Abstract
Objective
Patients suffering from psychosis are more likely than the general population to commit aggressive acts, but the therapeutics of aggressive behavior are still a matter of debate.
Methods
This pilot randomized, open-label study compared the efficacy of quetiapine versus olanzapine in reducing impulsive and aggressive behaviors (primary endpoints) and psychotic symptoms (secondary endpoints) from baseline to days 1, 7, 14, 28, 42, 56, and 70, in 15 violent schizophrenic patients hospitalized in a maximum-security psychiatric hospital.
Results
Quetiapine (525±45 mg) and olanzapine (18.5±4.8 mg) were both efficacious in reducing Impulsivity Rating Scale from baseline to day 70. In addition, both treatments reduced the Brief Psychiatric Rating Scale, Positive and Negative Syndrome Scale, and Clinical Global Impression Scale scores at day 70 compared to baseline, and no differences were observed between treatments. Moreover, quetiapine, but not olanzapine, yielded an improvement of depressive symptoms in the items “depression” in Brief Psychiatric Rating Scale and “blunted affect” in Positive and Negative Syndrome Scale. Modified Overt Aggression Scale scores were also decreased from baseline to the endpoint, but due to the limited number of patients, it was not possible to detect a significant difference.
Conclusion
In this pilot study, quetiapine and olanzapine equally decreased impulsive and psychotic symptoms after 8 weeks of treatment. Double-blind, large studies are needed to confirm the validity of these two treatments in highly aggressive and violent schizophrenic patients.
Introduction
Mentally ill patients present an elevated rate of aggressive behaviors. Psychotic patients are five times more likely than the general population to commit violent acts, defined as “physical attacks on other persons, or on one’s self (self mutilation), with deliberate destructive intent sufficiently severe to justify a legal measure”.Citation1 Impulsivity is a factor strictly linked to aggression which, when present, increases the risk of aggressive behavior.Citation2,Citation3
Aggression is a complex phenomenon associated with genetic, neurobiological, and psychosocial factors.Citation4 Impairments of many neurotransmitter systems, including the serotonin (5-HT), dopamine (DA), and norepinephrine (NE) systems, are implicated in the biology of aggression.Citation5,Citation6 The most consistent neurobiological data on the pathogenesis of aggression and impulsivity concern modifications of serotonergic (5-HT) neurotransmission.Citation7 For example, several studies have reported that violent and suicidal patients have attenuated levels of 5-hydroxyindoleacetic acid (5-HIAA), the primary metabolite of 5-HT, in their cerebrospinal fluid,Citation8–Citation12 although many studies have also failed to find an association between aggression and low cerebrospinal fluid 5-HIAA levels.Citation13 Depletion of tryptophan, which in turn reduces central 5-HT levels by decreasing the levels of available precursor, also increases violent and impulsive behaviors.Citation14–Citation17
Clozapine was the first atypical antipsychotic agent for which clear anti-aggressive properties were demonstrated.Citation18–Citation28 Because clozapine therapy can result in the development of agranulocytosisCitation29 and frequent blood tests are required to avoid such complications, clozapine may not be an optimal treatment, particularly in aggressive patients for whom collecting blood samples on a regular basis is not an easy task. Olanzapine, an atypical neurolepticCitation30 which, similarly to clozapine, shows high affinity for both the 5-HT2A and the 5-HT2C receptors,Citation31 has also demonstrated efficacy in reducing aggressive behaviors in psychotic patients.Citation32–Citation35
Quetiapine’s unique binding profile includes partial agonist action at 5-HT1A receptorsCitation36 and low-affinity antagonist action at 5-HT2A receptors,Citation37 along with a lack of action at 5-HT2C receptors.Citation38 Considering these effects on the serotonergic system, it is logical to hypothesize that quetiapine should have an effect on the control of aggression and impulsivity. Indeed, several randomized, double-blind, placebo-controlled studies have demonstrated that quetiapine therapy effectively attenuates aggressive behavior in patients suffering from schizophrenia.Citation39–Citation42
Only a few studies have compared the effect of olanzapine and quetiapine in patients suffering from schizophrenia over short-termCitation43 and long-termCitation44,Citation45 periods. Moreover, none of these studies were carried out in selective violent patients. Due to the shortage of relevant research, the goal of the present study is to compare the efficacy of olanzapine and quetiapine in treating aggression in patients suffering from schizophrenia. Moreover, unlike previous research, the present study employs a sample of extremely aggressive inpatients from a forensic psychiatric hospital, specifically investigating the drugs in a dangerously violent population.
