Abstract
Objective
While acetylcholinesterase inhibitors, such as donepezil, galantamine, and rivastig-mine, are beneficial in treating behavioral symptoms of patients with Alzheimer’s disease (AD), their dose-limiting effects include gastrointestinal disturbances, such as nausea, vomiting, and diarrhea. We aimed to predict the occurrence of these gastrointestinal disturbances with rivastigmine therapy for optimal drug choice and improved compliance.
Materials and methods
Thirty patients with mild-to-moderate AD (scores 10–22 on the MiniMental State Examination) were administered a rivastigmine 18 mg patch with domperidone 30 mg (RWD) and without domperidone (RWOD; n = 15 each) for 20 weeks. Gastrointestinal disturbances were evaluated using a frequency scale for symptoms of gastroesophageal reflux disease (FSSG), Bristol stool form scale, laboratory data (hemoglobin, albumin, total cholesterol), body weight, and amount of food intake.
Results
After 12 weeks, FSSG scores were higher in the RWOD group compared to baseline scores; however, no significant differences were noted between the RWD and RWOD groups. We then subdivided each group based on high and low baseline scores; the RWOD high-score (≥4) subgroup showed increased FSSG after 12 weeks compared with the baseline score. In both RWD and RWOD groups, the low-score (≤3) subgroups showed no changes during the dose-escalation phase.
Conclusion
For AD patients with higher FSSG scores at baseline, domperidone was effective in preventing rivastigmine-related gastrointestinal disturbances.
Introduction
Alzheimer’s disease (AD) is a progressive illness of the elderly. Patients show increasing declines in cognition, and behavioral symptoms occur in all stages of AD. While all AD patients can present with depression, agitation, and aggressive behaviors, behavioral difficulties are most pronounced in the advanced stages of the disease.Citation1–Citation3
Rivastigmine, inhibiting both acetylcholinesterase and butyrylcholinesterase, is the first transdermal treatment available for AD patients, and has been widely approved for the symptomatic treatment of AD.Citation4,Citation5 By providing continuous delivery of medication from the skin into the bloodstream, these patches achieve sustained, high drug concentration in the plasma over 24 hours.Citation6 However, the Investigation of transDermal Exelon in ALzheimer’s disease (IDEAL) study revealed that AD patients receiving a 10 cm2 patch frequently suffered from nausea (7.2%) and vomiting (6.2%).Citation4 Further, Japanese AD patients receiving an 10 cm2 patch also showed similar adverse events, including nausea (7.0%) and vomiting (8.0%).Citation5 AD patients have been reported to stop the medication because of these adverse effects; however, no technique is currently available to predict the occurrence of these adverse effects.
The antiemetic domperidone is often administered in order to administer higher, more effective dosages of cholinesterase inhibitors (ChEIs), and such a combination with domperidone has been shown to be effective in preventing rivastigmine-related gastrointestinal disturbances when administered orally in the rivastigmine capsule form.Citation7 In the present study, we examined the occurrence of gastrointestinal disturbances related to the use of the rivastigmine patch using the frequency scale for the symptoms of gastroesophageal reflux disease (FSSG),Citation8 Bristol stool form scale,Citation9 laboratory data, body weight, and amount of food intake. In addition, we compared the differences in gastrointestinal disturbances when using a rivastigmine patch with domperidone (RWD) and without domperidone (RWOD) in order to examine the efficacy of domperidone orally.
Materials and methods
Patients were recruited from February 2012 to January 2013 at the Department of Neurology, Toho University Omori Medical Center, Tokyo, Japan. Subjects included outpatients diagnosed with AD who lived with or had regular daily visits from a responsible caregiver. The diagnosis of AD was based on the criteria from the National Institute of Neurological Disorders and Stroke and the Alzheimer’s Association.Citation10 The criteria have been established by clinical and neuropsychological examination. Cognitive impairments have to be progressive and be present in two or more areas of cognition. The onset of the deficits has been between the ages of 40 and 90 years, and finally there must be an absence of other diseases capable of producing a dementia syndrome. Patients were excluded if they were undergoing therapy with antiemetic and/or antiulcer medications. A total of 30 patients with mild-to-moderate AD (scores 10–22 on the Mini-Mental State Examination [MMSE]Citation11) were enrolled and randomly allocated to two groups by the envelope method, ie, RWD and RWOD (n = 15 each). Dose titrations of rivastigmine patches were performed every 4 weeks using 4.5 mg/day increments from 4.5 mg/day to 18 mg/day. Domperidone was administered orally at a dose of 30 mg/day in the RWD group. Patients received rivastigmine-patch treatment for 20 weeks, and were evaluated for gastrointestinal disturbances at baseline and at weeks 4, 8, 12, 16, and 20.
