Abstract
Levomilnacipran (LVM, Fetzima®) was recently approved by the US Food and Drug Administration for the treatment of major depressive disorder. It is a unique dual neurotransmitter reuptake inhibitor. In contrast with other selective serotonin norepinephrine reuptake inhibitors, including duloxetine, venlafaxine, and desvenlafaxine, it has greater selectivity for inhibiting norepinephrine reuptake than serotonin reuptake. Our review focuses on the efficacy, safety, and tolerability data for five double-blind, placebo-controlled, short-term studies and two long-term studies. In the short-term studies, LVM was found to be more effective than placebo in reducing depression (Montgomery-Åsberg Depression Rating Scale) scores as well as improving functional impairment (Sheehan Disability Scale) scores. Long-term studies found LVM to be similarly effective but in the only placebo-controlled long-term study, LVM was not significantly superior to placebo. LVM is fairly well tolerated, with the most common adverse events being nausea, headache, dry mouth, hyperhidrosis, and constipation. Discontinuation rates were mildly increased in those being treated with LVM (9%) versus placebo (3%). Adverse events were not dose-related except for urinary hesitancy and erectile dysfunction. LVM was weight neutral, was not toxic to the liver, and did not cause clinically significant QTc prolongation. Consistent with being a predominant potentiator of norepinephrine, pulse and blood pressure were significantly elevated by LVM but rarely induced tachycardia or hypertension. LVM is a relatively safe alternative antidepressant treatment with minimal drug–drug interactions. It is the only antidepressant that has in its labeling that it is not only effective in improving depression but also effective in improving impaired functioning. Whether this important effect on functioning is unique to LVM must be researched. In addition, whether LVM might be effective in norepinephrine-deficit depression, refractory depression, atypical depression, or seasonal depression is yet to be evaluated. Ultimately, head-to-head studies comparing LVM with other antidepressants will determine the place of LM in antidepressant treatment.
Introduction
Although antidepressants clearly have been significantly effective in the treatment of major depressive disorder (MDD),Citation1 they have also been disappointing, in that many patients fail to respond, only have a partial response, cannot continue treatment due to intolerable side effects, or relapse despite continuing initially successful antidepressant treatment.Citation2,Citation3 This was highlighted in the STAR*D (Sequenced Treatment Alternatives to Relieve Depression) study in which only approximately 30% of patients had a remission and 50% had a clinical response (improvement of 50% from baseline) to a 12- to 14-week course of citalopram, a selective serotonin reuptake inhibitor (SSRI).Citation2,Citation3 In addition, over 40% of patients had significant side effects.Citation3 Lastly, even for patients who initially responded to treatment with citalopram, approximately 40% relapsed within one year of continued treatment.Citation2–Citation4 Thus, there is need for alternative antidepressants that are more effective and better tolerated than the currently approved antidepressants.
Recently, levomilnacipran (1S, 2R-milnacipran; LVM; Fetzima®, Forest Pharmaceuticals Inc, New York, NY, USA), was approved by the US Food and Drug Administration for the treatment of MDD. It is an enantiomer of the racemic drug, milnacipran, which is approved for the treatment of MDD in Europe and Japan and for fibromyalgia in the USA.Citation5–Citation7 Preclinical studies have found that LVM is a more potent inhibitor of norepinephrine and serotonin (50 and 13 times, respectively) than the less active enantiomer, F2696 (1R, 2S-). Furthermore, it has a better pharmacokinetic profile than F2696, having a longer elimination half-life with a higher maximal concentration and area under the curve.Citation8 Thus, LVM is a dual neurotransmitter reuptake inhibitor of norepinephrine and serotonin. It is unique amongst other dual neurotransmitter reuptake inhibitors in that it predominantly potentiates norepinephrine over serotonin; it has over a 15-fold higher selectivity for norepinephrine versus serotonin reuptake inhibition compared with duloxetine, desvenlafaxine, or venlafaxine.Citation8–Citation10 Interestingly, both in vitro and in vivo animal studies suggest that, at higher doses, serotonergic activity increases so that inhibition of norepinephrine reuptake approaches that of inhibition of serotonin reuptake. LVM lacks affinity for other receptors, including the dopaminergic, adrenergic, histaminic, muscarinic, and opioid receptors.Citation8
The pharmacokinetics of LVM follow linear dynamics between 25 mg/day and 300 mg/day. LVM has a half-life of approximately 12 hours, with a time to peak concentration of 6–8 hours. Absorption is not affected by food intake, and the drug is 22% bound to protein. Metabolism is primarily through cytochrome 3A4. The latter can contribute to potential drug–drug interactions if the concomitant drug is a strong inhibitor of cytochrome 3A4, such as ketoconazole, clarithromycin, or ritonavir. Therefore, in these situations, a maximal dose of 80 mg is recommended. Excretion of LVM is predominantly via the kidney. Thus, the package insert suggests a reduced maximal dose of 80 mg if moderate renal impairment exists (creatinine clearance 30–59 mL per minute) and 40 mg if severe renal impairment exists (creatinine clearance 15–29 mL per minute). LVM should not be used in patients with end-stage renal disease.Citation11
The purpose of this paper is to review the status of LVM, which was approved by the US Food and Drug Administration in July 2014, presenting an overview of its effectiveness, safety, and tolerability. Some speculation as to where LVM might fit in clinically is also presented.
Treatment studies
Short-term studies
Overall, there were five double-blind, placebo-controlled studies evaluating LVM during the acute phase of treatment (8–10 weeks).Citation5,Citation6,Citation12–Citation14 There was a single-blind, placebo, lead-in phase in four of the five studiesCitation5,Citation12–Citation14 (one study did not have a placebo lead-in)Citation6 of one weekCitation5,Citation12–Citation14 and a double-blind, down-taper period of one week in two trialsCitation6,Citation12 and 2 weeks in three trials.Citation5,Citation13,Citation14 An extended-release (ER) form of LVM (the preparation which is approved as Fetzima in the USA), developed for once daily administration was used in these studies.