Methods
This randomized, single-center, 10-week, open-label study compares the effects of quetiapine and olanzapine in psychotic patients presenting with serious problems related to aggression (Study code: 5077-9056). At the time of the study (January 2003–June 2005), the investigators were not aware that quetiapine possessed antidepressant effects. The primary purpose of the study was to compare the efficacy of quetiapine versus olanzapine in reducing impulsive and aggressive behaviors, measured with the Modified Overt Aggression Scale (MOAS)Citation46 and the Impulsivity Rating Scale (IRS),Citation47 in psychotic patients. The secondary purpose was instead to compare the efficacy of quetiapine versus olanzapine in reducing the intensity of psychotic symptoms measured with the Brief Psychiatry Rating Scale (BPRS),Citation48 the Positive and Negative Syndrome Scale (PANSS),Citation49 the Clinical Global Impression-Severity (CGI-S), and the CGI-improvement (CGI-I) scale.Citation50 In addition, the possibility of a correlation between the reduction of aggressive behaviors and that of the psychotic symptoms was also assessed.
After being admitted to the study, patients were randomly assigned to either the quetiapine (up to 800 mg/day) or olanzapine (up to 20 mg/kg) group for 10 weeks. Random numbers, generated from a set exhibiting statistical randomness, were communicated by an independent statistical center to the research nurse. This study included only inpatients treated for schizophrenia, schizoaffective disorder, or paranoid disorder.
Patients
Thirty patients were screened and a total of 15 participants were then selected for the study. The patients were inpatients at the Philippe Pinel Institute, a maximum-security Canadian psychiatric hospital (300 beds) located in Montreal, Québec, specialized in the treatment and evaluation of patients suffering from psychiatric diseases and showing aggressive or impulsive behavior. These patients come from other psychiatric hospitals, prisons, or other institutions of the Ministry of Health and Social Service of Québec. The study was originally designed to enroll 20–25 patients per group in order to have a power of 0.8 at an alpha level of 0.05. Due to the difficulty in the recruitment process (see Discussion), we then decided to carry out only a pilot study. The study was approved by the Philippe Pinel Institute Ethical Committee and was conducted according to the ethical principles for medical research involving human subjects as proposed by the World Medical Association Declaration of Helsinki. All patients signed an informed consent form before participating in the study.
Inclusion and exclusion criteria
Eligible patients were between 18 and 65 years of age and had a primary diagnosis of schizophrenia, schizoaffective disorder (any subtype), or paranoid disorder according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-R) criteria. A score of at least 15 on the positive scale of the PANSS was also required for study enrollment, and eligible patients had to have been considered as having a specific problem related to aggression or impulsivity. Finally, eligible patients had to have been receiving a stable daily dose (within approximately 20% of the current dose) of a single antipsychotic agent for the month prior to the study; patients receiving depot medication were also considered for the study, as long as their last dose of depot was administered at least 2 weeks or one treatment cycle prior to study entry, with no other antipsychotic agent administered during this time.
Principal exclusion criteria were acute exacerbation of the psychotic state having resulted in a recent modification of the antipsychotic medication; concurrent treatment with antipsychotic agents after randomization; treatment with an investigational agent within the previous month; diagnosis of substance abuse using DSM-IV criteria within the previous 3 months; organic mental disease, including mental retardation, rendering the response to investigators unreliable; history of psychosurgery; history of clinically significant physical illness or abnormal laboratory values at screening; inability to give informed consent; pregnancy; and female subjects of childbearing potential without adequate contraception (hormonal contraception, double-barrier methods, intrauterine devices, tubal ligation). Other clinical exclusion criteria were clinically significant electrocardiography abnormality at screening; a QTc greater than 450 milliseconds or administration of medications that prolong the QT interval; known sensitivity to quetiapine or olanzapine or unsuccessful treatment with adequate doses (similar to those used in the present protocol, for at least 4 weeks) of quetiapine or olanzapine during the previous 3 months; diagnosis of any clinically meaningful, unstable renal, hepatic, cardiovascular respiratory, cerebrovascular disease, or other serious, progressive physical disease; any clinically meaningful abnormal finding uncovered during physical examination and/or clinically significant abnormal laboratory results at screening; history of neuroleptic malignant syndrome; use of potent cytochrome P450 inhibitors during the 14 days preceding randomization (ketoconazole, itraconazole, fluconazole, erythromycin, clarithromycin, troleandomycin, indinavir, nelfinavir, ritonavir, and saquinavir), and use of potent cytochrome P450 inducers (eg, phenytoin, carbamazepine, barbiturates, rifampin, and glucocorticoids) during the 14 days preceding randomization. Patients deemed unable or unlikely to follow the study protocol were also excluded from the study.