The FSSG is a simplified questionnaire for evaluation of the symptoms of gastroesophageal reflux disease (GERD), and has been demonstrated to be a useful tool for objectively evaluating symptoms in GERD patients.Citation8 The questionnaire consists of twelve items that are scored as 0–4 based on the frequency of the symptom (0 = never, 1 = occasionally, 2 = sometimes, 3 = often, and 4 = always) with total scores ranging from 0 to 48. The twelve questions are as follows: (1) Do you get heartburn? (2) Does your stomach get bloated? (3) Does your stomach ever feel heavy after meals? (4) Do you sometimes subconsciously rub your chest with your hand? (5) Do you ever feel sick after meals? (6) Do you get heartburn after meals? (7) Do you have an unusual (eg, burning) sensation in your throat? (8) Do you feel full while eating meals? (9) Do some things get stuck when you swallow? (10) Do you get bitter liquid (acid) coming up into your throat? (11) Do you burp a lot? (12) Do you get heartburn if you bend over?
The Bristol stool form scale consists of seven types (1, separate hard lumps, like nuts; 2, sausage-shaped but lumpy; 3, like a sausage or snake, but with cracks on its surface; 4, like a sausage or snake, smooth and soft; 5, soft blobs with clear-cut edges; 6, fluffy pieces with ragged edges, a mushy stool; and 7, watery, no solid pieces). The FSSG and Bristol stool form scale scores were obtained not only from patients but also from their caregivers to obtain accurate information. If the scores differed between the patient and his/her caregiver, we usually took the caregiver’s score, because of the patient’s cognitive impairment. In addition, we evaluated the changes in laboratory data (hemoglobin [Hb], albumin [Alb], and total cholesterol [T-Chol]), body weight, and amount of food intake to evaluate the nutritional status of AD patients. The amount of food intake per day was set as a score of 10 at baseline, and any improvement or loss in appetite was reflected as increased or decreased scores, respectively, based on patient and caregiver feedback.
Statistical analysis was performed by StatMate (Graph-Pad Software, La Jolla, CA, USA), with the Mann–Whitney U test and Wilcoxon signed-rank test, with P < 0.05 used as the threshold for statistical significance. This study was approved by the Ethical Committee of Toho University Omori Medical Center. All patients and legally accepted caregivers participating in the trial were given an explanation of the trial by the investigators before the start of study, and informed consent to participate in the study was obtained. Consent of patients was obtained orally or in writing, and the consent of legally accepted representatives was always obtained in writing.
Results
Patient characteristics
We enrolled and screened 30 patients in this study. Overall, three patients from the RWD group discontinued their participation due to skin irritation, and one patient from the RWOD group withdrew because of nausea. The patient demographic data are shown in . No differences were observed between the RWD and RWOD groups with respect to mean age, the sex ratio, MMSE score, or body weight.
Table 1 Demographic data
Changes during the dose-escalation phase
The FSSG scores for the RWOD group showed significant increases after 12 weeks compared with baseline (versus 12 weeks, P = 0.035; versus 16 weeks, P = 0.034; versus 20 weeks, P = 0.034); however, this was not observed in the RWD group (). Before initiating rivastigmine-patch therapy, 23 of 30 patients (RWD group, eleven of 15; ROWD group, twelve of 15 patients) showed the presence of some gastrointestinal symptoms based on the FSSG questionnaire. Therefore, we divided each group into two subgroups based on the baseline scores. The median value of the FSSG at baseline was 3.8 in the RWD group and 3.1 in the RWOD group; therefore, we set the high-score threshold ≥4 points and low-score threshold ≤3 points. In the RWOD group, the high-score subgroup showed increased FSSG scores after 12 weeks compared with baseline scores (versus 12 weeks, P = 0.038; versus 16 weeks, P = 0.035; versus 20 weeks, P = 0.034); however, no such changes were observed in the high-score subgroup in the RWD group (). In both RWD and RWOD groups, the low-score subgroups showed no changes in FSSG scores during the dose-escalation phase. The data for MMSE, laboratory results (Hb, Alb, and T-Chol), body weight, amount of food intake, and Bristol stool form scale scores are shown in ; no differences were noted between the baseline values and those at week 20 for either group.
Figure 1 Mean (±standard error) frequency scale for symptoms of gastroesophageal reflux disease (FSSG) scores of the rivastigmine patch (18 mg) with domperidone (30 mg) (RWD) and rivastigmine patch (18 mg) without domperidone (RWOD) groups.