A total of 2,598 subjects entered the studies (1,588 on LVM ER and 1,032 on placebo). Patients were aged 18–80 years (mean age approximately 43 years) and predominantly female (63.8%). The study population had a long history of depression with multiple episodes and were moderately to severely ill; about 80% had a history of recurrent depressive episodes, with 25% having a current depressive episode of 12 or more months, 46% having a history of MDD for at least 10 years, and the mean Montgomery-Åsberg Depression Rating Scale (MADRS) score at baseline was over 33.Citation15 These studies consisted of two fixed-dose trialsCitation5,Citation12 and three flexible-dose trials.Citation6,Citation13,Citation14 The dose ranged from 40 mg/day to 120 mg/day. All studies were USA-based except for one that took place in Europe, South Africa, and India.Citation6 Four studies were Phase IIICitation5,Citation12–Citation14 and one study was Phase II.Citation6
Continuation phase of treatment
Overall, there were two long-term studies, comprising one study that treated patients openly (not placebo-controlled) with LVM for 48 weeksCitation16 and one that was a 24-week, placebo-controlled, relapse prevention study.Citation17
The relapse prevention study treated patients openly with a flexible LVM dose (40–120 mg/day) for 12 weeks. Those patients who had a clinical response at weeks 10 and 12, defined as a MADRSCitation18 score of ≤12 and a Clinical Global Impression-IllnessCitation19 score of ≤2, were randomized to LVM versus placebo. Those who were randomized to placebo had a down-taper of LVM over one week; those who were randomized to LVM stayed on the same dose that they were on at the end of week 12 in the open-label study. Relapse was defined as a MADRS score of ≥22 and an increase of ≥2 points in the Clinical Global Impression score above baseline just prior to randomization.Citation17
Efficacy measures
The primary efficacy measure for the short-term studies was change from baseline depression rating at the end of the study (week 8 for all studies, except Montgomery et alCitation6 which was week 10) as assessed with the MADRS; the latter is a 10-item rating scale focusing on symptoms of depression. Each item gets a score of 0 to 6, with a total MADRS score potentially ranging from 0 to 60.Citation18 The least squares mean differences (LSMDs) between LVM and placebo (change in score from baseline to the end of the study) using a mixed-effect model for repeated-measures analyses was the main statistical model utilized. In addition, a number of secondary measures were also assessed, such as change in functionality, which was evaluated using the Sheehan Disability Scale (SDS), a self-rating scale.Citation20 The latter included three subscales of functionality, ie, work/school, social life, and family life, with ratings ranging from 0 (not at all) to 10 (extremely). Thus, an SDS total score of 0–30 was derived by adding the scores of the three subscales. LSMDs were also assessed for the SDS results similar to the statistical evaluation applied for the primary efficacy variable, ie, change in depression scores. Response rates and remission rates were assessed for the MADRS and SDS scores. Response regarding the depression variable was defined as a ≥50% decline in the MADRS score from baseline to the end of treatment and remission was defined as a MADRS score of ≤10 at the end of study. Response regarding the functionality variable was defined as a total SDS score of ≤12 and all subscale scores of ≤4 by the end of treatment; a functional remission was defined as a total SDS score of ≤6 and all subscale scores of ≤2 by the end of treatment.
Efficacy results
Primary efficacy variable
A pooled analysis of the total population (five short-term clinical trials) found LVM to be more effective than placebo (LSMD −3.0, P<0.001).Citation15 Four of the five clinical trials found LVM to be significantly more effective than placebo in improving depression (MADRS, ).Citation5,Citation6,Citation12,Citation13 The one study that did not find a significant difference (LSMD −1.5, P=0.260) did find that LVM had a numerically greater response than placebo ().Citation14 The authors of that study suggested an explanation of these negative findings, ie, that although the LVM group showed an antidepressant response similar to that in the positive trials, the placebo group had a particularly marked antidepressant response (2–3 points lower MADRS score than the results of the other positive LVM trials).Citation14 A marked placebo response is occasionally seen in clinical trials, which is known to be contributory to a lack of separation of the test drug from placebo and inconclusive results.Citation21
Table 1 Summary of five double-blind, placebo-controlled, short-term LVM studies
An LSMD score difference of >2 has been described in the literature as noting a clinically meaningful difference between a test drug and placebo.Citation22 Thus, the more than 3-point LSMD score differences between LVM and placebo found for the positive LVM trialsCitation5,Citation6,Citation12,Citation13 suggest that LVM is indeed an effective antidepressant for short-term use (8–10 weeks). The only long-term study that was placebo-controlled (a double-blind, placebo discontinuation study) found that the time to relapse was longer for patients on LVM versus placebo but was not significantly different (P=0.165).Citation17 The authors suggest that the unexpected negative findings probably relate to the low relapse rate of 20.5% seen in the placebo group (versus a relapse rate of 13.9% for the LVM group). The number of patients selected for the study in order to achieve statistical power was based on the assumption that 38% of the placebo group would relapse versus 20% of the LVM group. Clearly, the long-term LVM study may have been underpowered. Thus, the efficacy of LVM for long-term use is unclear at this point.
Secondary efficacy variables
A meta-analysis of the five short-term LVM studies found that the LVM group had a significantly greater response rate (46% versus 36%, P<0.001) and remission rate (28% versus 22%, P<0.05) regarding their depression (MADRS) score then the placebo group.Citation15 The MADRS response rate and remission rate for LVM were significantly greater than placebo in four of the five LVM trials;Citation5,Citation6,Citation12,Citation13 the one trial that failed to show significant superiority of LVM over placebo had a greater placebo response rate (a rate of 34.8%) than the others.Citation14 Of the long-term studies, the open-label, 48-week trial found a MADRS response rate of 73% and a remission rate of 53%.Citation16
Functional impairment has recently become a focus of attention regarding antidepressant treatment of patients with MDD. Clearly, a successful treatment course must not only lead to symptomatic improvement but also lead to a normalization of functioning. Although symptomatic improvement is usually accompanied by improvement in functioning, the latter can still remain impaired.Citation23 This appears to be an important clinical point in that euthymic patients who still have impaired functioning are less likely to have a full recovery and more likely to suffer recurrences.Citation24 Recently, some investigators have suggested that antidepressants with potent noradrenergic mechanisms may be particularly helpful in normalizing impaired functioning in depressed patients partly due to their positive effect on cognitive functioning and concentration.Citation25 Since LVM is unique in having a potent noradrenergic component and one’s functioning is so important, SDS was assessed in the LVM trials.