Scales
Patients were rated by a psychiatrist for aggression (MOAS), impulsivity (IRS), and psychotic symptoms (BPRS, CGI-S, and CGI-I) at baseline and days 1, 7, 14, 28, 42, 56, and 70. In addition, the PANSS scale was completed at baseline and days 1, 28, 42, 56, and 70.
Drugs posology
Quetiapine
Quetiapine treatment was initiated at a dosage of 50 mg twice daily for days 1 and 2, then increased to 100 mg twice a day (bid) on day 3, 150 mg bid on day 4, and 200 mg bid on day 5. The dosage was subsequently adjusted on the basis of clinical response; three daily dosage levels were allowed: 200 mg, 300 mg, and 400 mg. The maximal total daily dose of quetiapine allowed was 800 mg/day.
Olanzapine
Olanzapine was initiated at a dosage of 2.5 mg at bedtime for days 1 and 2, and was increased to 5 mg on day 3, 7.5 mg on day 4, and 10 mg on day 5. The dosage of olanzapine was subsequently adjusted on the basis of clinical response between three daily dosage levels: 10 mg, 15 mg, and 20 mg. The maximal total daily dose of olanzapine allowed was 20 mg/day. The maximal doses of quetiapine and olanzapine followed the recommendation by Health Canada at the time of the study.
Other medications
Benzodiazepines were administered when necessary, at the investigator’s discretion, during the course of the study. Patients taking typical antipsychotics were also allowed to enter the study if the dosage was stable over the 3 months prior to the study.
Statistical analyses
Statistical analyses were carried out using the Predictive Analytical Software (PASW; v18; SPSS Inc., Chicago, IL, USA) and Sigma Plot (v12; SPSS Inc.). Numerical values are presented as mean ± standard deviation (SD) unless otherwise specified. Group comparison on baseline demographic and clinical characteristics was performed using Student’s unpaired t-test for continuous variables and chi-square test for categorical variables to test lack of balance between groups. MOAS score differences between treatments and time of treatment (baseline and endpoint) were analyzed using Wilcoxon rank-sum test. For all the other scales, score changes between treatments and time were evaluated using the two-way repeated measure ANOVA (factors treatment and time) followed by Bonferroni post-hoc test for multiple comparisons. Statistical significance was set at an alpha of 0.05 (two-sided).
Efficacy analyses were performed using an intent-to-treat data set and an evaluable data set. The intent-to treat data set included all patients who received the treatment but who missed one, two, or three evaluation points. To address missing evaluations, a last observation carried forward (LOCF) analysis was conducted.Citation51
Results
Demographic and clinical characteristics
The demographic and clinical characteristics of the study population are represented in . Eight patients were randomly assigned to receive quetiapine and seven to receive olanzapine. The two treatment groups were similar at baseline based on age, sex, diagnosis, and severity of illness ().
Table 1 Baseline demographic and clinical characteristics of the study population
The majority of subjects were male (14 of 15) and Caucasian (9 of 15), three patients were Afro-American, two Hispanic, and one American Native. No baseline differences in scores on the MOAS, IRS, BPRS, PANSS positive, or PANSS negative scales between the quetiapine and olanzapine groups were observed ( and ). Ten patients received a diagnosis of schizophrenia, as assessed by a qualified psychiatrist following the DSM-IV criteria, while four patients were diagnosed with schizoaffective disorder and one with delusional disorder. The majority of patients (14 out of 15) received a sentence for a crime prior to hospitalization such as murder, attempted murder, or physical or verbal harassment, but were found to be not responsible due to their mental illnesses (these data have not been shown in detail for legal reasons).
Figure 1 Effect of treatments. IRS, BPRS, CGI –Severity of illness, PANSS – Positive subscale, PANSS – Negative subscale, and PANSS – General subscale scores at baseline (day 0) and endpoint (day 70) in patients treated with quetiapine (n=8) or olanzapine (n=7).
Abbreviations: BPRS, Brief Psychiatric Rating Scale; CGI, Clinical Global Impression Scale; IRS, Impulsivity Rating Scale; PANSS, Positive and Negative Syndrome Scale.
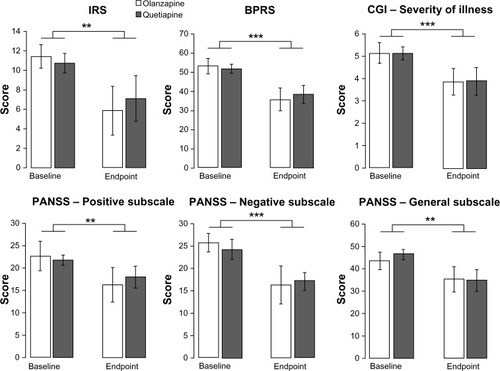
Table 2 Effects of treatments
Medication
Mean doses at day 7 were 50±00 mg/day for quetiapine and 2.5±00 mg/day for olanzapine; at day 28, 475±46 mg/day for quetiapine and 15±3.2 mg/day for olanzapine; and at day 70, 525±45 mg/day for quetiapine and 18.5±4.8 mg/day for olanzapine.
