 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Dual dependence on alcohol and nicotine is common, with many reports suggesting that more than 80% of alcoholics also smoke cigarettes. Even after cessation of alcohol consumption, many recovering alcoholics continue to smoke. In this exploratory study, we examined how current smoking and a history of alcoholism interacted in relation to brain volumes and neuropsychological performance.
Methods
Participants were 14 abstinent long-term alcoholics (seven current smokers and seven nonsmokers), and 13 nonalcoholics (six current smokers and seven nonsmokers). The groups were equivalent in age, gender, education, and intelligence quotient. Two multiecho magnetization-prepared rapid acquisition with gradient echo (MP-RAGE) scans were collected for all participants using a 3T magnetic resonance imaging scanner with a 32 channel head coil. Brain volumes for each gray and white matter region of interest were derived using FreeSurfer. Participants completed a battery of neuropsychological tests measuring intelligence quotient, memory, executive functions, personality variables, and affect.
Results
Compared to nonsmoking nonalcoholics, alcoholics who smoke (the comorbid group) had volumetric abnormalities in: pre- and para-central frontal cortical areas and rostral middle frontal white matter; parahippocampal and temporal pole regions; the amygdala; the pallidum; the ventral diencephalic region; and the lateral ventricle. The comorbid group performed worse than nonsmoking nonalcoholics on tests of executive functioning and on visually-based memory tests. History of alcoholism was associated with higher neuroticism scores among smokers, and current smoking was associated with higher sensation seeking scores and lower extraversion scores among nonalcoholics.
Conclusion
Results from this exploratory study support and extend prior reports showing that alcoholism and smoking, alone and in combination, are associated with structural brain abnormalities and poorer performance on neuropsychological tests. Therefore, it is important to consider smoking status in alcoholism studies and vice versa.
Introduction
Cigarette smoking among alcoholics occurs at a considerably higher rate than in the general population. Historically, cigarette smoking rates among heavy drinkers were consistently as high as 90%, although this number has decreased since the early 1990s.Citation1 In 2011, 19% of Americans in the general population were regular smokers.Citation2 Among currently drinking alcoholics, the rate is over twice as high at 45%, and it is as high as 80% in treatment-seeking populations.Citation3 While rates of tobacco use and nicotine dependence vary among alcohol abusers and those who are alcohol dependent,Citation4 it is generally the case that individuals who are the heaviest drinkers also are the heaviest smokers.Citation5 Similarly, smoking alcoholics consume alcohol more frequently than nonsmoking and former-smoking alcoholics,Citation6 and the correlation between smoking and drinking exists for both use and dependence.Citation7 Addressing cigarette smoking in treatment for alcoholism provides an opportunity for improved treatment outcomes, as reductions in smoking are associated with greater success in cessation of alcohol abuse.Citation8
Alcohol and smoking have differential and synergistically harmful effects on many systems in the body, but alcoholics are more likely to die from smoking-related health problems than from those associated with alcohol abuse.Citation9 Chronic smoking and chronic drinking are known to be associated with neurocognitive deficits and brain injury.Citation10–Citation15 As such, it has become clear that studies investigating the effects of either alcohol abuse or cigarette smoking cannot be properly interpreted without information about the use of both substances by the participants studied.
While gray and white matter tissue loss and enlarged ventricles have been well-established in association with long-term alcoholism,Citation16–Citation18 cigarette smoking has been identified as a highly relevant confound in the alcoholism neuroimaging literature.Citation14,Citation15 Both alcoholism and smoking have been shown to be associated with abnormal volumes in numerous brain regions, but many alcoholism studies have failed to report or control for the contribution of cigarette smoking. We have identified ten brain areas that have been shown to be impacted both by alcoholism and by smoking (see for references to studies reporting abnormalities for each area). Therefore, we examined the differential and interacting contributions of each condition within these regions. Of the ten areas, six of these areas are cortical gray matter, including prefrontal cortex,Citation14,Citation17,Citation19–Citation24 precentral (motor) cortex,Citation25–Citation27 anterior cingulate cortex,Citation21,Citation25,Citation28,Citation29 the insula,Citation17,Citation21–Citation25 inferior temporal/lingual cortex,Citation21,Citation23,Citation25,Citation26 and superior temporal cortex.Citation21,Citation23,Citation26 Subcortical gray matter areas include the nucleus accumbensCitation5,Citation17,Citation30 and the thalamus.Citation21–Citation23,Citation27,Citation29,Citation31 The primary white matter structure is the corpus callosum.Citation16,Citation21,Citation32–Citation35 Finally, abnormalities of the cerebellum also have been reported in association with alcoholismCitation21 and smoking.Citation23,Citation26,Citation36 Generally, volumetric reductions in these regions were reported in alcoholic and smoking populations,Citation11 but some studies would suggest that nicotine has a protective effect,Citation37 or that larger regions might represent a risk factor for nicotine addiction and/or an effect of chronic nicotine exposure.Citation30
Table 1 References to studies reporting brain volumetric abnormalities associated with alcoholism and smoking
Neuropsychological tests have shown that alcoholics and smokers suffer from deficits in several overlapping domains, including various aspects of memory. For example, alcoholics are impaired on auditory verbal memory tasks such as the Logical Memory subtest of the Wechsler Memory Scale (WMS), which requires participants to remember and recall a short story.Citation38,Citation39 Likewise, smokers perform poorly on other auditory verbal tasks such as a free recall taskCitation40 and the Rey Auditory Verbal Learning Task.Citation41 Visual memory, which requires recollection of information seen, also has been found to be impaired in alcoholics (for the Rey-OsterriethCitation42–Citation44 and WMS Visual RecallCitation45) and in smokers (for the WMS Visual Memory IndexCitation46). Finally, working memory, which for the purposes of this paper is defined as the mental function involved in storing and manipulating transitory information (as a component of executive functioning), is impaired in alcoholics and in smokers. For example, poor performance on the working memory tasks of Digit Symbol-Coding has been reported in alcoholicsCitation17,Citation39,Citation45,Citation47–Citation51 and smokers.Citation52–Citation54
Processing speed, which may be a component of executive function, can be measured by cancellation tests and has been found to be impaired in conjunction with alcoholismCitation42,Citation48 and with smoking.Citation41,Citation55 Another measure of executive function, the Wisconsin Card Sorting Test (WCST), with its measures of categories completed, perseverative responses, and conceptual responses, has been associated with alcoholism-related deficitsCitation21,Citation43,Citation44,Citation48,Citation51,Citation56,Citation57 and smoking-related deficits.Citation58 Visuospatial cognition requires the ability to identify stimuli; locate objects in space; navigate; and conceptualize distances, areas, and volumes. Alcoholics and smokers perform poorly on the Block Design subtest of the Wechsler Adult Intelligence Scale (WAIS), a test that measures visuospatial capacities.Citation40,Citation42,Citation44,Citation51
Finally, scores on the extraversion scale of the Eysenck Personality Questionnaire (EPQ) have been shown to be positively associated with drinking,Citation59 and scores on the extraversion, psychoticism, and neuroticism scales have been positively associated with smoking.Citation60,Citation61
In the present exploratory study, we compared those alcoholics who were smoking while abstaining from alcohol to those who either had quit smoking or never smoked. We sought to answer these questions: What are the independent and combined effects of alcoholism and smoking? Does the presence of a comorbid smoking addiction mask the effects of the alcohol history under investigation? Many research studies have not considered smoking as a variable in investigations of morphological and neuropsychological sequelae of alcoholism (perhaps due to recruitment challenges, project complexity, or statistical power). Thus, if alcoholic participants are observed to be impaired, researchers might misattribute those impairments to the alcohol history when in fact the deficits could be tied to current or past smoking history. To examine this confounding effect, we examined long-term chronic alcoholic participants who had been sober for months or years.