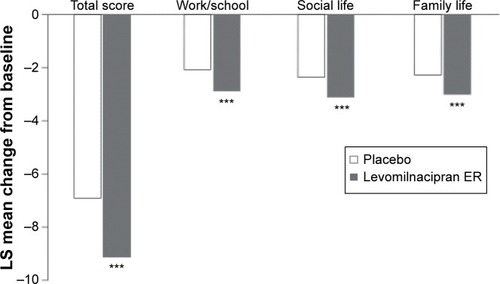
In the overall study population (meta-analysis of the five short-term LVM studies), the mean change in SDS score (end of treatment minus baseline score) was significantly greater for LVM over placebo (LSMD −2.2, P<0.001). In addition, LVM was significantly more effective than placebo in each of the SDS subscales, ie, work/school (LSMD −0.6, P<0.001), social life (LSMD −0.8, P<0.001), and family life (LSMD −0.6, P<0.001, ).Citation26 Four of the five LVM trials found that LVM had a significantly greater positive effect on functionality than placebo ().Citation5,Citation6,Citation12,Citation13 Again, as was the case with the primary efficacy variable, ie, MADRS, the LVM study conducted by Gommoll et al failed to find that LVM was significantly more effective than placebo (LSMD −0.6, not statistically significant).Citation14
Figure 1 Meta-analysis of the five short-term LVM studies evaluating the effect of LVM versus placebo on SDS.
Abbreviations: LS, least squares; LVM, levomilnacipran; SDS, Sheehan Disability Scale; ER, extended-release.
In summary, LVM is effective in improving impaired functioning and improves depressive symptoms in MDD. Whether LVM is more effective than other antidepressants that are not predominantly noradrenergic, such as other serotonin-norepinephrine reuptake inhibitors (SNRIs) or SSRIs, awaits clarification. One recent meta-analysis of duloxetine, another SNRI, found that an asynchrony existed between symptomatic remission and functional remission. Clinical remission frequently was not accompanied by a functional remission.Citation27 Thus, LVM may be unique here.
Clinical dimensions associated with LVM responsiveness
In the five short-termCitation5,Citation6,Citation12–Citation14 and two long-term LVM studies,Citation16,Citation17 ie, a total of seven publications, no clear clinical dimensions were identified as being associated with LVM treatment response. Recently, a meta-analysis of the five short-term LVM studies by Montgomery et alCitation15 and Sambunaris et alCitation26 was conducted using a large database to evaluate whether various baseline clinical dimensions were related to the LVM clinical response (on depression ratings, MADRS scores, and SDS functionality scores). Baseline clinical dimensions assessed included demographics, symptom severity, and MDD history.
Regarding the antidepressant response to LVM, age was evaluated by breaking the groups into <45 years and ≥45 years; <60 years and ≥60 years. Significant effects of LVM on MADRS were seen in all age groups, with the group aged 60 years and older having the greatest response of all the age groups (LSMD −4.4, P=0.002). In addition, drug-placebo differences in response rates were greatest in the group aged ≥60 years. The latter group also had drug-placebo differences in remission rates that were higher than in the group aged <45 years but similar to the other age groups. This is of major interest in that the literature suggests that the elderly may not have as good an antidepressant effect compared with younger depressed populations.Citation28 Thus, uniquely, the antidepressant effect of LVM does not appear to decrease in the elderly.
Regarding sex, both males and females had a greater LVM antidepressant response than placebo, with minimal sex differences in response and remission rates.Citation15 This is an important point, in that the literature suggests a differential response to some antidepressants based on sexCitation29 and that women tend to have a better response than men.Citation30
Severity of illness was also evaluated for its effect on LVM antidepressant responsiveness. The baseline severity of illness of the depressed population in the five acute studies in general was predominantly moderate to severe, with 80% having a MADRS score ≥30 and 40% having a MADRS score ≥35. For these severely ill groups, significant improvement in MADRS over placebo occurred (for the group with MADRS score ≥30 and for the group with MADRS score ≥35, LSMD −2.9 and −3.2 respectively, P<0.001). Approximately 17% of the population had a more mild to moderately severe depression MADRS score of <30. LVM was also significantly more effective than placebo (LSMD −33.5, P<0.01). Thus, LVM was effective in treating depression in both more severe and less severe populations of MDD.Citation15 This is of particular interest in that some studies have suggested that antidepressants may be less effective in severe depressionCitation3 while other studies have suggested the contrary.Citation31 A recent post hoc analysis of desvenlafaxine found that the latter was more effective than placebo, irrespective of the baseline depression scores.Citation32 Thus, LVM also appears to be highly effective as an antidepressant in patients with a full range of severity of illness.