Primary scales endpoints: MOAS and IRS
During the study, no severe episodes of Physical Aggression Against Self, and Physical Aggression Against Other People were reported in the MOAS; for this reason, these two items of the MOAS are not reported in the results. MOAS verbal aggression, episodes of aggression against objects, number of interventions, and the duration of aggression episodes (verbal + against objects) scores were not different comparing baseline versus endpoint (day 70) for both olanzapine and quetiapine (). Moreover, no differences were detected between the two treatments (data not reported). However, the olanzapine group showed a trend in decreasing the number and duration of episodes of verbal aggression at the end of the treatment.
As showed in , the treatment with both quetiapine and olanzapine significantly and equally decreased IRS scores comparing baseline versus endpoint (P=0.009). Indeed, two-way ANOVA for repeated measures indicated an effect of time (F1,13=9.25, P=0.009) but not of treatment and no interaction between factors. For the quetiapine group, the endpoint was equal to 33.7% of the baseline score for the IRS; for the olanzapine group, the endpoint was equal to 48.7% of the baseline score for the IRS.
Secondary scales endpoints: PANSS, BPRS, and CGI
Two-way ANOVA for repeated measures revealed a significant effect of time for scores on the PANSS General (F1,13=13.61, P=0.003), Positive (F1,13=9.02, P=0.01) and Negative (F1,13=18.99, P<0.001) subscales, as well as BPRS (F1,13=26.35, P<0.001) and CGI Severity of Illness scale (F1,13=16.48, P=0.001), all of which decreased over the treatment period (). Of note, for all scales, there was no significant effect of treatment and no time × treatment interaction, meaning that the two treatments produced a similar decrease of psychotic symptoms. For the quetiapine group, the endpoint score was equal to 70.6% of the baseline score for the PANSS Negative subscale, 82.8% for the PANSS Positive subscale, 74.9% for PANSS General subscale, and 75.8% for the CGI Severity of Illness scale. For the olanzapine group, the endpoint score was equal to 68.3% of the baseline score for the PANSS Negative subscale, 71.7% for the PANSS Positive subscale, 81.3% for PANSS General subscale, and 75.1% for the CGI Severity of Illness scale.
Scales sub-items: depression
Using two-way repeated measure ANOVA followed by Bonferroni post-hoc test for multiple comparisons, we looked at possible changes over time and differences between treatments for all the sub-items of the IRS, PANSS, BPRS, and CGI scales. No significant effects were detected except for the sub-items of BPRS (depression) and PANSS (blunted affect) related to depression.
Analysis of the scores on the depression sub-item of the BPRS scale (depression) indicated that quetiapine compared to olanzapine better improved the symptoms of depression at day 70. Indeed, analysis of the changes in BPRS depression sub-item scores between the different time points and baseline revealed an interaction time per treatment (F6,78=2.26, P=0.046) and Bonferroni post-hoc analysis for multiple comparisons indicated a difference between olanzapine and quetiapine treatment at day 70 (P=0.001; ).
Figure 2 Time course of the depression items mean score change from baseline in the BPRS (top) and PANSS (bottom) in patients treated with quetiapine (n=8) or olanzapine (n=7).
Abbreviations: BPRS, Brief Psychiatric Rating Scale; PANSS, Positive and Negative Syndrome Scale.
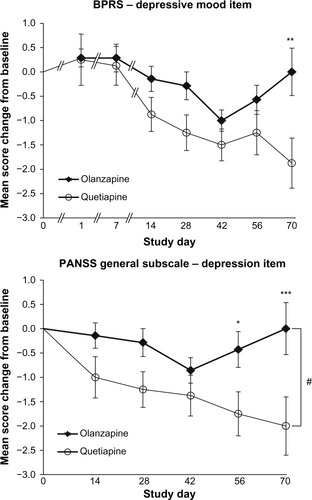
Similar results were found for the PANSS sub-item related to depressive mood (blunted affect): there was a significant interaction time per treatment (F4,52=2.60, P=0.050) and post-hoc analysis indicated a difference between olanzapine and quetiapine treatment at day 56 (P=0.036) and day 70 (P=0.003; ). In addition, there was a significant effect of treatment (F1,52=5.06, P=0.042).