Among alcoholics, cigarettes have been identified as the most widely used substance at 88% prevalence.Citation62 However, many abstinent alcoholics have had experience with illicit drugs. As with nicotine dependence, there also is high comorbidity between alcoholism and illicit drugs. Dawson and colleagues identified rates of past-year illicit drug use exceeding 20% among an emergency department screening group characterized by individuals with at least monthly drinking sessions of four drinks or more.Citation63 Therefore, in order to isolate the effects of cigarette smoking, we excluded participants with any drug history of more than once per week, and those taking any psychiatric medications.
We sought to confirm the morphometric and neuropsychological abnormalities described above, and extend them by examining the effects of alcoholism and smoking together to distinguish the contributions of each. Thus, we examined several independently justified research questions. We expected that the effects of alcoholism and smoking would vary by the absence or presence of the other. That is, we predicted that alcoholism might have differential effects on smokers and nonsmokers, and as such, we planned to explore alcoholism as a factor in smokers and nonsmokers considered separately (regardless of the interaction effect). Likewise, we predicted that smoking would have differential effects on alcoholics and nonalcoholics, and thus, we explored smoking effects in each of those groups separately. Moreover, the specific brain regions and neuropsychological functions we selected to examine were planned based upon regions of interest identified in the literature on alcoholism and smoking (see ). However, we also sought to discover new relationships with additional morphometric and neuropsychological measures as exploratory analyses.
Materials and methods
Participants
This study included 14 abstinent long-term alcoholics (seven current smokers [sAL] and seven nonsmokers [nsAL]), and 13 nonalcoholic controls (six current smokers [sNA] and seven nonsmokers [nsNA]) (see ). Participation was solicited from newspaper and web-based advertisements and from flyers placed in and around the Boston University Medical Campus, the Veterans Affairs Boston Healthcare System, and the Massachusetts General Hospital. This study was approved by the Institutional Review Boards of all participating institutions, and informed consent was obtained from each subject prior to neuropsychological testing and scanning. Participants were reimbursed for time and travel expenses. Neurobehavioral and psychiatric evaluations typically required 6 to 9 hours over 3 or more days. Participants had frequent breaks, and sessions were discontinued and rescheduled if a participant indicated fatigue.
Table 2 Participant characteristics
Participants underwent a medical history interview and vision testing, plus a series of questionnaires (eg, handedness, alcohol and drug use) to ensure they met inclusion criteria. The groups also were similar in racial and gender distributions and in body mass index (). In order to minimize confounding effects from illicit drug use, psychoactive drug use, and psychiatric comorbidity, participants were given an extensive battery of screening tests. They performed a computer-assisted, shortened version of the Diagnostic Interview Schedule Version IVCitation64 that provides lifetime psychiatric diagnoses according to the Diagnostic and Statistical Manual Fourth Edition criteria.Citation65 Individuals were excluded from further participation if any source (Diagnostic Interview Schedule scores, hospital records, referrals, or personal interviews) indicated that English was not their first language, or if they had any of the following: Korsakoff’s syndrome; human immunodeficiency virus; hepatitis; cirrhosis; major head injury with loss of consciousness greater than 20 minutes; stroke; epilepsy or seizures unrelated to alcoholism; Hamilton Rating Scale for DepressionCitation66 score over 14; major depressive disorder; bipolar I or II disorder; schizoaffective disorder; schizophreniform disorder; schizophrenia; generalized anxiety disorder; or electroconvulsive therapy. All participants reported that they were not currently taking psychiatric medication and had never used illicit drugs more than once a week.
Participants received a structured interview regarding their drinking patterns, including length of abstinence and duration of heavy drinking, ie, the number of years they consumed more than 21 drinks per week (one drink: 355 mL beer, 148 mL wine, or 44 mL hard liquor). A Quantity Frequency Index,Citation67 which roughly corresponds to number of daily drinks, was calculated for each participant. This measure factors the amount, type, and frequency of alcohol usage over the last 6 months (for the nonalcoholic groups), or over the 6 months preceding cessation of drinking (for the alcoholic groups). For one alcoholic participant with a Quantity Frequency Index value lower than three daily drinks, the last 6 months of heavy drinking was used instead. The alcoholic participants met Diagnostic and Statistical Manual Fourth Edition criteria for lifetime alcohol abuse or dependence for a period of at least 5 years, and had abstained from alcohol for at least 4 weeks prior to testing. Inclusion criteria for the currently-smoking group was based on self-reported cigarettes currently smoked per day, and duration of smoking was the number of years that the participants smoked their current amount of cigarettes.
Clinical evaluation and neuropsychological assessment
In order to assess the neuropsychological measures needed for our planned comparisons described in the Introduction, tests of memory, executive function, visuospatial cognition, affect, social cognition (including facial processing), and personality were administered. These assessments included: the WAIS-IV,Citation68 the WMS-IV,Citation69 the EPQCitation70 for extraversion, neuroticism, and psychoticism, the Hamilton Rating Scale for Depression,Citation66 and the Multiple Affect Adjective Check ListCitation71 for depression, anxiety, and sensation seeking. Additionally, the Advanced Clinical SolutionsCitation72 (ACS) for the WAIS-IV was administered to assess social perception, affect recognition from faces and prosody, affect naming, and face recognition. Subjects also were given executive function tests sensitive to frontal brain system disruption, including the WCST,Citation73 two measures from the Delis–Kaplan Executive Function System,Citation74 a modified Trail Making Test versions A and B,Citation75 and the Controlled Oral Word Association Test or FAS test.Citation76,Citation77
Magnetic resonance imaging (MRI) acquisition and processing
Because we were particularly interested in identifying regions with abnormalities that have been implicated in alcoholism and in smoking, we investigated regional volumes while taking into account total brain volumes, so as to highlight regions especially susceptible to the effects of alcoholism and smoking relative to the rest of the brain (or those regions especially relevant as risk factors). This approach has the additional benefit of controlling for the brain size differences associated with gender simply due to differences in head size.