The clinical subgroups of current depressive episode ≥12 months and duration of illness <2 years did not show a significant LVM versus placebo difference; the difference in remission rates between LVM and placebo was 0.1% for the group with a current depressive episode ≥12 months and 1.9% for the group with an illness duration of <2 years. Montgomery et al suggest that these negative findings could relate to the idea that the group with a longer duration of illness may be more refractory to antidepressants and, therefore, perhaps to LVM also, and that depressives with a short current episode (<2 years) had a high placebo response rate, factors that could decrease the likelihood of finding drug-placebo differences.Citation15
Regarding baseline clinical dimensions that may be associated with an effect of LVM on functional impairment, Sambunaris et al found that age, sex, functional impairment at baseline, and severity of illness at baseline were not significant factors.Citation26
Treatment-emergent adverse events
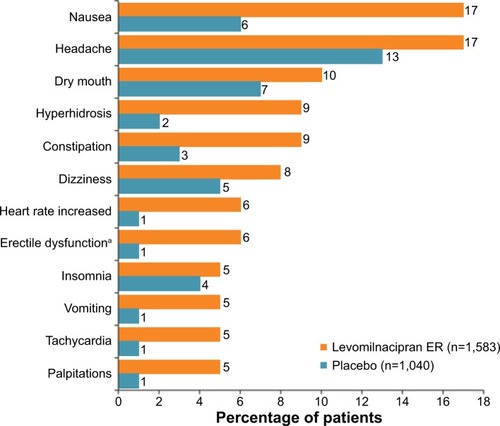
Treatment-emergent adverse events were frequently reported for LVM and placebo (77% versus 61%, respectively) and were thought to be predominantly unrelated to the study medication.Citation33 As can be seen in , the incidence of many adverse events was greater for LVM than for placebo. The treatment-emergent adverse events were mainly of mild to moderate severity. The most common adverse events to LVM were nausea, headache, hyperhidrosis, constipation, and dizziness. Nausea and headache tended to occur early on in treatment and were frequently transient.Citation11,Citation33 Adverse events rarely led to discontinuation of medication. In the above short-term studies, 9% of LVM patients discontinued medication due to an adverse event in comparison with 3% of placebo patients; these rates are similar to those with other antidepressants, eg, venlafaxine (12%) and fluoxetine (7%).Citation34 The only adverse event that led to discontinuation of ≥1% of patients on LVM was nausea (1.5% for LVM and 0.4% for placebo).Citation11,Citation33 The incidence of serious adverse events was similar for LVM (0.7%) and placebo (1.3%). No deaths occurred during the LVM trials.Citation11,Citation33
Figure 2 Common treatment-emergent adverse events (occurring in ≥5% of patients on levomilnacipran ER and ≥2% of patients on placebo.
Abbreviation: ER, extended-release.
There were no dose-related adverse events noted for LVM in the dose range of 40–120 mg/day other than urinary hesitation (4%, 5%, and 6% of patients on LVM 40 mg, 80 mg, and 120 mg, respectively, versus 0% of patients on placebo) and erectile dysfunction (6%, 8%, and 10% of patients on LVM 40 mg, 80 mg, and 120 mg, respectively, versus 2% of patients on placebo); higher doses were associated with an increased incidence of erectile dysfunction and urinary hesitation.Citation11,Citation33 Regarding the latter, males appear to be particularly vulnerable.Citation35–Citation36
Although none of the cases of LVM-induced urinary hesitancy led to urinary retention or failure, cases where other antidepressants induced urinary hesitancy occasionally progressed rapidly to a medical emergency.Citation37 Thus, caution is advisable in patients who develop urinary hesitancy. The package insert suggests consideration of drug discontinuation if urinary hesitancy occurs. The package insert also suggests caution if using LVM in patients prone to obstructive urinary disorders.Citation11 A prior history of a urinary obstructive disorder appears to predispose patients to urinary hesitancy secondary to antidepressants.Citation36
Cardiovascular effects
LVM was associated with tachycardia, increased heart rate, and palpitations (). In addition, increased blood pressure (BP), hypertension, and hypotension occasionally occurred.Citation11,Citation33 During the course of treatment in the short-term (8- to 10-week) studies, systolic and diastolic BP increased at the end of treatment by 3.0 mmHg and 3.2 mmHg, respectively, in contrast with placebo, which was associated with a negligible effect on systolic BP (−0.04 mmHg) and diastolic BP (−0.0 mmHg). Further, 1.8% of LVM patients and 1.2% of placebo patients met broad criteria for hypertension defined a priori (systolic BP ≥140 mmHg and an increase ≥15 mmHg or diastolic BP ≥90 mmHg and an increase ≥10 mmHg on at least three consecutive visits); 0.3% of LVM patients and 0.1% of placebo patients met strict criteria for hypertension defined a priori (systolic BP ≥140 mmHg and an increase ≥15 mmHg and diastolic BP ≥90 mmHg and an increase ≥10 mmHg on at least three consecutive visits).Citation11 Hypotension was seen in 11.6% of patients on LVM versus 9.7% of patients on placebo. There was no dose relationship between LVM and BP findings.Citation11,Citation33 Regarding the long-term LVM studies, the open-label, 48-week trial found an increase in systolic BP of 3.9 mmHg and an increase in diastolic BP of 3.3 mmHg.Citation16 During the long-term, double-blind, placebo-discontinuation study, BP changes were minimal in the LVM and placebo groups.Citation17 Thus, although LVM clearly can increase BP and occasionally induces a hypertensive state, this is relatively uncommon. If sustained hypertension develops, LVM may have to be discontinued. No data has been accumulated as yet in hypertensive states existing prior to initiating LVM treatment; thus, significant caution is suggested. It is strongly recommended that hypertensive patients should first be aggressively treated for their BP problems and hopefully become normotensive prior to initiating antidepressant treatment with LVM. Clinicians should carefully monitor BP prior to and periodically during treatment with LVM.Citation11
In addition to having effects on BP, LVM is also known to affect heart rate; short-term studies found that the LVM group had an increase in heart rate of 7.4 beats per minute versus the placebo group, which had a decrease in heart rate of 0.3 beats per minute.Citation11,Citation33 During the long-term trials, there was an increase of 9.1 beats per minute in the open-label LVM study,Citation16 and in the placebo-controlled study, there was an increase of 12.3 beats per minute for LVM and 3.6 beats per minute for placebo.Citation17 The package insert suggests that if people experience an increased heart rate that is sustained, the drug may have to be discontinued or the event be adequately treated. Since minimal data exist on LVM in depressed patients with a cardiac rhythm disorder, the package insert recommends that patients with a cardiac rhythm disorder at baseline should be adequately treated for this condition prior to LVM therapy.Citation11
The main reason for a focus on the potential of an antidepressant to prolong the QTc interval is that prolongation is associated with torsades de pointes and sudden cardiac death.Citation38 A number of psychotropic medications, including antidepressants such as citalopram, venlafaxine, and mirtazapine, have been associated with QTc prolongation.Citation39,Citation40 Thus, knowing what effect LVM might have on the QTc interval is clinically relevant.