Metabolic parameters
Glucose, triglycerides, and HDL-cholesterol values were evaluated at baseline and endpoint. No significant differences in these three parameters were noticed at baseline and endpoint in the olanzapine and quetiapine groups (data not shown).
Discussion
The results of this pilot study suggest that both quetiapine and olanzapine are effective in improving impulsivity and psychotic symptoms after long-term treatment in a population of criminally violent patients with schizophrenia. We showed that the decrease in impulsivity was paralleled by the decrease in psychotic symptoms for both treatments. Moreover, we found an improvement in depressive items at day 70 after quetiapine but not olanzapine treatment. MOAS scores were also decreased from baseline to the endpoint, but due to the limited number of patients, it was not possible to detect a significant difference.
Remarkably, despite the fact that this randomized clinical trial included a limited number of patients, it was able to detect a beneficial effect of quetiapine and olanzapine treatments for both impulsivity and psychotic symptoms. This study reflects the “real life” in a psychiatric world; in fact the psychotic and violent patients enrolled in the study for 70 days were participating in daily activities despite the high risk of aggression relapse and the consequent safety risk for staff who were in constant contact with these patients. The major drawback of the study was the limited numbers of patients, we could not reach the planned number as indicated in the Methods section, due to the difficulty in keeping these very violent and unpredictable patients in a research protocol for 70 days with limited pharmacological adjustments in case of violent behavior relapse, causing major distress and danger for nurses and staff dealing with these patients.
It is difficult to compare the present research work with that in previous literature due to differences in patient population, scales for evaluation of aggression/impulsivity, and time endpoint.
Three studies have compared the efficacy of quetiapine versus olanzapine in psychotic patients. In one rater-blinded study, 101 acutely psychotic patients were orally administered quetiapine, olanzapine, haloperidol, or olanzapine for agitation; over 72 hours, all four treatments were equally effective in attenuating aggression and hostility scores.Citation43 In a prospective, open-label, naturalistic study, 3,135 schizophrenic patients were prescribed olanzapine (N=2,118), quetiapine (N=104), clozapine, risperidone, or haloperidol. All treatments decreased the number of patients expressing hostile behavior and this decrease was not significantly different between the olanzapine and quetiapine groups.Citation44 In a randomized, double-blind study that was part of the National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project, 1,445 patients with schizophrenia were prescribed either olanzapine, quetiapine, risperidone, ziprasidone, or perphenazine, a typical antipsychotic.Citation45 In this study, the olanzapine group (N=185) experienced an equal reduction in violence risk compared to the perphenazine group (N=114), and the quetiapine group (N=131) showed a lower risk reduction than perphenazine.
Other studiesCitation32,Citation33,Citation52–Citation56 have evaluated the effects of olanzapine using the PANSS excited component to evaluate the effects on aggression, reporting a positive effect of olanzapine in the control of extreme behavior, but none of these used validated scales for the measure of impulsivity and aggression.
Krakowski et al,Citation33 in a large randomized double-blind study comparing the effect of clozapine, olanzapine, and haloperidol using the MOAS, found that olanzapine decreased verbal and physical aggression, similar to haloperidol, but to a lesser extent than clozapine. Other studies have instead evaluated the anti-aggressive properties of quetiapine using sub-items of the BPRS in schizophrenic patientsCitation40,Citation41 or PANSS aggression/hostility cluster.Citation42
Ganesan et alCitation57 used the Overt Aggression Scale in evaluating aggression in schizophrenic patients in a naturalistic study and found that quetiapine reduced the physical aggression score compared to the other scores by 83% at day 2, and that the decrease was maintained until day 5; similar results were also obtained in a small cohort of patients with traumatic brain injuryCitation58 and adolescents with attention deficit hyperactivity disorder and other disruptive disorders.Citation59
Our results indicate that both quetiapine and olanzapine may have an incisive effect on impulsivity as measured by the IRS. In fact, olanzapine and quetiapine both act as antagonist at the 5-HT2A receptors, increasing noradrenergic firing activity,Citation60 which in turn increases 5-HT firing activity,Citation61 thus reversing the impulsivity that is characterized by low 5-HT activity (see Introduction).
In this trial, the equivalent doses of olanzapine (18.5 mg) were relatively higher compared to those of quetiapine (525 mg), the latter not reaching optimal anti-psychotic and likely anti-aggression efficacy. However, our results suggest that even at lower doses, quetiapine was effective in decreasing psychotic symptoms and improving depression items in schizophrenic patients.