MRI scans were obtained at the Martinos Center for Biomedical Imaging at Massachusetts General Hospital on a 3 Tesla Siemens (Munich, Germany) MAGNETOM Trio Tim scanner with a 32 channel head coil. Image acquisitions included two T1-weighted multiecho magnetization-prepared rapid acquisition with gradient echo (MP-RAGE) scans collected for volumetric analysis and averaged to aid in motion correction (TR = 2530 ms, TE = 1.79 ms, 3.71 ms, 5.63 ms, 7.55 ms [RMS average used], flip angle = 7 degrees, field of view = 256 mm, matrix = 256 × 256, slice thickness = 1 mm with 50% distance factor, 176 interleaved sagittal slices, GRAPPA acceleration factor of 2).
Scans were analyzed using the FreeSurfer processing stream version 5.3.0 (https://surfer.nmr.mgh.harvard.edu) in order to obtain the a priori regional brain volumes described in the Introduction. Volumes of cortical gray matter regions were assessed using FreeSurfer’s automated cortical parcellation algorithm.Citation78,Citation79 Subcortical gray matter regions, corpus callosum regions, ventricular volumes, and other congregate total volumes were derived using a segmentation algorithm.Citation80,Citation81 Volumes of cortically-associated white matter regions were defined according to the overlying gyrus as delineated by the Desikan atlas for FreeSurfer.Citation78,Citation82 The FreeSurfer brain segmentation volume (which excludes the brainstem) was used to define total brain volume.
Statistical analyses
As described in the Introduction, our analyses consisted of independent planned comparisons conducted to confirm and extend literature findings within our conceptual model, followed by exploratory analyses to identify novel associations. The planned comparisons were independently justified (by prior literature) research questions, and the second set of analyses were exploratory. Thus, for both planned and exploratory analyses, multiple comparisons corrections were not applied. As such, results obtained from exploratory analyses should be considered as preliminary findings. All statistical analyses were performed using JMP Pro Version 10.0.1 (SAS Institute Inc, Cary, NC, USA).
Brain volume differences were assessed using a 2 × 2 factorial analysis of covariance (ANCOVA) model, with alcohol history, current smoking status, and their interaction as between-group factors, with age and total brain volume included as covariates. Next, six simple effects comparisons of volumetric differences were performed using ANCOVA: nsNA versus sNA, nsNA versus nsAL, nsNA versus sAL, sNA versus nsAL, sNA versus sAL, and nsAL versus sAL. Results are reported in five sections: 1) interaction effects (with component simple effects), 2) the simple contrast of sAL (the comorbid group) with nsNA, 3) the simple effects associated with alcoholism, 4) the simple effects associated with smoking, and 5) differences between nonalcoholics who smoke and alcoholics who do not smoke.
Neuropsychological scores were scaled by age using normative data, so further correction for age effects was unnecessary. For each subtest score, the interaction of alcohol history and current smoking status was examined using a 2 × 2 factorial analysis of variance (ANOVA) model with all three factors (main effect of smoking, main effect of alcoholism, and the interaction between them). Next, significant simple effects of alcohol history and current smoking on neuropsychological performance were identified using independent samples Student’s t-tests for each of the same six planned comparisons among the groups (as performed for the brain volume analyses). Similarly, results are reported using the same five sections as used for brain volume differences: 1) interactions, 2) comorbid effects, 3) alcoholism, 4) smoking, and 5) differences between smoking nonalcoholics and nonsmoking alcoholics.
Effect size percentages were calculated as the absolute difference between the mean scores of the groups divided by the mean score of all the participants:
Effect sizes for significant findings are presented in , , , , and . Hypothesis test statistics are presented in , , and .
Table 3 Least squares mean volumes (in cm3) of regions of interest
Table 4 Scores of neuropsychological tests
Normality assumptions for all analyses were assessed using normal probability plots, and it was determined that only one score, WCST Categories Completed, was not normally distributed. Those scores were rank transformed prior to ANOVA examination of the interaction of smoking and alcoholism, and the Wilcoxon rank sums test was used in place of the t-test. All models reported did not violate homoscedasticity assumptions as determined by Levene’s test.Citation84 For the volume measures, the interactions of covariates (age and brain volume) and group effects were examined to determine if they satisfied the homogeneity-of-regression assumption. Only one model indicated such an interaction: the right white matter underlying the fusiform. Thus, this finding is not included in our results. Outliers were assessed first in regard to the distributions of the outcome measures (volumes and neuropsychological performance), and then leverage points were identified within significant models. There were no outliers within outcome measures greater than three standard deviations from the mean in each group. Leverage points were defined as any individual observation with a Cook’s distance above 1.0, for each analysis separately.Citation85 There were five leverage points identified above 1.0. When the participant exerting leverage for right ventral diencephalon was removed, the group difference (sAL versus sNA) in volumes no longer was significant, and thus, this contrast was not included in our results. Similarly, when a leverage point for the subcortical gray matter volume was removed, the group difference (nsAL versus nsNA) in volume no longer was significant, and thus, this contrast was not included in our results. For all other results, when the participant exerting the leverage point was removed, all models remained significant. Thus, all statistics reported included all observations.
Results
Participant characteristics
The extent to which the smoking and alcoholism groups differed on demographics and other characteristics is summarized in and . None of the groups varied significantly by age, gender, race, education, WAIS Full Scale IQ, nor total brain volume. By definition, both of the smoking groups (sAL and sNA) smoked more than both of the nonsmoking groups (nsNA and nsAL). Likewise, both of the alcoholic groups (nsAL and sAL) drank more heavily and for a longer duration than nonalcoholics (sNA and nsNA). The alcoholic smoking group smoked an average of 8.4 more cigarettes per day compared to the nonalcoholic smoking group.
Brain volume regions of interest
We evaluated several regional volumes of interest: cortical regions, subcortical gray matter, cortically associated white matter regions, the corpus callosum, and the cerebellum, as specified in the Introduction and Methods sections. Within the cortical areas, we observed significant results for the following regions: four prefrontal areas (left and right caudal middle frontal, right pars orbitalis, right rostral middle frontal), the right precentral cortex, and the anterior cingulate cortex (caudal portion). Within the subcortical structures, we observed significant results for the left nucleus accumbens and the right thalamus. Results are summarized in and , and , and are described below.
Figure 1 Significant group contrasts for brain regions.
Abbreviations: L, left hemisphere; R, right hemisphere; nsAL, alcoholic participants – currently not smoking; nsNA, nonalcoholic participants – currently not smoking; sAL, alcoholic participants (the comorbid group); sNA, nonalcoholic participants – current smokers.
