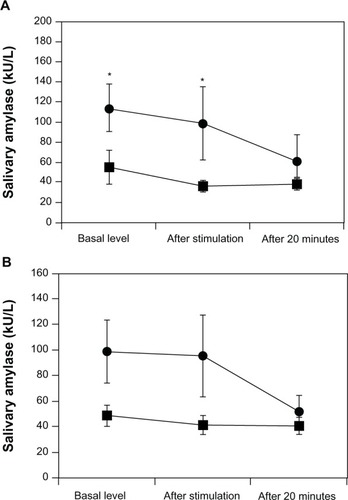
Figure 2 Salivary cortisol responses to electrical stimulation stress in patients with bipolar disorder (BP) and healthy matched control subjects.
Discussion
In the present study, we showed that an acute physical stress induced increased sAA levels in BP patients. sAA levels in BP patients were significantly elevated compared with controls both before and after electrical stimulation. sAA levels have been proposed as a marker of sympathetic nervous system activity, and sAA levels also seem to be a useful measure of SAM axis activation. This study suggests a possible role of sAA before and after electrical stimulation as a potential biomarker of BP.
The influence of physical stress on sAA levels has been reported previously.Citation17,Citation18 In the present acute stress study, we found sAA levels were significantly higher in BP patients than in healthy controls after the stimulation. These results suggest that BP patients may experience fundamentally higher anxiety even before the electrical stimulation. Therefore, sAA levels before an electric stimulus may increase due to anticipatory anxiety in BP patients. The decrease in the vigor score in this group may also support this observation.
We considered a sex difference in the sAA response before and after the electric stimulus. sAA levels in female BP patients significantly increased compared with those in the female controls. A trend toward higher sAA levels before and after the stimulus was observed in male patients with BP compared with male controls. Tanaka et al previously reported that sAA levels in females with MDD are significantly higher than those in healthy controls.Citation18 Filaire et al suggests that there are no sex differences between sAA levels and stress,Citation26 whereas van Stegeren et al suggests that men displayed higher sAA levels compared with women during stressful tasks.Citation27 Thus, further studies are required to examine the relationship between stress and sAA levels in BP patients of both sexes.
Differences might exist between psychological status and hormonal responses. Anticipatory anxiety presumably caused increases in sAA levels in BP patients, whereas prolonged stress suppressed SAM activity in healthy controls. In contrast, prolonged stress did not suppress SAM activity in BP patients, suggesting the maintenance of hormonal responsiveness in these individuals. However, there is not one clear reason for the observed differences between these groups. One possibility is that the duration of BP may have influenced the results. A second possibility is that individual biological or genetic backgrounds in BP patients may have affected the results, since specific genetic polymorphisms are associated with stress sensitivity.Citation28,Citation29 No differences in HRV values or strength of the applied electrical stimulation were observed between the two groups, so biological background did not influence the different hormonal reaction. A third possibility is that the genetic backgrounds of the depressed patients may have influenced the results. We did not check for genetic differences, such as single nucleotide polymorphisms, between the two groups; however, we will be evaluating the influence of SNPs on stress, HPA axis activity, and SAM axis activity in future studies. Further studies are necessary to evaluate the relationships between hormonal responsiveness in relation to depression and genetic composition.
We did not find any differences in salivary cortisol levels between BP patients and controls before or after electrical stimulation. HPA axis functions are usually measured by responses to the combined DEX/CRH test, the DEX suppression test, and basal cortisol levels. It has been reported that the function of the HPA axis is abnormal in BP patients.Citation30 The DEX/CRH test clarifies dysregulated HPA axis function in both remitted and unremitted BP patients. In particular, BP patients show an enhanced cortisol response in the DEX/CRH test in comparison with controls, and this reaction does not significantly differ between remitted and unremitted patients.Citation30 As our participants were examined in the afternoon, between 1 and 5 pm, we may not have observed basic cortisol changes in BP patients. Furthermore, the strength of electrical stimulation may have been insufficient to change cortisol levels, as BP patients and controls in our study did not significantly differ in response to the electrical stimulation.