In this study, we have noticed that MOAS might not be a sensitive enough scale in a high-security environment where patients are strictly watched and prevented from committing aggressive acts against themselves and other people. In fact, MOAS items at baseline were very low considering this type of patient; nevertheless, we were able to detect episodes of verbal aggression and aggression against objects, but not physical aggression or aggression against self and others. Finally, considering that impulsivity is one of the main causes of aggressive acts,Citation2,Citation3 the fact that both quetiapine and olanzapine decreased impulsive behavior can be considered as a positive outcome for long-term treatment of aggression, independently from the effects on the MOAS. Other than decreases in impulsivity and psychotic symptoms, it is worthy to mention that this pilot study showed that quetiapine, but not olanzapine, improved the depressive symptoms in the sub-items of PANSS and BPRS. As mentioned in the Materials and Methods section, this study was carried out before the studies on the antidepressant effects of quetiapine were publishedCitation62 thus confirming that these results were not biased by the clinical judgments of raters. The neurobiological mechanism explaining this difference may lie in the partial agonist activity of quetiapine for the 5-HT1A receptors and in the NE reuptake pump affinity of the metabolite norquetiapine; remarkably, these two pharmacological features are not shared by olanzapine.Citation31 Our results suggest that quetiapine, rather than olanzapine, should be the first choice in schizophrenic patients with depressive symptomatology.
Finally, in our study, we did not notice significant differences at the endpoint in glucose, cholesterol, or triglycerides (except for one patient in the quetiapine group and another patient in the olanzapine group) as reported in other studies,Citation63 but this lack of secondary effects on metabolism could be due to the relatively short duration of the clinical trial.
In conclusion, this study shows that olanzapine and quetiapine equally decrease impulsivity and improve psychotic symptoms in people suffering from schizophrenia and violent behavior, and that these two treatments have a distinct effect on depression, suggesting that atypical antipsychotics, because of their different receptorial affinities, may modulate the range of psychotic symptoms in different ways.
Because of the limited number of patients in the protocol, all the results and statements presented in this research need further extended double-blind studies in order to be confirmed and the presence of a positive P-value could simply be due to chance alone (type I error). Specifically, more trials are needed to further characterize the effects of atypical anti-psychotics in specific clinical subpopulations and to evaluate their efficacy considering the type of symptomatology.
Acknowledgments
The authors thank Rita Bhatti for clinical assistance in the study and Mr Ian Debonnel, Dr Michael Tau, and Dr Francis Rodriguez Bambico for helping with the data bank and preliminary statistics. The authors thank the patients, psychiatrists, nurses, and all the staff of the “Institute Pinel de Montreal” for their kind and patient collaboration in the study.
Disclosure
This research was an investigator-initiated trial sponsored by AstraZeneca (Study code: 5077-9056, granted to GD); GG has received speaker honoraria from AstraZeneca Canada, Eli Lilly Canada Inc., and Merck Canada. The authors have no other conflicts of interest to declare.
References
- ArseneaultLMoffittTECaspiATaylorPJSilvaPAMental disorders and violence in a total birth cohort – results from the Dunedin studyArch Gen Psychiatry2000571097998611015816
- SeroczynskiADBergemanCSCoccaroEFEtiology of the impulsivity/aggression relationship: genes or environment?Psychiatry Res1999861415710359481
- ApterAPlutchikRvan PraagHMAnxiety, impulsivity and depressed mood in relation to suicidal and violent behaviorActa Psychiatr Scand1993871158424318
- ComaiSTauMGobbiGThe psychopharmacology of aggressive behavior: a translational approach: part 1: neurobiologyJ Clin Psychopharmacol2012321839422198449
- NelsonRJChiavegattoSMolecular basis of aggressionTrends Neurosci2001241271371911718876
- GobbiGDebonnelGWhat is a recommended treatment for aggression in a patient with schizophrenia?J Psychiatry Neurosci200328432032012921225
- LeeRCoccaroEThe neuropsychopharmacology of criminality and aggressionCan J Psychiatry2001461354411221488