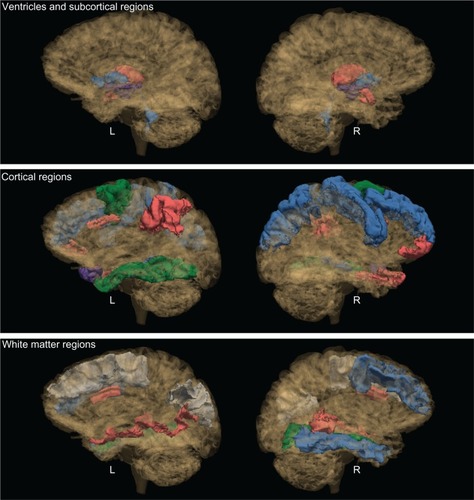
Volume differences associated with the interaction of alcoholism and smoking
A significant interaction between current smoking status and alcoholism history was indicated for the right pars orbitalis (a prefrontal region). The volumes for sNA were found to be 16% smaller than nsNA, whereas no difference was detected among alcoholics between smokers and nonsmokers in this region.
Volume differences between the comorbid group and nonsmoking nonalcoholic controls
The comorbid group also had 7% smaller right precentral cortex volumes than the nsNA group.
Volume differences associated with alcoholism
Among nonsmokers, the left nucleus accumbens was found to be 20% larger for alcoholics (nsAL versus nsNA). Also among nonsmokers, the rostral middle frontal cortex was 10% larger in association with alcoholism. Likewise, the volume of the right precentral cortex was 7% smaller among sAL than sNA.
Volume differences associated with smoking
Among nonalcoholics, the right thalamus was found to be 6% smaller for smokers (sNA versus nsNA). Conversely, the right caudal anterior cingulate was 26% larger (sNA versus nsNA).
Volume differences between smoking nonalcoholics and nonsmoking alcoholics
For both the left and right hemispheres, the caudal middle frontal cortex was found to be smaller in association with nsAL as compared to sNA (9% on the left and 19% on the right).
Volume differences not confirmed
We did not identify significant interactions or simple effects among the groups on the other brain areas shown to be impacted by alcoholism and/or smoking: the insula, inferior temporal/lingual cortex, superior temporal cortex, the corpus callosum, and the cerebellum.
Exploratory regional brain volume analyses
Volume differences associated with the interaction of alcoholism and smoking
Interactions between current smoking status and alcoholism history were observed for several regions: the left pallidum, along with the white matter associated with the left inferior parietal gyrus, the left middle temporal gyrus, the left superior frontal gyrus, and the right banks of the superior temporal sulcus. The left pallidum was found to be about 25% larger in nsNA than in sAL, nsAL, and sNA. The significant interaction indicated that for the left pallidum, the effects of smoking and alcoholism did not appear to be additive. For the white matter of the left inferior parietal gyrus, the simple effects were not significant, but a significant interaction was observed wherein the nsAL and sNA had the largest volumes. The left middle temporal white matter volume was 15% larger in sNA than nsNA, with the interaction indicating that the effect was smaller for sAL versus nsAL. For the left superior frontal white matter, there were no significant pairwise simple effects, but the significant interaction indicated unexpected opposite effects of smoking and alcoholism: nsAL and sNA had the largest volumes, while sAL and nsNA had smaller volumes. The white matter underlying the right banks of the superior temporal sulcus was about 20% smaller in sAL than both nsAL and sNA, indicating a significant additive effect for this region.
No other interactions were observed. Simple effects for further exploratory results are presented in .
Neuropsychological performance
In addition to measures of mood and social cognition, we evaluated several a priori domains of neuropsychological function: memory, executive functioning, visuospatial cognition, and personality, as specified in the Introduction and Methods. Within these domains, we observed significant results for the following tests: WMS Designs (four scores), WAIS Cancellation, WCST (two scores), and the EPQ (two scores). Results are summarized in and , and .
Figure 2 Shown here are the significant findings of differences in neuropsychological measures.
Abbreviations: ACS, advanced clinical solutions; EPQ, Eysenck Personality Questionnaire; MAACL, Multiple Affective Adjective Check List; nsAL, alcoholic participants – currently not smoking; nsNA, nonalcoholic participants – currently not smoking; sAL, alcoholic participants (the comorbid group); sNA, nonalcoholic participants – current smokers; WAIS, Wechsler Adult Intelligence Scale; WCST, Wisconsin Card Sorting Test; WMS, Wechsler Memory Scale.
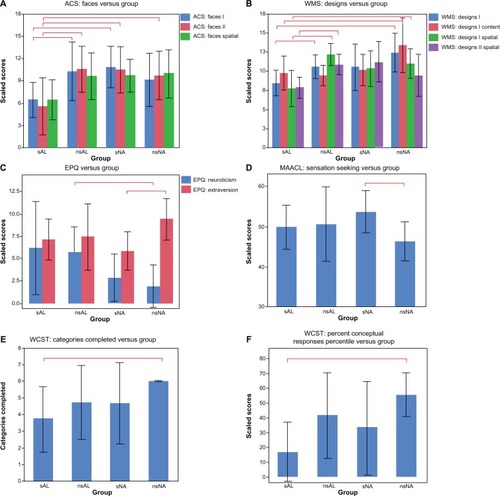
Differences associated with the interaction of alcoholism and smoking
The interaction effect between alcoholism and smoking was significant for Designs I Spatial and Designs II Spatial (see and ). For Designs I Spatial (immediate recall), the scores of the sAL group were significantly worse than those of both the nsAL and nsNA groups; sNA scores were in between. The significant interaction indicated a large effect of smoking for alcoholics in comparison to the significantly smaller impairment associated with smoking observed for controls. In other words, there was a large effect of alcoholism for smokers in comparison to the significantly smaller impairment associated with alcoholism observed for nonsmokers. For Designs II Spatial (delayed recall), sAL scored significantly worse than nsAL, and a similar interaction was observed.
Differences between the comorbid group and nonsmoking nonalcoholics
In addition to the effect of smoking described above, Designs I and Designs I Content scaled scores were observed to be about a third lower for the sAL than for nsNA (). Additionally, several subscores of the WCST showed significant differences between the two groups. All nsNA participants completed all six categories of the test, while the sAL group completed only an average of 3.7 categories (). Conceptual level responses also indicated a strong disadvantage for the sAL group, which scored at the 17th percentile, performing significantly below average, while nsNA scored at the 55th percentile ().
Differences associated with alcoholism
For the EPQ, nsAL were found to be 93% more neurotic than nsNA ().
Differences associated with smoking
For the Designs I subtest of the WMS, nsAL performed 20% better than sAL (). For the EPQ: extraversion, sNA were 48% less extroverted than nsNA ().
Differences between smoking nonalcoholics and nonsmoking alcoholics
For the Cancellation subtest of the WAIS, nsAL performed 31% better than sNA.