The QTcB (Bazett’s correction) was mildly higher in the LVM group than in the placebo group, but none of the patients in the short-term studies experienced a potentially clinically significant prolongation (>500 msec) of QTcB or QTcF (Fridericia correction). At doses that were 2.5 times the maximum recommended dose (300 mg), LVM did not elevate QTc to a clinically relevant extent.Citation11 Since LVM is associated with an increased heart rate, the QTcF is a preferred measurement of ventricular repolarization since it adjusts for heart rate. In the long-term, open-label LVM study, one patient met the criteria for abnormal QTcB but had a normal QTcF value.Citation16 In the placebo-controlled, double-blind, discontinuation study, no patient had an abnormal QTcB or QTcF interval; the QTcF interval on LVM was −1.3 msec and on placebo was 1.4 msec.Citation17 Thus, it appears that LVM can be used without much concern for inducing a cardiac arrhythmia.
Chemistry, hematological, and urinalysis changes
Regarding the potential effect of antidepressants on chemistries, considerable attention has always been paid to liver function. Occasionally, antidepressants and other drugs can induce a drug-induced liver injury which, if accompanied by jaundice and hepatocellular damage, can lead to a liver transplant or death in over 10% of cases.Citation41 To help guard against this possible adverse event, Hy’s law was established to predict liver failure. Hy’s law includes having an alanine aminotransferase or aspartate aminotransferase level greater than three times the upper limit of normal, a total bilirubin greater than twice the upper limit of normal, and an alkaline phosphatase level less than twice the upper limit of normal.Citation41 No patient in the LVM trials met Hy’s law. Alanine aminotransferase and aspartate aminotransferase were found to be, at most, mildly higher in LVM-treated versus placebo-treated patients.Citation5,Citation12 Thus, LVM appears not to be toxic to the liver. Even when there is baseline pathology in the liver (mild, moderate, or severe liver impairment), the dose of LVM does not need to be modified since it is minimally metabolized by the liver.Citation11 Further, the LVM-treated and placebo-treated groups did not differ regarding other chemistry, hematological findings, and urinalysis.Citation11
Body weight
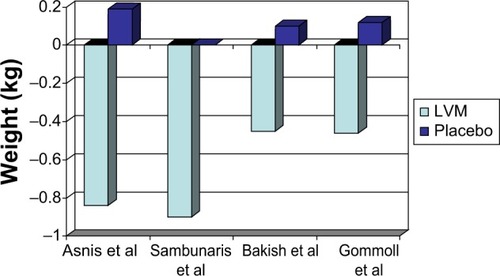
Four of the five short-term LVM trials reported weight change during the trial in their publications.Citation5,Citation12–Citation14 There was a mean weight change of −0.5 kg for those treated with LVM and 0.1 kg for those treated with placebo ().
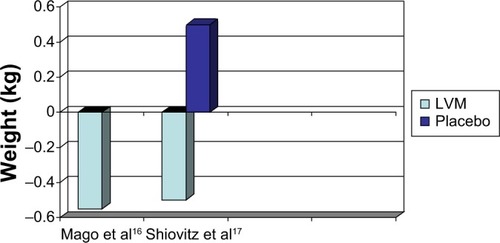
Regarding the long-term studies (), there was a −0.55 kg weight change by the end of treatment in the 48-week, open-label study.Citation16 In the other long-term LVM study (a placebo-controlled, randomized, discontinuation study), there was a −0.5 kg weight change for LVM versus a 0.5 kg weight change for placebo at the end of 24 weeks.Citation17 Clearly, LVM was weight neutral both for short-term use (8 weeks) and long-term use (24–48 weeks).
Suicidal behavior
For the last decade, there has been careful scrutiny as to whether an antidepressant is associated with the development or worsening of suicidal ideation and behavior in patients with depression and other psychiatric disorders. Retroactive analysis of a number of short-term clinical trials with various SSRIs, SNRIs, and tricyclic antidepressants have reported a mild but significant increase in suicidal ideation and suicidal behavior in those treated with antidepressants versus placebo in children, adolescents, and young adults (aged 18–24 years)Citation11,Citation42 but a neutral to reduced suicidal risk for adults over the age of 24 years.Citation11,Citation43 In a review of the clinical trials leading up to this warning, no actual suicides were found in the database regarding children, adolescents, and young adults.Citation11,Citation42 In spite of the fact that the original database for this analysis only evaluated nine antidepressants in children and adolescents and eleven antidepressants in adults including some SSRIs, SNRIs and tricyclic antidepressants, all antidepressants, including LVM, have a black box warning. Regarding specific treatment-emergent suicidal ideation and suicidal behaviors in the short-term LVM studies, four of the five trialsCitation5,Citation12–Citation14 evaluated and monitored suicidal ideation and behavior at baseline and during the treatment program using a structured rating instrument, the Columbia-Suicide Severity Rating Scale.Citation44 The treatment-emergent adverse event of suicidal ideation and behavior was no different between LVM and placebo.Citation11,Citation33 Suicidal ideation worsened or developed in three patients taking LVM and in one patient on placebo,Citation5,Citation14 whereas suicidal behavior occurred in two patients on LVM and two patients on placebo.Citation5,Citation12 No completed suicides occurred in any of the short-term LVM studies. Thus, regarding antidepressant-induced suicidal ideation and behavior, LVM appears to be safe. The two long-term studies also found that LVM rarely induced suicidal ideation and behavior.Citation16,Citation17 The one long-term study that was placebo-controlled found that three patients (2.7%) on placebo developed suicidal ideation versus eleven patients (4.8%) on placebo. No suicidal behavior occurred in either treatment group.Citation17 A note of caution should be made here. The LVM trials excluded patients who had a significant suicidal risk-prior suicide attempt in the past year or severe suicidal ideation during the screening period. It is possible that such at-risk patients may be more vulnerable to LVM (antidepressant)-induced worsening or development of suicidal ideation and behavior. Out of caution, the package insert states that clinicians must monitor carefully the risk of suicidal behavior, both prior to initiating and during LVM treatment, as is requested for all treatment with antidepressant medications in general.Citation11
Administration guidelines