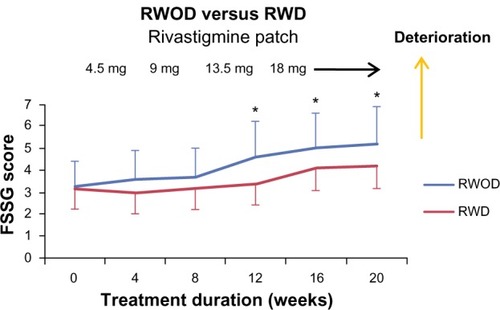
Figure 2 Comparison between high- and low-frequency subgroups for symptoms of gastroesophageal reflux disease (FSSG) scores (±standard error) in the rivastigmine patch (18 mg) with domperidone (30 mg) (RWD) and rivastigmine patch (18 mg) without domperidone (RWOD) groups.
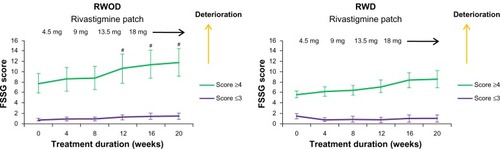
Table 2 Comparison between the RWD and RWOD groups at baseline and week 20
Comparison between RWD and RWOD groups
We compared the data for the RWD and RWOD groups for all the parameters examined. No significant differences were observed between the two groups for the FSSG (), MMSE, laboratory data, body weight, amount of food intake, or Bristol stool form scale scores ().
Discussion
Vomiting is a highly specific physical event, defined as “the rapid, forceful evacuation of gastric contents in retrograde fashion from the stomach up to and out of the mouth.” In contrast, nausea is an entirely subjective experience, defined as “the sensation that immediately precedes vomiting,” wherein patients feel that they are about to vomit. Rivastigmine patch-related gastrointestinal disturbances have been reported in less than 10% of patients receiving this therapy.Citation4,Citation5 In our study, only one patient from the RWOD group discontinued the therapy due to nausea. However, our FSSG questionnaire findings revealed that 23 of 30 AD patients (>75%) suffered from at least one gastrointestinal disturbance before the initiation of the rivastigmine patch. Furthermore, AD patients with higher FSSG were observed to show a further increase in the FSSG score during the dose-escalation phase. Our results may indicate that AD patients do not report gastrointestinal disturbances because of the memory deficits involved in AD.
Domperidone, a dopamine antagonist that acts by blocking dopamine receptors in the chemoreceptor trigger zone, has been previously shown to prevent gastrointestinal disturbances in rivastigmine therapy administered orally.Citation7 In the present study, domperidone was effective in reducing or preventing gastrointestinal disturbances during the dose-escalation phase of rivastigmine-patch therapy in AD patients with higher FSSG scores at baseline.
The FSSG is a convenient tool for evaluating the symptoms of GERD; in our experience, patients/caregivers require approximately 1 minute to complete the questionnaire. The questionnaire consists of twelve detailed items; therefore, the slightest gastrointestinal problem can be easily detected. In order to obtain accurate information in the present study, we confirmed the symptoms described by the AD patients using FSSG questionnaires completed by their caregivers.
At present, there is no technique for predicting the occurrence of gastrointestinal disturbances with rivastigmine-patch therapy. It has been suggested that pretreatment with domperidone may protect against the onset of centrally induced nausea and vomiting induced by ChEIs.Citation12 However, our findings indicate that AD patients with low FSSG scores at baseline may not require additional antiemetic and/or antiulcer medications. On the other hand, AD patients with initial high FSSG scores showed elevations in the FSSG scores during the dose-escalation phase, indicating an increased frequency of gastrointestinal disturbances. Therefore, clinicians should pay careful attention to AD patients with high FSSG scores at baseline during the dose-escalation phase to prevent gastrointestinal disturbances and to avoid patient discontinuation of rivastigmine-patch therapy. Domperidone has, on rare occasions, been associated with acute extrapyramidal syndromes;Citation13 however, our AD patients did not show such side effects.
Randomized, double-blind clinical trials conducted with other ChEIs, such as donepezil and galantamine, have provided evidence that these are safe and effective therapies against cognitive dysfunction and behavioral symptoms.Citation14–Citation16 However, similar to the side effects of rivastigmine-patch therapy, gastrointestinal disturbances are the primary adverse effects associated with this therapy. We speculate that the FSSG may be a useful tool for predicting and preventing ChEI-related gastrointestinal disturbances for these drugs.
The results of this study must be viewed in light of its limitations. This study did not include a placebo for domperidone, and was limited by the small sample size; therefore, improvements in cognitive dysfunction with rivastigmine-patch therapy could not be demonstrated fully. Further studies are needed to confirm our findings.