Neuropsychological effects not confirmed
The groups did not differ significantly on tests measuring auditory verbal memory (WAIS: Logical Memory I and II), working memory (WAIS: Letter-Number Sequencing and WAIS: Digit Symbol-Coding), or visuospatial abilities (WAIS: Block Design).
Exploratory neuropsychological analyses
Differences associated with the interaction of alcoholism and smoking
The interaction effect between history of alcoholism and current smoking was significant for ACS Faces I and II (see ). For both immediate and delayed recall, the scores for sAL were significantly worse than both nsAL and sNA. For delayed recall, sAL additionally scored significantly worse than nsNA (). In summary, for both scores, the sAL group scored about 50% worse than nsAL and sNA. As was observed for Designs, the interaction indicated a large effect of alcoholism for smokers in comparison to the significantly smaller impairment associated with alcoholism observed for nonsmokers.
No other significant interactions were observed. Simple effects for further exploratory results are presented in .
Discussion
Both alcoholism and smoking have been shown to be associated with impairments in brain and behavior, as measured by regional brain volumes and neuropsychological performances. However, the literature examining the effects of comorbid addictions, and comparing the effects of alcoholism with those of smoking, is sparse. We examined these effects and found evidence of associations of both alcoholism and smoking with regard to: 1) brain volumes for cortical regions, subcortical regions, gyrally-associated white matter regions, and ventricles; and 2) measures of memory, executive functioning, personality, and social cognition.
A number of our findings confirmed and extended the a priori hypotheses suggested by previous literature.Citation20,Citation31 Exploratory analyses revealed further evidence of a relationship of alcoholism and smoking to brain volume and neuropsychological performance.
Brain morphometry
Comorbidity
In studies by Durazzo et alCitation83 and Gazdzinski et al,Citation31 the alcoholic participants had been abstinent for a brief duration (1 week). By comparison, our alcoholics had been abstinent for a much longer duration (about 4 years). Thus, our findings suggested that the abnormalities continue and are persistent. Nevertheless, findings from all of these studies indicated abnormalities associated with alcoholism and smoking, despite differences in the exact brain regions involved.
Parahippocampal volumes were found to be smaller in the comorbid group (sAL) than in nonalcoholics with or without a smoking history. The parahippocampal gyrus abuts the hippocampus and is a crucial structure for memory processing. The neuropsychological measures involving memory reported here indicated that sAL performed worse on WMS Designs and ACS Faces subtests, which require the ability to remember designs and faces, respectively.
Separate effects of alcoholism or smoking
Although Makris et al reported that the right nucleus accumbens was smaller in alcoholics than in nonalcoholics (smoking was not reported),Citation17 unexpectedly, the present study found that nsAL had larger left nucleus accumbens volumes than nsNA, an effect that was not observed for sAL. The nucleus accumbens is a core component of the mesocorticolimbic reward circuitry, which is important for emotional and motivational functions and for memory processing. Further research could help explain why larger nucleus accumbens volumes would be associated with addiction, eg, predisposition to alcoholism or risk-taking behaviors.Citation86 Our results revealed similar findings in another region: the right rostral middle frontal cortex, a component of the prefrontal cortex. This region was smaller in nonalcoholics compared to alcoholics. Previous research has indicated opposite findings,Citation19 although smoking effects were not examined in the same manner. A study by Fein et al showed that abstinent alcoholics had significantly smaller primary motor cortex compared to normal controls.Citation25 Within our sample, we observed smaller primary motor cortex in association with smoking.
Our results revealed that the right thalamus was smaller in sNA compared to nsNA. This confirmed findings from literature suggesting that smokers had a smaller right thalamus.Citation23,Citation27 It is interesting to note that [3H] nicotine binding studies have shown that the thalamus has the highest density of nicotinic acetylcholine receptors in the human brain.Citation87 Das et al speculated that the highest number of nicotinic receptors are found in the same regions where smokers had smaller volumes, eg, in the cerebral cortex, thalamus, substantia nigra, and striatum.Citation30 Thus, these regions might be more susceptible to damage caused by excessive amounts of nicotine.
Among nonalcoholics, smoking was associated with smaller right pars orbitalis (a component of the prefrontal cortex), a region that has been implicated in language production. Pars orbitalis is part of a well-mapped neural circuitry involved in social cognition.Citation88 In the present study, the results of the social cognition tests that involved identifying faces indicated that among alcoholics, smokers performed worse than nonsmokers. In both cases (volume of the pars orbitalis and scores on ACS Faces I and II), we detected an interaction wherein the combination of smoking and alcoholism was significantly worse than either alone.
Our results indicated that smokers had a larger right caudal anterior cingulate gray matter volume than nonsmokers. This result is in contrast with the finding presented in the meta analysis by Pan et al, which indicated that smokers had a smaller anterior cingulate.Citation28 Only one morphometric study was identified that examined the white matter adjacent to the anterior cingulate,Citation26 and, as in the present study, those researchers reported that smokers had a larger volume of anterior cingulate white matter.
Besides looking at the interaction effects, one way to address whether smoking or alcoholism had a larger effect is to compare smoking nonalcoholics and alcoholics who do not smoke (sNA versus nsAL). For both left and right caudal middle frontal volumes, nsAL had smaller volumes than sNA, implying that alcoholism had more of an effect on these volumes than smoking.
From the exploratory data, we also found that the left and the right pallidum were smaller in nsAL than nsNA. The ventral pallidum is a part of the mesocorticolimbic circuitry, which is involved in motivation and emotion, and activated by environmental stimuli associated with rewards. Further, it has been suggested in the literature that the addictive properties of alcohol are regulated by GABAA1 receptors in the ventral pallidum.Citation89,Citation90 Thus, we may speculate that the binding of ethanol to GABA receptors may result in cytoarchitectonic modifications which cause reduced volumes.
Neuropsychological performance
Comorbidity
The associations of these addictions to abnormalities in brain morphometry occur in conjunction with neuropsychological deficits. Among alcoholics, smoking was associated with a variety of impaired spatial memory functions (as measured by WMS Designs subtests and ACS Faces subtests), and executive functions (as measured by the WCST). The Designs subtests assess the ability to remember correct location (Designs Spatial) and correct detail (Designs Content) of the design immediately after seeing the design (Designs I), and after some delay (Designs II). Our findings indicated that the combination of smoking and alcoholism was associated with worse scores than either alone. Of note, although others also have reported learning and memory deficits associated with comorbidity,Citation20,Citation31 we expanded such findings to include abnormalities in memory for spatial aspects of facial stimuli. Regarding executive functioning, only the comorbid group performed worse on the WCST task for both measures: categories completed and percent conceptual responses. The conceptual response score measures understanding of the sorting principle needed for the task by totaling contiguous responses consisting of three or more correct answers. Thus, these results could be interpreted to mean that abstinent alcoholics who smoke are impaired with regard to the reasoning needed to understand an abstract categorical system.