- AsbergMTräskmanLThorénP5-HIAA in cerebrospinal fluid. A biochemical suicide predictor?Arch Gen Psychiatry1976331011931197971028
- BrownGLGoodwinFKBallengerJCGoyerPFMajorLFAggression in humans correlates with cerebrospinal fluid amine metabolitesPsychiatry Res19791213113995232
- LinnoilaMVirkkunenMScheininMNuutilaARimonRGoodwinFKLow cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behaviorLife Sci19833326260926146198573
- VirkkunenMGoldmanDNielsenDALinnoilaMLow brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violenceJ Psychiatry Neurosci19952042712757544158
- CremniterDJamainSKollenbachKCSF 5-HIAA levels are lower in impulsive as compared to nonimpulsive violent suicide attempters and control subjectsBiol Psychiatry199945121572157910376117
- RoggenbachJMuller-OerlinghausenBFrankeLSuicidality, impulsivity and aggression – is there a link to 5HIAA concentration in the cerebrospinal fluid?Psychiatry Res20021131–219320612467958
- CleareAJBondAJThe effect of tryptophan depletion and enhancement on subjective and behavioral aggression in normal-male subjectsPsychopharmacology1995118172817597125
- LeMarquandDGBenkelfatCPihlROPalmourRMYoungSNBehavioral disinhibition induced by tryptophan depletion in nonalcoholic young men with multigenerational family histories of paternal alcoholismAm J Psychiatry1999156111771177910553742
- LeMarquandDGPihlROYoungSNTryptophan depletion, executive functions, and disinhibition in aggressive, adolescent malesNeuropsychopharmacology19981943333419718596
- RogersRDBlackshawAJMiddletonHCTryptophan depletion impairs stimulus-reward learning while methylphenidate disrupts attentional control in healthy young adults: implications for the monoaminergic basis of impulsive behaviourPsychopharmacology1999146448249110550499
- BellusSBStewartDKostPPClozapine in aggressionPsychiatr Serv19954621871877712263
- BuckleyPBartellJDonenwirthKLeeSTorigoeFSchulzSCViolence and schizophrenia: clozapine as a specific antiaggressive agentBull Am Acad Psychiatry Law19952346076118639988
- ChengappaKNREbelingTKangJSLevineJParepallyHClozapine reduces severe self-mutilation and aggression in psychotic patients with borderline personality disorderJ Clin Psychiatry199960747748410453803
- FavaMPsychopharmacologic treatment of pathologic aggressionPsychiatr Clin North Am19972024274519196923
- GlazerWMDicksonRAClozapine reduces violence and persistent aggression in schizophreniaJ Clin Psychiatry1998598149541332
- PabisDJStanislavSWPharmacotherapy of aggressive behaviorAnn Pharmacother19963032782878833564
- RabinowitzJAvnonMEffect of clozapine on physical and verbal aggressionSchizophr Res1996182–32492559000322
- RateyJJLeveroniCKilmerDGutheilCSwartzBThe effects of clozapine on severely aggressive psychiatric inpatients in a state hospitalJ Clin Psychiatry19935462192238331090
- SpivakBMesterRWittenbergNMamanZWeizmanAReduction of aggressiveness and impulsiveness during clozapine treatment in chronic neuroleptic-resistant schizophrenic patientsClin Neuropharmacol19972054424469331521
- VolavkaJZitoJMVitraiJCzoborPClozapine effects on hostility and aggression in schizophreniaJ Clin Psychopharmacol19931342872898376618
- WilsonWHClinical review of clozapine treatment in a state hospitalHosp Community Psychiatry19924377007031516900
- AlvirJMJLiebermanJASaffermanAZSchwimmerJLSchaafJAClozapine-induced agranulocytosis. Incidence and risk-factors in the United StatesN Engl J Med199332931621678515788
- BymasterFPCalligaroDOFalconeJFRadioreceptor binding profile of the atypical antipsychotic olanzapineNeuropsychopharmacology199614287968822531
- FultonBGoaKLOlanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychosesDrugs19975322812989028746
- KinonBJStaufferVLKollack-WalkerSChenLSniadeckiJOlanzapine versus aripiprazole for the treatment of agitation in acutely ill patients with schizophreniaJ Clin Psychopharmacol200828660160719011427
- KrakowskiMICzoborPCitromeLBarkNCooperTBAtypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorderArch Gen Psychiatry200663662262916754835
- StrousRDKupchikMRoitmanSComparison between risperidone, olanzapine, and clozapine in the management of chronic schizophrenia: a naturalistic prospective 12-week observational studyHum Psychopharmacol200621423524316783815
- VolavkaJCzoborPNolanKOvert aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidolJ Clin Psychopharmacol200424222522815206671
- Newman-TancrediAGavaudanSConteCAgonist and antagonist actions of antipsychotic agents at 5-HT1A receptors: a [S-35] GTPgammaS binding studyEur J Pharmacol19983552–32452569760039
- TysonPJRobertsKHMortimerAMAre the cognitive effects of atypical antipsychotics influenced by their affinity to 5HT-2A receptors?Int J Neurosci2004114659361115204055