It is important to optimize the drug for AD in individuals to slow the rate of cognitive dysfunction and behavioral and psychological symptoms of dementia.Citation3–Citation5,Citation17–Citation20 Of note, behavior symptoms in patients with AD increase direct costs of care. For example, a 1-point increase in the Neuropsychiatric Inventory scoreCitation21 was calculated to be equivalent to an increased cost of $247–$409 in total annual direct costs.Citation22 In addition, compared with those who never used cognitive enhancers, patients who used ChEIs had a significant delay in nursing home admission.Citation23
Conclusion
In summary, the group receiving rivastigmine-patch therapy without domperidone showed significant increases in the FSSG score after 12 weeks (dose-escalation phase) compared with baseline scores. Domperidone was effective in preventing rivastigmine patch-related gastrointestinal disturbances in AD patients with higher FSSG scores at baseline. Further, the FSSG questionnaire may be a useful tool for the prediction of gastrointestinal symptoms associated with rivastigminepatch therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
- JostBCGrossbergGTThe evolution of psychiatric symptoms in Alzheimer’s disease: a natural history studyJ Am Geriatr Soc1996449107810818790235
- LopezOLBeckerJTSweetRAPsychiatric symptoms vary with the severity of dementia in probable Alzheimer’s diseaseJ Neuropsychiatry Clin Neurosci200315334635312928511
- KanoOItoHTakazawaTClinically meaningful treatment responses after switching to galantamine and with addition of memantine in patients with Alzheimer’s disease receiving donepezilNeuropsychiatr Dis Treat2013925926523431041
- WinbladBGrossbergGFrölichLIDEAL: a 6-month, double-blind, placebo-controlled study of the first skin patch for Alzheimer diseaseNeurology2007694 Suppl 1S14S2217646619
- NakamuraYImaiYShigetaMA 24-week, randomized, double-blind, placebo-controlled study to evaluate the efficacy, safety and tolerability of the rivastigmine patch in Japanese patients with Alzheimer’s diseaseDement Geriatr Cogn Dis Extra20111116317922163242
- CevcGDrug delivery across the skinExpert Opin Investig Drugs199761218871937
- ScarzellaLCostanzaAVastolaKDomperidone is effective in the prevention of rivastigmine-related gastrointestinal disturbancesFunct Neurol200722210110417637213
- KusanoMShimoyamaYSugimotoSDevelopment and evaluation of FSSG: frequency scale for the symptoms of GERDJ Gastroenterol200439988889115565409
- LewisSJHeatonKWStool form scale as a useful guide to intestinal transit timeScand J Gastroenterol19973299209249299672
- McKhannGDrachmanDFolsteinMKatzmanRPriceDStadlanEMClinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s DiseaseNeurology19843479399446610841
- FolsteinMFFolsteinSEMcHughPR“Mini-mental state.” A practical method for grading the cognitive state of patients for the clinicianJ Psychiatr Res19751231891981202204
- ChampionMCHartnettMYenMDomperidone, a new dopamine antagonistCMAJ198613554574613527396
- ReddymasuSCSoykanIMcCallumRWDomperidone: review of pharmacology and clinical applications in gastroenterologyAm J Gastroenterol200710292036204517488253
- RogersSLDoodyRSMohsRCFriedhoffLTDonepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil Study GroupArch Intern Med19981589102110319588436
- RaskindMAPeskindERWesselTYuanWGalantamine in AD: a 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study GroupNeurology200054122261226810881250
- WinbladBMaintaining functional and behavioral abilities in Alzheimer diseaseAlzheimer Dis Assoc Disord200115Suppl 1S34S4011669508
- TariotPNFarlowMRGrossbergGTMemantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trialJAMA2004291331732414734594
- HowardRMcShaneRLindesayJDonepezil and memantine for moderate-to-severe Alzheimer’s diseaseN Engl J Med20123661089390322397651
- CummingsJLSchneiderETariotPNGrahamSMBehavioral effects of memantine in Alzheimer disease patients receiving donepezil treatmentNeurology2006671576316832078
- WilcockGHoweIColesHA long-term comparison of galantamine and donepezil in the treatment of Alzheimer’s diseaseDrugs Aging2003201077778912875613
- CummingsJLThe neuropsychiatric inventory: assessing psychopathology in dementia patientsNeurology1997485 Suppl 6S10S169153155
- MurmanDLChenQPowellMCKuoSBBradleyCJColendaCCThe incremental direct costs associated with behavioral symptoms in ADNeurology200259111721172912473759
- LopezOLBeckerJTWahedASLong-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer diseaseJ Neurol Neurosurg Psychiatry200980660060719204022