When we checked the differences in POMS and STAI scores between the BP and control groups, we found that the tension–anxiety, depression–dejection, anger–hostility, fatigue, and confusion scores of the POMS significantly increased in BP patients compared with those in healthy controls, whereas the vigor scores significantly decreased in BP patients. Our results agree with another study that the POMS depression–dejection scale exactly classifies persons with and without BP using the Structured Clinical Interview for DSM-IV diagnoses.Citation18,Citation30 STAI was chosen to evaluate state and trait anxiety. State and trait scores increased in BP patients in comparison with healthy controls, in agreement with another recent study.Citation18 It is important to note that anticipatory anxiety might be related to increases in basal sAA levels. Finally, the Beck Depression InventoryCitation31 and the STAI have correlation coefficients of 0.50 to 0.80,Citation32 and an ongoing depressive state may also have influenced our results.
Our study had four major limitations. One is that the number of patients and healthy controls was relatively small; further studies with a larger study sample should be performed. The second limitation was that the number of hormonal assessments was limited, so the number of tested hormonal responses should be increased in future. The third limitation is that BP patients took medication some point prior to the study, and this may have influenced the results. A fourth limitation is that we set the time period prior to the stress as the baseline, and sAA and salivary cortisol levels may have been different based on the length of time a participant had been in the hospital.Citation33 In future studies, we will try to include patients who are not undergoing drug treatment and make comparisons between treated and non-treated patients.
Conclusion
These preliminary results suggest that sAA could be a biological marker of autonomic function in BP patients. It differentiates between sympathetic and parasympathetic responses, and has been a confounder in autonomic biomarker research. It distinguishes between the generic stress response and the possibly specific stress response associated with BP. It seemed that the current electrical stimulation paradigm did not sufficiently activate the HPA axis, and further studies should consider the strength of the applied stress. The current stress consistently activated the SAM system but was not strong enough to stimulate the HPA axis. Additional studies incorporating more frequent measurements and additional combinations of stress markers will be needed to examine the pathophysiology of BP patients.
Acknowledgments
The study was supported by a Grant-in-Aid for Scientific Research (C) 23591719 from the Ministry of Health and Welfare, Japan.
Disclosure
The authors report no conflicts of interest in this work. All authors declare that the funding source had no impact on the study design or the collection, analysis, and interpretation of data, writing of the report, or decision to submit the study for publication.
References
- SteenNEMethliePLorentzenSIncreased systemic cortisol metabolism in patients with schizophrenia and bipolar disorder: a mechanism for increased stress vulnerability?J Clin Psychiatry2011721515152121367348
- OstiguyCSEllenbogenMAWalkerCDWalkerEFHodginsSSensitivity to stress among the offspring of parents with bipolar disorder: a study of daytime cortisol levelsPsychol Med2011412447245721524333
- DabanCVietaEMackinPYoungAHHypothalamic-pituitary-adrenal axis and bipolar disorderPsychiatr Clin North Am20052846948015826743
- WatsonSGallagherPRitchieJCFerrierINYoungAHHypothalamic-pituitary-adrenal axis function in patients with bipolar disorderBr J Psychiatry200418449650215172943
- HermanJPOstranderMMMuellerNKFigueiredoHLimbic system mechanisms of stress regulation: hypothalamo-pituitary- adrenocortical axisProg Neuropsychopharmacol Biol Psychiatry2005291201121316271821
- ParianteCMGlucocorticoid receptor function in vitro in patients with major depressionStress2004720921916019586