- RauserLSavageJEMeltzerHYRothBLInverse agonist actions of typical and atypical antipsychotic drugs at the human 5-hydroxytryptamine(2C) receptorJ Pharmacol Exp Ther20012991838911561066
- ArangoCBernardoMThe effect of quetiapine on aggression and hostility in patients with schizophreniaHum Psychopharmol2005204237241
- CantillonMGoldsteinJMQuetiapine fumarate reduces aggression and hostility in patients with schizophreniaPoster presented at: American Psychiatric Association 151st Annual MeetingMay 30–June 4, 1998Toronto, Canada
- ChengappaKNGoldsteinJMGreenwoodMJohnVLevineJA post hoc analysis of the impact on hostility and agitation of quetiapine and haloperidol among patients with schizophreniaClin Ther200325253054112749512
- KalaliASchulzSCKahnRSEffectiveness of once-daily extended release quetiapine fumarate (quetiapine xr) for excitability, hostility and aggression in schizophreniaSchizophr Res200898Suppl 1164
- VillariVRoccaPFonzoVMontemagniCPandulloPBogettoFOral risperidone, olanzapine and quetiapine versus haloperidol in psychotic agitationProg Neuropsychopharmacol Biol Psychiatry200832240541317900775
- BitterICzoborPDossenbachMVolavkaJEffectiveness of clozapine, olanzapine, quetiapine, risperidone, and haloperidol monotherapy in reducing hostile and aggressive behavior in outpatients treated for schizophrenia: a prospective naturalistic study (IC-SOHO)Eur Psychiatry2005205–640340816084068
- SwansonJWSwartzMSVan DornRAComparison of antipsychotic medication effects on reducing violence in people with schizophreniaBr J Psychiatry20081931374318700216
- FoleySRKellyBDClarkeMIncidence and clinical correlates of aggression and violence at presentation in patients with first episode psychosisSchizophr Res2005722–316116815560961
- LecrubierYBraconnierASaidSPayanCThe impulsivity rating scale (IRS): preliminary resultsEur Psychiatry199510733133819698364
- OverallJE GDThe brief psychiatric rating scalePsychol Rep196210799812
- KaySRFiszbeinAOplerLAThe positive and negative syndrome scale (PANSS) for schizophreniaSchizophr Bull19871322612763616518
- GuyWECDEU Assessment Manual for Psychopharmacology – Revised, 1976Rockville, MAUS Department of Health, Education, and Welfare1976218222
- StreinerD GJIntention to treat analysis in clinical trials when there are missing dataEvid Based Ment Health20014707112004740
- BreierAMeehanKBirkettMA double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophreniaArch Gen Psychiatry200259544144811982448
- LambertMHuberCGNaberDTreatment of severe agitation with olanzapine in 166 patients with schizophrenia, schizoaffective, or bipolar I disorderPharmacopsychiatry200841518218918763220
- SanLArranzBQuerejetaIBarrioSDe la GandaraJPerezVA naturalistic multicenter study of intramuscular olanzapine in the treatment of acutely agitated manic or schizophrenic patientsEur Psychiatry200621853954316697151
- WangXSavageRBorisovAEfficacy of risperidone versus olanzapine in patients with schizophrenia previously on chronic conventional antipsychotic therapy: a switch studyJ Psychiatr Res200640766967616762371
- WrightPBirkettMDavidSRDouble-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophreniaAm J Psychiatry200115871149115111431240
- GanesanSLevyMBilskerDKhanbhaiIEffectiveness of quetiapine for the management of aggressive psychosis in the emergency psychiatric setting: a naturalistic uncontrolled trialInt J Psychiatry Clin Pract200593199203
- KimEBijlaniMA pilot study of quetiapine treatment of aggression due to traumatic brain injuryJ Neuropsychiatry Clin Neurosci200618454754917135382
- KronenbergerWGGiauqueALLafataDEBohnstedtBNMaxeyLEDunnDWQuetiapine addition in methylphenidate treatment-resistant adolescents with comorbid ADHD, conduct/oppositional-defiant disorder, and aggression: a prospective, open-label studyJ Child Adolesc Psychopharmacol200717333434717630867
- DaweGSHuffKDVandergriffJLSharpTO’NeillMJRasmussenKOlanzapine activates the rat locus coeruleus: in vivo electrophysiology and c-Fos immunoreactivityBiol Psychiatry200150751052011600104
- BlierPSzaboSTPotential mechanisms of action of atypical antipsychotic medications in treatment-resistant depression and anxietyJ Clin Psychiatry200566Suppl 8304016336034
- CalabreseJRKeckPEJrMacfaddenWA randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depressionAm J Psychiatry200516271351136015994719
- AtmacaMKulogluMTezcanEGeciciOUstundagBWeight gain, serum leptin and triglyceride levels in patients with schizophrenia on antipsychotic treatment with quetiapine, olanzapine and haloperidolSchizophr Res20036019910012505146