LVM is an ER preparation and is to be taken once a day. The package insert suggests that it be taken at the same time every day, and that it is not affected by food intake and should be swallowed whole (“not chewed, opened or crushed”).Citation11 As with most medications that have nausea as a common side effect, we agree with Mago et al that LVM should be taken after the intake of food to possibly buffer such an effect.Citation45 LVM comes in four capsule strengths, ie, 20 mg, 40 mg, 80 mg, and 120 mg. The package insert suggests that the starting dose should be 20 mg/day for a minimum of 2 days and increased by 20 mg/day every 2 days, depending on tolerance and efficacy, up to a maximum dose of 120 mg/day. The therapeutic dose is 40–120 mg/day.Citation11 Thus, dose increases can be made rather quickly; nonetheless, we suggest a somewhat slower upward titration of the dose since some of the potential adverse events are dose-dependent (urinary hesitancy, erectile dysfunction) and possibly can be avoided and/or minimized with a slower dose titration. In addition, it is somewhat unclear how effective “pushing the dose” is, since there is a question as to whether there is in fact a clear dose-response relationship. A recent presentation by Asnis et al found that although LSMD scores appeared larger at higher doses for MADRS and SDS scores, there were no statistically significant differences between the lowest dose of 40 mg and the highest dose of 120 mg.Citation46 Clearly, many patients respond to lower doses, ie, 40 mg and 80 mg, as was demonstrated in the two fixed-dose studies.Citation5,Citation12 One can always increase the dosage if lack of response becomes an issue.
When discontinuing LVM, the package insert suggests to gradually reduce the dose over time if possible in order to avoid and/or minimize “discontinuation symptoms”.Citation11 We suggest that the discontinuation follow a slow down-taper of the medication from 1 to 2 weeks as was the design of the LVM clinical trials; if a patient develops any symptoms during this period, the down-taper can always be stretched out longer. “Discontinuation symptoms” have been well documented in the literature, occurring in about 20% of patients who suddenly discontinue their antidepressant. Discontinuation symptoms may include nausea, dizziness, headaches, and insomnia.Citation47 Interestingly, the LVM trials found comparable rates of discontinuation symptoms between LVM and placebo. Nonetheless, the possibility of discontinuation symptoms exists. Thus, caution should be applied when discontinuing LVM.
Is there a special role for LVM?
How will LVM be used by mental health professionals? Will it target, eg, particular depressive symptoms? LVM has just been approved without comparator studies with other antidepressants, minimal post-clinical trial experience, and no post-marketing studies as yet. Nonetheless, it is possible to speculate on its possible usefulness in the treatment of MDD based partially on its uniqueness. LVM is an SNRI, but unique among SNRIs, in that it is the only SNRI that preferentially inhibits norepinephrine reuptake over serotonin reuptake.Citation8 It might be a particularly effective antidepressant for a patient with MDD who has a hypothesized underlying norepinephrine deficit. The latter subgroup has been supported by biochemical and imaging studies. Further, the data suggest that norepinephrine-deficit depressions are associated with decreased concentration, low motivation, poor energy, inattention, poor self-care, and cognitive difficulties, whereas serotonin-deficit depressions are associated with anxiety, suicidality, and appetite disturbances.Citation25,Citation48 A secondary analysis of an LVM study by Montgomery et al found that LVM was superior to placebo for most depressive symptoms identified and rated by the MADRS, including those implicated in norepinephrine-deficit depression as well as serotonin-deficit depression.Citation49 This broad effect may be due to its potentiating effect on serotonin despite its main effect being on norepinephrine.Citation8
LVM not only has significant antidepressant effects but also has significant effects on improving and/or normalizing functioning in patients with MDD. Whether this relates to its predominant norepinephrine effects is unclear, although there are some data suggesting a major role of norepinephrine on functionality.Citation25 Studies comparing LVM with other SNRIs and SSRIs regarding functionality are awaited. At this point, only LVM has in its labeling a statement that it not only significantly improves depressive symptoms but also significantly improves functional impairment.
LVM is an antidepressant that is fairly well tolerated, with most adverse events being of mild to moderate severity. Discontinuation rates are modest (9% for LVM versus 3% for placebo).Citation11,Citation33 The two main adverse events that lead to discontinuation by patients on antidepressants in general are weight gain and sexual dysfunction.Citation50 Regarding LVM, it has been shown to be weight neutral in both short-term studiesCitation5,Citation12–Citation14 and long-term studies.Citation16,Citation17 In addition, spontaneous reports of sexual dysfunction were low, but in males, LVM did have a small increased incidence of erectile dysfunction (5.9%), ejaculatory disorder (4.7%), and testicular pain (3.8%) when compared with placebo (≤1% for each sexual dysfunction).Citation11
LVM is easy to use. There are few drug-drug interactions other than the concomitant use of strong cytochrome 3A4 inhibitors. It can be used safely in moderate and severe hepatic impairment and in mild renal impairment without any dose reductions.Citation11
One potential problem for LVM is adverse events that are related to its noradrenergic mechanisms. There are significant increases in BP and pulse which can occasionally become clinically significant, necessitating periodic monitoring of vital signs. Patients who have hypertension and or arrhythmias should be stabilized prior to treatment. If that is not possible, an alternative antidepressant should be considered. In addition, urinary hesitancy, a dose-related adverse event, occasionally occurs (4% for the 40 mg dose, 5% for the 80 mg dose, and 6% for the 120 mg dose versus 0% for placebo).Citation11,Citation33 The latter appears to be predominantly related to norepinephrine; supporting this suggestion is the finding that other noradrenergic antidepressants like reboxetine that are also associated with urinary hesitancy have been successfully treated with an alpha-1-adrenoceptor antagonist.Citation36 In addition, Asnis et al were successful in treating a few cases of LVM-induced urinary hesitancy with tamsulosin, an alpha-1-adrenoceptor antagonist (Asnis et al, unpublished data, 2014). Patients who have symptoms of urinary obstruction or even a history of such symptoms should consider an alternative antidepressant.