- CarrollBJFeinbergMGredenJFA specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utilityArch Gen Psychiatry19813815227458567
- SchüleCNeuroendocrinological mechanisms of actions of antidepressant drugsJ Neuroendocrinol20071921322617280595
- ZobelAWYassouridisAFrieboesRMHolsboerFPrediction of medium-term outcome by cortisol response to the combined dexamethasone-CRH test in patients with remitted depressionAm J Psychiatry199915694995110360139
- Aston-JonesGRajkowskiJKubiakPAlexinskyTLocus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance taskJ Neurosci199414446744808027789
- BaumBJPrinciples of saliva secretionAnn N Y Acad Sci19932017238105741
- GordisEBGrangerDASusmanEJTrickettPKSalivary alpha amylase-cortisol asymmetry in maltreated youthHorm Behav2008539610317945232
- NaterUMRohlederNGaabJHuman salivary alpha-amylase reactivity in psychosocial stress paradigmInt J Psychophysiol20055533334215708646
- ChattertonRTJrVogelsongKMLuYCEllmanABHudgensGASalivary alpha-amylase as a measure of endogenous adrenergic activityClin Physiol1996164334488842578
- NaterUMBohusMAbbruzzeseEIncreased psychological and attenuated cortisol and alpha-amylase responses to acute psychosocial stress in female patients with borderline personality disorderPsychoneuroendocrinology2010351565157220630661
- IshitobiYAkiyoshiJTanakaYElevated salivary α-amylase and cortisol levels in unremitted and remitted depressed patientsInt J Psychiatry Clin Pract20101426827324917438
- HamiltonMRating depressive patientsJournal of Clinical Psychiatry19804121247440521
- TanakaYIshitobiYMaruyamaYSalivary alpha-amylase and cortisol responsiveness following electrical stimulation stress in major depressive disorder patientsProg Neuropsychopharmacol Biol Psychiatry20123622022422063648
- SheehanDVLecrubierYSheehanKHThe Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10J Clin Psychiatry199859Suppl 2022339881538
- TanakaYIshitobiYMaruyamaYSalivary α-amylase and cortisol responsiveness following electrical stimulation stress in panic disorder patientsNeurosci Res201273808422391145
- ShettyVZiglerCRoblesTFElashoffDYamaguchiMDevelopmental validation of a point-of-care, salivary α-amylase biosensorPsychoneuroendocrinology20113619319920696529
- ShettyVYamaguchiMSalivary biosensors for screening trauma-related psychopathologyOral Maxillofac Surg Clin North Am20102226927820403559
- StricklandPLDeakinJFPercivalCDixonJGaterRAGoldbergDPBio-social origins of depression in the community. Interactions between social adversity, cortisol and serotonin neurotransmissionBr J Psychiatry200218016817311823330
- SpielbergerCDEdwardsCDMontuoriJLusheneRState-Trait Anxiety Inventory for ChildrenMenlo Park, CAMind Garden Inc1973
- McNairDMLorrMDropplemanLFPoms Manual: Profile of Mood StatesSan Diego, CAMulti-Health Systems Inc1992
- FilaireEDreuxBMassartANourritBRamaLMTeixeiraASalivary alpha-amylase, cortisol and chromogranin A responses to a lecture: impact of sexEur J Appl Physiol2009106717719190932
- van StegerenAHWolfOTKindtMSalivary alpha amylase and cortisol responses to different stress tasks: impact of sexInt J Psychophysiol200869334018417235
- ColeSWElevating the perspective on human stress genomicsPsychoneuroendocrinology20103595596220630660
- DeRijkRHde KloetERZitmanFGvan LeeuwenNMineralocorticoid receptor gene variants as determinants of HPA axis regulation and behaviorEndocr Dev20112013714821164267
- PattersonKYoungCWoodsSPVigilOGrantIAtkinsonJHHIV Neurobehavioral Research Center GroupScreening for major depression in persons with HIV infection: the concurrent predictive validity of the Profile of Mood States Depression-Dejection ScaleInt J Methods Psychiatr Res200615758219722288
- BeckATWardCHMendelsonMMockJErbaughJAn inventory for measuring depressionArch Gen Psychiatry1961456157113688369
- WatsonDKendallPCUnderstanding anxiety and depression: Their relation to negative and positive affective statesKendallPCWatsonDAnxiety and Depression: Distinctive and Overlapping FeaturesSan Diego, CAAcademic Press1989326
- BalodisIMWynne-EdwardsKEOlmsteadMCThe other side of the curve: examining the relationship between pre-stressor physiological responses and stress reactivityPsychoneuroendocrinology2010351363137320456867