Separate effects of alcoholism or smoking
The evidence presented regarding personality could provide a partial explanation of the deficits observed. The neuroticism measure derived from the EPQ represents temperament aspects characterized by levels of negative affect, including depression and anxiety. In contrast, the EPQ questionnaire also assesses extraversion, which is characterized by high levels of positive affect, such as talkativeness and outgoing qualities. These two personality traits distinguished smokers from drinkers: alcoholics were found to be more neurotic, while smokers were found to be less extraverted. The exploratory analyses revealed a significant pattern of deficits associated with face discrimination, recognition, and perception, in addition to location information associated with the faces. Taken together, these results suggested that both alcoholism and smoking have an impact in relation to cognitive abilities, mental function, and personality.
Limitations
This is a preliminary retrospective study for which we carefully chose, from our larger sample of alcoholics, those participants who did not differ significantly with respect to demographic characteristics, and who were free from potential confounds (eg, psychiatric medications and history of excessive drug use). Consequently, our sample size was small, and additional participants would be needed to increase the statistical strength of the data and to make strong inferential claims. As such, these results should be considered tentative, interpreted with caution, and confirmed in future studies with larger sample sizes. Some studies have shown that aging effects may be nonlinear,Citation82,Citation91 while our analyses assumed a linear impact of age. Detrimental impacts of smoking and alcoholism can also vary by age,Citation92 but we did not find evidence for this within our sample. Gender effects also exist, and thus, the relationships of smoking and alcoholism should be explored in a sample large enough to explore gender effects.
Despite the specific planned nature of the independent comparisons we made based upon regions of interest identified in the alcoholism and smoking literatures, the exploratory analyses we conducted were not corrected for multiple comparisons. This approach may inflate the possibility of making a Type I error. However, it reveals the effects of alcoholism for smokers separately from the effects of alcoholism for nonsmokers, and thereby provides useful avenues for validation and future research. Additionally, this study is cross-sectional, which limits the causal interpretation of results: the findings may be risk factors, consequences, or caused by other factors (eg, body weight, nutrition, exercise, genetic predispositions, etc).
It was difficult to differentiate the effects of smoking history in alcoholic individuals, because we examined alcoholics who were current smokers at the time of testing. However, based on the qualitative data we had obtained, we determined that many of the currently nonsmoking recovering alcoholics had a history of smoking, whereas the nonsmoking nonalcoholic participants had never smoked. Moreover, the comorbid group smoked more than the nonalcoholic smokers. Because the alcoholic smoking group smoked more cigarettes per day compared to the nonalcoholic smoking group, the brain volume changes and poorer performances in the comorbid group could be attributed either to alcoholism or to the larger quantity of cigarettes. Lifetime smoking is known to be associated with volumetric abnormalities, including increased volumes, which perhaps represent predispositions for addiction.Citation30,Citation93–Citation97
Conclusion
Compared to nonsmoking nonalcoholics, alcoholics who smoke (the comorbid group) had the greatest number of cortical and subcortical gray matter volume abnormalities and neuropsychological deficits. Therefore, we recommend that researchers who study alcoholism should, at a minimum, aim to equate their groups by smoking status, ie, include a similar number of smokers within alcoholic and nonalcoholic groups. Structuring the sample in such a way would address confounding but not interaction effects; these need to be examined independently of group matching. Further, when considered in future studies, the interaction of smoking and alcoholism may elucidate methods for smoking cessation programs or alcohol treatment.Citation98–Citation101
Acknowledgments
This study was supported by funds from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants R01-AA007112 and K05-AA000219 to Dr Marlene Oscar Berman, and the Department of Veterans Affairs Medical Research Service, as well as the Center for Functional Neuroimaging Technologies, P41RR14075. The authors thank all research participants as well as Pooja Parikh, Diane Merritt, Mary M Valmas, and Steven Lehar, who assisted with recruitment, neuropsychological assessment, and MRI data collection. We also thank our collaborator Dr Gordon Harris who provided us mentorship at the Massachusetts General Hospital Athinoula A Martinos Center for Biomedical Imaging.
Supplementary tables
Table S1 Statistical comparisons for alcoholism and smoking
Table S2 Volumetric variables presented within models containing age and total brain volume as covariates
Table S3 Neuropsychological variables
Disclosure
The authors report no conflicts of interest in this work.
References
- BoboJKHustenCSociocultural influences on smoking and drinkingAlcohol Res Health200024422523215986717
- Adult Cigarette Smoking in the United States: Current Estimate [webpage on the Internet]AtlantaCenter for Disease Control and Prevention2011 Available from: http://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htmAccessed July 29, 2013
- KalmanDKimSDiGirolamoGSmelsonDZiedonisDAddressing tobacco use disorder in smokers in early remission from alcohol dependence: the case for integrating smoking cessation services in substance use disorder treatment programsClin Psychol Rev2010301122419748166
- FalkDEYiHYHiller-SturmhofelSAn epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related ConditionsAlcohol Res Health200629316217117373404
- NarahashiTSoderpalmBEricsonMMechanisms of alcohol-nicotine interactions: alcoholics versus smokersAlcohol Clin Exp Res2001255 Suppl ISBRA152S156S11391065
- JohnUMeyerCRumpfHJSchumannAThyrianJRHapkeUStrength of the relationship between tobacco smoking, nicotine dependence and the severity of alcohol dependence syndrome criteria in a population-based sampleAlcohol Alcohol200338660661214633650
- National Institute on Alcohol Abuse and Alcoholism (NIAAA)Alcohol and tobaccoAlcohol Alert2007No 7116
- FriendKBPaganoMEChanges in cigarette consumption and drinking outcomes: findings from Project MATCHJ Subst Abuse Treat200529322122916183471