Although LVM appeared to significantly help anxiety symptoms in the LVM trials as measured by the MADRS (ie, the MADRS item for inner tension) and Hamilton Depression Rating Scale (HAMD17, ie, the HAMD items of anxiety-psychological, somatic symptoms-gastrointestinal, and somatic symptoms-general),Citation49 what effect LVM has on comorbid anxiety disorders or other comorbid psychiatric diagnoses is unknown, since the presence of a comorbid psychiatric diagnosis was an exclusion criterion in three of the five LVM trials;Citation5,Citation6,Citation14 unfortunately, the effect of allowable comorbid anxiety disorders, ie, social anxiety disorder, generalized anxiety disorder, and specific phobias, seen in two trials was never analyzed.Citation12,Citation13 What effect LVM might have on comorbid anxiety disorders is an important question, given that approximately 60% of patients with MDD have a comorbid anxiety disorder.Citation51
In addition, although LVM was significantly superior to placebo in the short-term studies, it failed to be significant in the one placebo-controlled, double-blind, long-term study.Citation16 Another study is currently underway to reassess this important issue.
Despite having a number of positive reasons to consider LVM early in treatment, the economics of LVM may be prohibitive for many patients. Since LVM at this point does not have a generic equivalent, antidepressants which have generic formulations may be necessary due to lack of insurance coverage and restricted formularies.
Since refractory depression is such a prevalent problem (33% of patients with MDD fail to respond to a course of two sequential antidepressants),Citation2,Citation3 what effect LVM might have on refractory depression is an important question. The five short-term LVM trials all excluded patients who failed to respond to two antidepressants for their current depressive episode. Thus, whether LVM is effective in refractory depression needs to be studied. In addition, the efficacy of LVM in atypical depression, “anxious depression”, psychotic depression, and seasonal depression needs to be studied. The true place of LVM will be learned from head-to-head, placebo-controlled studies comparing LVM with other antidepressants.
Disclosure
GMA is on the speaker’s bureaus for Forest Research Institute and Shire Pharmaceuticals, and has received clinical research grants from Shire Research Institute, Lunbeck, and Takeda. MAH has no conflicts of interest to declare.
References
- American Psychiatric AssociationPractice guidelines for the treatment of patients with major depressive disorder (third edition)Am J Psychiatry2010167Suppl111820068118
- RushAJTrivediMHWisniewskiSRAcute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D reportAm J Psychiatry20061631905191717074942
- TrivediMHRushAJWisniewskiSREvaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practiceAm J Psychiatry2006163284016390886
- NierenbergAAHusainMMTrivediMHResidual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D reportPsychol Med201040415019460188
- AsnisGMBoseAGommollCPChenCGreenbergWMEfficacy and safety of levomilnacipran sustained release 40 mg, 80 mg, or 120 mg in major depressive disorder: a phase 3, randomized, double-blind, placebo-controlled studyJ Clin Psychiatry20137424224823561229
- MontgomerySAMansuyLRuthABoseALiHLiDEfficacy and safety of levomilnacipran sustained release in moderate to severe major depressive disorder: a randomized, double-blind, placebo-controlled, proof-of-concept studyJ Clin Psychiatry20137436336923656841
- CitromeLLevomilnacipran for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant – what is the number needed to treat, number needed to harm and likelihood to be helped or harmed?Int J Clin Pract2013671089110424016209
- AuclairALMartelJCAssiéMBLevomilnacipran (F2695), a norepinephrine-preferring SNRI: profile in vitro and in models of depression and anxietyNeuropharmacology20137033834723499664
- DeecherDCBeyerCEJohnstonGDesvenlafaxine succinate: a new serotonin and norepinephrine reuptake inhibitorJ Pharmacol Exp Ther200631865766516675639
- BlierPSaint-AndréEHébertCde MontignyCLavoieNDebonnelGEffects of different doses of venlafaxine on serotonin and norepinephrine reuptake in healthy volunteersInt J Neuropsychopharmacol200710415016690005
- Forest Pharmaceuticals, IncFetzima (levominacipran extended-release capsules). United States Prescribing Information Revised July 2014. Available from: http://www.frx.com/pi/fetzima_pi.pdfAccessed November 2, 2014
- BakishDBoseAGommollCLevomilnacipran ER 40 mg and 80 mg in patients with major depressive disorder: a phase III, randomized, double-blind, fixed-dose, placebo-controlled studyJ Psychiatry Neurosci201439404924144196
- SambunarisABoseAGommollCPChenCGreenbergWMSheehanDVA phase III, double-blind, placebo-controlled, flexible-dose study of levomilnacipran extended-release in patients with major depressive disorderJ Clin Psychopharmacol201434475624172209
- GommollCPGreenbergWMChenCA randomized, double-blind, placebo-controlled study of flexible doses of levomilnacipran ER (40–120 mg/day) in patients with major depressive disorderJ Drug Assess201431019
- MontgomerySAGommollSAChenCGreenbergWMEfficacy of levomilnacipran extended-release in major depressive disorder: pooled analysis of 5 double-blind, placebo-controlled trialsCNS Spectr201451924902007
- MagoRForeroGGreenbergWMGommollCChenCSafety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension studyClin Drug Investig201333761771
- ShiovitzTGreenbergWMChenCForeroGGommollCA randomized, double-blind, placebo-controlled trial of the efficacy and safety of levomilnacipran ER 40–120 mg/day for prevention of relapse in patients with major depressive disorderInnov Clin Neurosci201411102224653937