- LittletonJBarronSPrendergastMNixonSJSmoking kills (alcoholics)! shouldn’t we do something about it?Alcohol Alcohol200742316717317526626
- DurazzoTCRothlindJCGazdzinskiSBanysPMeyerhoffDJChronic smoking is associated with differential neurocognitive recovery in abstinent alcoholic patients: a preliminary investigationAlcohol Clin Exp Res20073171114112717451399
- GlassJMAdamsKMNiggJTSmoking is associated with neurocognitive deficits in alcoholismDrug Alcohol Depend200682211912616169161
- MeyerhoffDJTizabiYStaleyJKDurazzoTCGlassJMNixonSJSmoking comorbidity in alcoholism: neurobiological and neurocognitive consequencesAlcohol Clin Exp Res200630225326416441274
- Oscar-BermanMValmasMSawyerKRuizSMLuharRGravitzZProfiles of impaired, spared, and recovered processes in alcoholismPfefferbaumASullivanEVAlcohol and the Nervous SystemNew YorkElsevierIn Press2013
- DurazzoTCMeyerhoffDJNixonSJChronic cigarette smoking: implications for neurocognition and brain neurobiologyInt J Environ Res Public Health20107103760379121139859
- DurazzoTCFryerSLRothlindJCMeasures of learning, memory and processing speed accurately predict smoking status in short-term abstinent treatment-seeking alcohol-dependent individualsAlcohol Alcohol201045650751320923865
- RuizSMOscar-BermanMSawyerKSValmasMMUrbanTHarrisGJDrinking history associations with regional white matter volumes in alcoholic men and womenAlcohol Clin Exp Res201337111012222725728
- MakrisNOscar-BermanMJaffinSKDecreased volume of the brain reward system in alcoholismBiol Psychiatry200864319220218374900
- PfefferbaumASullivanEVRosenbloomMJMathalonDHLimKOA controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year intervalArch Gen Psychiatry199855109059129783561
- PfefferbaumALimKOZipurskyRBBrain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI studyAlcohol Clin Exp Res1992166107810891471762
- CardenasVAStudholmeCGazdzinskiSDurazzoTCMeyerhoffDJDeformation-based morphometry of brain changes in alcohol dependence and abstinenceNeuroimage200734387988717127079
- ChanraudSMartelliCDelainFBrain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioningNeuropsychopharmacology200732242943817047671
- FroeligerBKozinkRVRoseJEBehmFMSalleyANMcClernonFJHippocampal and striatal gray matter volume are associated with a smoking cessation treatment outcome: results of an exploratory voxel-based morphometric analysisPsychopharmacology (Berl)2010210457758320424827
- GallinatJMeisenzahlEJacobsenLKSmoking and structural brain deficits: a volumetric MR investigationEur J Neurosci20062461744175017004938
- ZhangXSalmeronBJRossTJGengXYangYSteinEAFactors underlying prefrontal and insula structural alterations in smokersNeuroimage2011541424820699124
- FeinGShimotsuRChuRBarakosJParietal gray matter volume loss is related to spatial processing deficits in long-term abstinent alcoholic menAlcohol Clin Exp Res200933101806181419645730
- YuRZhaoLLuLRegional grey and white matter changes in heavy male smokersPLoS One2011611e2744022076160
- AlmeidaOPGarridoGJLautenschlagerNTHulseGKJamrozikKFlickerLSmoking is associated with reduced cortical regional gray matter density in brain regions associated with incipient Alzheimer diseaseAm J Geriat Psychiat20081619298
- PanPShiHZhongJChronic smoking and brain gray matter changes: evidence from meta-analysis of voxel-based morphometry studiesNeurol Sci201334681381723207549
- LiaoYTangJLiuTChenXHaoWDifferences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry studyAddict Biol201217697798020731627
- DasDCherbuinNAnsteyKJSachdevPSEastealSLifetime cigarette smoking is associated with striatal volume measuresAddict Biol201217481782521392170
- GazdzinskiSDurazzoTCStudholmeCSongEBanysPMeyerhoffDJQuantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumesAlcohol Clin Exp Res20052981484149516131857
- AgartzIMomenanRRawlingsRRKerichMJHommerDWHippocampal volume in patients with alcohol dependenceArch Gen Psychiatry199956435636310197833
- PfefferbaumALimKODesmondJESullivanEVThinning of the corpus callosum in older alcoholic men: a magnetic resonance imaging studyAlcohol Clin Exp Res19962047527578800395
- PfefferbaumAAdalsteinssonESullivanEVDysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholismNeurobiol Aging2006277994100915964101
- ChoiMHLeeSJYangJWDifference between smokers and non-smokers in the corpus callosum volumeNeurosci Lett20104851717320804817
- KühnSRomanowskiASchillingCBrain grey matter deficits in smokers: focus on the cerebellumBrain Struct Funct2012217251752221909705
- CeballosNATobacco use, alcohol dependence, and cognitive performanceJ Gen Psychol2006133437538817128957
- SullivanEVRosenbloomMJPfefferbaumAPattern of motor and cognitive deficits in detoxified alcoholic menAlcohol Clin Exp Res200024561162110832902
- DaviesSJPanditSAFeeneyAIs there cognitive impairment in clinically ‘healthy’ abstinent alcohol dependence?Alcohol Alcohol200540649850316186142
- HillRDNilssonLGNybergLBackmanLCigarette smoking and cognitive performance in healthy Swedish adultsAge Ageing200332554855012958006
- StarrJMDearyIJFoxHCWhalleyLJSmoking and cognitive change from age 11 to 66 years: a confirmatory investigationAddict Behav2007321636816650620
- BeattyWWHamesKABlancoCRNixonSJTivisLJVisuospatial perception, construction and memory in alcoholismJ Stud Alcohol19965721361438683962
- SullivanEVRosenbloomMJLimKOPfefferbaumALongitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: relationships to changes in brain structureNeuropsychology200014217818810791858
- SullivanEVFamaRRosenbloomMJPfefferbaumAA profile of neuropsychological deficits in alcoholic womenNeuropsychology2002161748311853359
- ReedRJGrantIRourkeSBLong-term abstinent alcoholics have normal memoryAlcohol Clin Exp Res19921646776831530129
- LiuJTLeeIHWangCHChenKCLeeCIYangYKCigarette smoking might impair memory and sleep qualityJ Formos Med Assoc2013112528729023660225
- BeattyWWTivisRStottHDNixonSJParsonsOANeuropsychological deficits in sober alcoholics: influences of chronicity and recent alcohol consumptionAlcohol Clin Exp Res200024214915410698365
- RattiMTBoPGiardiniASoragnaDChronic alcoholism and the frontal lobe: which executive functions are imparied?Acta Neurol Scand2002105427628111939939
- Oscar-BermanMKirkleySMGanslerDACoutureAComparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioningAlcohol Clin Exp Res200428466767515100620
- RosenbloomMJPfefferbaumASullivanEVRecovery of short-term memory and psychomotor speed but not postural stability with long-term sobriety in alcoholic womenNeuropsychology200418358959715291737
- Oscar-BermanMValmasMMSawyerKSFrontal brain dysfunction in alcoholism with and without antisocial personality disorderNeuropsychiatr Dis Treat2009530932619557141