- MontgomerySAAsbergMA new depression scale designed to be sensitive to changeBr J Psychiatry1979134382389444788
- GuyWECDEU Assessment Manual for Psychopharmacology Clinical Global ImpressionsDHEW Publication No. 76-338Rockville, MD, USANational Institute of Mental Health1976
- SheehanDVHarnett-SheehanKRajBAThe measurement of disabilityInt Clin Psychopharmacol199611Suppl 389958923116
- KhinNAChenYFYangYYangPLaughrenTPExploratory_analyses of efficacy data from major depressive disorder trials submitted to the US Food and Drug Exploratory Administration in support of new drug applicationsJ Clin Psychiatry20117246447221527123
- MontgomerySAMollerHJIs the significant superiority of escitalopram compared to other antidepressants clinically relevant?J Clin Psychopharmacol200924111118
- SolomonDALeonACEndicottJPsychosocial impairment and recurrence of major depressionCompr Psychiatry20044542343015526252
- SpijkerJGraafRBijlRVBeekmanATOrmelJNolenWAFunctional disability and depression in the general population. Results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS)Acta Psychiatr Scand200411020821415283741
- MoretCBrileyMThe importance of norepinephrine in depressionNeuropsychiatr Dis Treat20117Suppl 191321750623
- SambunarisAGommollCGreenbergWMEfficacy of levomilnacipran extended-release in improving functional impairment associated with major depressive disorder: pooled analyses of five double-blind, placebo-controlled trialsInt Clin Psychopharmacol20142919720524667487
- SheehanDVHarnett-SheehanKSpannMEThompsonHFPrakashAAssessing remission in major depressive disorder and generalized anxiety disorder clinical trials with the discan metric of the Sheehan Disability ScaleInt Clin Psychopharmacol201126758321102344
- NelsonJCDelucchiKSchneiderLSJEfficacy of second generation antidepressants in late-life depression: a meta-analysis of the evidenceAm J Geriatr Psychiatry20081655856718591576
- KornsteinSGSchatzbergAFThaseMEGender differences in treatment response to sertraline versus imipramine in chronic depressionAm J Psychiatry20001571445145210964861
- YoungEAKornsteinSGMarcusSMSex differences in response to citalopram: a STAR*D reportJ Psychiatr Res20094350351118752809
- PapakostasGICulpepperLFayadRSMusgnungJGuico-PabiaCJEfficacy of desvenlafaxine 50 mg compared with placebo in patients with moderate or severe major depressive disorder: a pooled analysis of six randomized, double-blind, placebo-controlled studiesInt Clin Psychopharmacol20132831232123881185
- PapakostasGIFavaMDoes the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDDEur Neuropsychopharmacol200919344018823760
- ThaseMEGreenbergWMBoseAGummollCChenCSafety and tolerability of levomilnacipran SR in major depressive disorder: analysis of 5 short-term double-blind, placebo-controlled studiesPresented at the 166th Annual American Psychiatric Association MeetingSan Francisco, CA, USAMay 20, 2013
- NemeroffCBThaseMEEPIC 014 Study GroupA double-blind, placebo-controlled comparison of venlafaxine and fluoxetine treatment in depressed outpatientsJ Psychiatr Res20074135135916165158
- DemyttenaereKHuygensRVan BuggenhoutRTamsulosin as an effective treatment for reboxetine-asociated urinary retentionInt Clin Psychopharmacol20011635335511712624
- KasperSWolfRSuccessful treatment of reboxetine-induced urinary hesitancy with tamsulosinEur Neuropsychopharmacol20021211912211872327
- AggarwalAKhandelwalAJilohaRCMilnacipran-associated urinary retention: a case reportJ Clin Psychopharmacol20103064120841968
- StrausSMSturkenboomMCBleuminkGSNon-cardiac QTc-prolonging drugs and the risk of sudden cardiac deathEur Heart J2005262007201215888497
- Van NoordCStrausSMSturkenboomMCPsychotropic drugs associated with corrected QT interval prolongationJ Clin Psychopharmacol20092991519142100
- JasiakNMBostwickJRA review of the risk of QT/QTc prolongation among newer non-SSRI antidepressantsAnn Pharmacother2014481620162825204465
- RegevABjornssonESDrug-induced liver injury: morbidity, mortality, and Hy’s lawGastroenterology2014147202424880009
- HammadTALaughrenTRacoosinJSuicidality in pediatric patients treated with antidepressant drugsArch Gen Psychiatry20066333233916520440
- StoneMLaughrenTJonesMLRisk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug AdministrationBMJ2009339b288019671933
- PosnerKBrownGKStanleyBThe Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adultsAm J Psychiatry20111681266127722193671
- MagoRMahajanRThaseMELevomilnacipran: a newly approved drug for treatment of major depressive disorderExpert Rev Clin Pharmacol2014713714524524592
- AsnisGMGommollCPChenCTreating major depressive disorder with levomilnacipran ER: efficacy and tolerability across the dose rangePresented at the 167th Annual American Psychiatric Association MeetingNew York, NY, USAMay 5, 2014
- WarnerCHBoboWWarnerCReidSRachalJAntidepressant discontinuation syndromeAm Fam Physician20067444945616913164
- NuttDJRelationship of neurotransmitters to the symptoms of major depressive disorderJ Clin Psychiatry200869Suppl E14718494537
- MontgomerySAMansuyLRuthACLiDGommollCThe efficacy of extended-release levomilnacipran in moderate to severe major depressive disorder: secondary and post-hoc analyses from a randomized, double-blind, placebo-controlled studyInt Clin Psychopharmacol201429263524172160
- AshtonAKJamersonBDL WeinsteinWWagonerCAntidepressant-related adverse effects impacting treatment compliance: results of a patient surveyCurr Ther Res Clin Exp2005669610624672116
- KesslerRCBerglundPDemlerOThe epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R)JAMA200382893095310512813115