- WhalleyLJFoxHCDearyIJStarrJMChildhood IQ, smoking, and cognitive change from age 11 to 64 yearsAddict Behav2005301778815561450
- CerhanJRFolsomARMortimerJACorrelates of cognitive function in middle-aged adults. Atherosclerosis Risk in Communities (ARIC) Study InvestigatorsGerontology1998442951059523221
- StewartMCDearyIJFowkesFGPriceJFRelationship between lifetime smoking, smoking status at older age and human cognitive functionNeuroepidemiology2006262839216352911
- SpilichGJJuneLRennerJCigarette smoking and cognitive performanceBr J Addict1992879131313261392553
- SullivanEVMathalonDHZipurskyRBKersteen-TuckerZKnightRTPfefferbaumAFactors of the Wisconsin Card Sorting Test as measures of frontal-lobe function in schizophrenia and in chronic alcoholismPsychiatry Res19934621751998483976
- FamaRPfefferbaumASullivanEVPerceptual learning in detoxified alcoholic men: contributions from explicit memory, executive function, and ageAlcohol Clin Exp Res200428111657166515547452
- RazaniJBooneKLesserIWeissDEffects of cigarette smoking history on cognitive functioning in healthy older adultsAm J Geriatr Psychiatry200412440441115249278
- TateDLCharetteLPersonality, alcohol consumption, and menstrual distress in young womenAlcohol Clin Exp Res19911546476521928639
- AraiYHosokawaTFukaoAIzumiYHisamichiSSmoking behaviour and personality: a population-based study in JapanAddiction1997928102310339376772
- SpielbergerCDJacobsGAPersonality and smoking behaviorJ Pers Assess19824643964037120020
- BatelPPessioneFMaitreCRueffBRelationship between alcohol and tobacco dependencies among alcoholics who smokeAddiction19959079779807663320
- DawsonDAComptonWMGrantBFFrequency of 5+/4+ drinks as a screener for drug use and drug-use disordersJ Stud Alcohol Drugs201071575176020731982
- RobinsLNCottlerLBMaking a structured psychiatric diagnostic interview faithful to the nomenclatureAm J Epidemiol2004160880881315466503
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders4th edWashington, DCAmerican Psychiatric Association2000
- HamiltonMA rating scale for depressionJ Neurol Neurosurg Psychiatry196023566214399272
- CahalanDCisinICrossleyHAmerican Drinking Practices: A National Study of Drinking Behavior and AttitudesNew Brunswick, NJRutgers Center for Alcohol Studies1969
- WechslerDWechsler Adult Intelligence Scale4th edLondonPearson2008
- WechslerDWechsler Memory Scale4th edS LondonPearson2009
- EysenckHJEysenckMWPersonality and Individual Differences: A Natural Science ApproachNew York, NYSpringer Dordrecht1985
- ZuckermanMLubinBRobinsSValidation of the multiple affect adjective check list in clinical situationsJ Consult Psychol19652965945846133
- PearsonNAdvanced Clinical Solutions for WAIS-IV and WMS-IV: Administration and Scoring ManualLondonPearson2009
- NelsonHEA modified card sorting test sensitive to frontal lobe defectsCortex19761243133241009768
- DelisDCKaplanEKramerJHDelis-Kaplan Executive Function System (D-KEFS)LondonPearson2001
- ArmitageSGAn analysis of certain psychological tests used for the evaluation of brain injuryPsychol Monogr1946601i48
- BentonAHamsherKSivanAMultilingual Aphasia Examination: Manual of InstructionsIowa CityAJA Associates1994
- SpreenOStraussEA Compendium of Neuropsychological Tests: Administration, Norms, and Commentary1st edNew YorkOxford Univerity Press1991
- DesikanRSSegonneFFischlBAn automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interestNeuroimage200631396898016530430
- FischlBvan der KouweADestrieuxCAutomatically parcellating the human cerebral cortexCereb Cortex2004141112214654453
- FischlBSalatDHBusaEWhole brain segmentation: automated labeling of neuroanatomical structures in the human brainNeuron200233334135511832223
- FischlBSalatDHvan der KouweAJSequence-independent segmentation of magnetic resonance imagesNeuroimage200423Suppl 1S69S8415501102
- SalatDHGreveDNPachecoJLRegional white matter volume differences in nondemented aging and Alzheimer’s diseaseNeuroimage20094441247125819027860
- DurazzoTCCardenasVAStudholmeCWeinerMWMeyerhoffDJNon-treatment-seeking heavy drinkers: effects of chronic cigarette smoking on brain structureDrug Alcohol Depend2007871768216950573
- HowellDCStatistical Methods for Psychology5th edPacific Grove, CADuxbury Thomson Learning2002
- CookRDWeisbergSResiduals and Influence RegressionNew York, NYChapman and Hall1982
- ThayerRECrotwellSCallahanTHutchisonKBryanANucleus accumbens volume is associated with frequency of alcohol use among juvenile justice-involved adolescentsBrain Sci201242605618
- CourtJAMartin-RuizCGrahamAPerryENicotinic receptors in human brain: topography and pathologyJ Chem Neuroanat2000203–428129811207426
- SkuseDHGallagherLDopaminergic-neuropeptide interactions in the social brainTrends Cogn Sci (Regul Ed)2009131273519084465
- HarveySCFosterKLMcKayPFThe GABA(A) receptor alpha1 subtype in the ventral pallidum regulates alcohol-seeking behaviorsJ Neurosci20022293765377511978852
- JuneHLFosterKLMcKayPFThe reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidumNeuropsychopharmacol2003281221242137
- WestlyeLTWalhovdKBDaleAMLife-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetryCereb Cortex20102092055206820032062
- DurazzoTCPenningtonDLSchmidtTPMonAAbeCMeyerhoffDJNeurocognition in 1-month-abstinent treatment-seeking alcohol-dependent individuals: interactive effects of age and chronic cigarette smokingAlcohol Clin Exp Res2013
- MaddenPABucholzKKMartinNGHeathACSmoking and the genetic contribution to alcohol-dependence riskAlcohol Res Health200024420921415986715
- DickDMBierutLJThe genetics of alcohol dependenceCurr Psychiatry Rep20068215115716539893
- FunkDMarinelliPWLeADBiological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factorsAlcohol Res Health200629318619217373407
- GruczaRABierutLJCo-occurring risk factors for alcohol dependence and habitual smoking: update on findings from the Collaborative Study on the Genetics of AlcoholismAlcohol Res Health200629317217817373405
- HendricksonLMGuildfordMJTapperARNeuronal nicotinic acetylcholine receptors: common molecular substrates of nicotine and alcohol dependenceFront Psychiatry201342923641218
- GulliverSBKamholzBWHelstromAWSmoking cessation and alcohol abstinence: what do the data tell us?Alcohol Res Health200629320821217373411
- KodlMFuSSJosephAMTobacco cessation treatment for alcohol-dependent smokers: when is the best time?Alcohol Res Health200629320320717373410
- ZiedonisDMGuydishJWilliamsJSteinbergMFouldsJBarriers and solutions to addressing tobacco dependence in addiction treatment programsAlcohol Res Health200629322823517373414
- PenningtonDLDurazzoTCSchmidtTPMonAAbeCMeyerhoffDJThe effects of chronic cigarette smoking on cognitive recovery during early abstinence from alcoholAlcohol Clin Exp Res20133771220122723432133