Abstract
Blood platelets are considered to be a peripheral marker in schizophrenia and other psychiatric disorders. Oxidative stress in schizophrenia may be responsible for changes in platelet metabolism and function; therefore, the aim of this study was to examine and compare the generation of superoxide anions and activity of an antioxidant enzyme (glutathione peroxidase [GPx]) in blood platelets in patients with schizophrenia and healthy subjects. The level of superoxide anions generated in platelets after thrombin and platelet-activating factor stimulation and activity of GPx in patients with schizophrenia and healthy volunteers was estimated. The results obtained from the study indicate that the generation of superoxide anions in platelets as a response of platelets in patients with schizophrenia to such activating factors as thrombin or platelet-activating factor is higher than in the response of platelets of healthy subjects. In platelets from schizophrenic patients, suppressed GPx activity of about 67% was observed.
Introduction
Schizophrenia is one of the mental disorders in which the pathogenic mechanisms are complex and poorly understood. A growing body of evidence appears to implicate the role of oxidative stress with an impaired antioxidant defense system in this disease.Citation1–Citation9 Molecular processes, including oxidative stress, that play a role in the etiopathology of schizophrenia cannot be studied directly in human brain tissue. Postmortem brain tissue has also been used extensively to study schizophrenia.Citation1 For studies of oxidative stress in mental diseases, various cellular models are helpful (erythrocytes, lymphocytes, blood platelets, and cultured skin fibroblasts), as well as peripheral tissue models, since molecular processes associated with oxidative damage occur in all tissues.Citation3,Citation10 In the present study of oxidative stress in schizophrenic patients, blood platelets were used. Blood platelets are recognized as cellular models in neurobiological studies, because of their similarity to neurons, having the same embryological origin; platelets and neurons of the central nervous system show many similarities, including monoamine oxidase activity, serotonin reuptake, presence of neurotransmitter receptors, and exocytosis.Citation11–Citation14 Blood platelets are highly reactive, and within seconds of exposure to such agonists as thrombin or platelet-activating factor (PAF), they undergo activation, with a dramatic change in their morphology and biochemical events. PAF is a phospholipid mediator of inflammation with a wide range of biological activities, including alteration of the barrier function of endothelium. It is a strong platelet activator involved in migraine.Citation15,Citation16 Free radical-mediated pathological reactions (oxidative modification of biomolecules) may play a role in schizophrenia. A variety of different physiological agonists can activate platelets, and upon the activation of platelets, a cascade of morphological changes and biochemical reactions concomitant with the generation of free radicals and reactive oxygen species (ROS) takes place.Citation17,Citation18 ROS, including superoxide radicals (O2•−), are generated during platelet activation induced by strong platelet agonists, and may be involved in platelet-signaling pathways.Citation17–Citation20 Under physiological conditions, superoxide radicals are constantly produced in the mitochondrial electron-transport chain. Platelets generate superoxide radicals mainly via the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase or xanthine oxidase. The main source of superoxide radicals in activated platelets is the metabolism of arachidonate and the glutathione (GSH) cycle.Citation17–Citation20
The aim of the present study was to determine and compare the generation of superoxide radicals in resting as well as in thrombin- and PAF-stimulated platelets from patients with schizophrenia and healthy subjects. Polyunsaturated fatty acids (PUFAs; including arachidonic acid present in platelet-membrane phospholipids) are especially vulnerable to attack by ROS and the formation of lipid hydroperoxides, because of the high concentration of allylic hydrogen in their structure. Antioxidative enzymes, including GSH peroxidase (GPx), protect cells against the ROS-mediated changes. GPx converts peroxides and hydroxyl radicals into nontoxic forms, together with the concomitant oxidation with reduced GSH. Reduced GSH levels in cerebrospinal fluid and the prefrontal cortex in patients with schizophrenia have been described,Citation21 and the deficit of GSH and its metabolite – γ-glutamylglutamine – in the cerebrospinal fluid of patients with schizophrenia who were drug-naïve or drug-free at that time was also found.Citation22 Recently, the results of several studies have indicated the efficacy of N-acetylcysteine, a GSH precursor that is a useful agent in the treatment of various psychiatric disorders, including schizophrenia.Citation23,Citation24 The activity of GPx reducing lipid hydroperoxides in the platelets of schizophrenic patients is not well known; therefore, the purpose of the study was also to estimate the activity of this key antioxidant enzyme in platelets from patients with schizophrenia. There are conflicting data in the literature on the activities of antioxidant enzymes in patients with schizophrenia, measured mainly in erythrocytes.Citation1,Citation6,Citation7
Materials and methods
Criteria for schizophrenic patient inclusion
Thirty schizophrenic patients were included in the study: 20 male and ten female, aged 18–36 years, who were treated in the Department of Psychiatry of the Medical University of Lodz, Poland. All patients were interviewed and completed a special questionnaire on their treatment, course of disease, and extrapyramidal symptoms, measured by the Simpson–Angus Scale and Barnes Akathisia Scale.
The Mini-International Neuropsychiatric Interview for psychiatric examination was performed.Citation25 All patients were diagnosed with paranoid schizophrenia, according to Diagnostic and Statistical Manual of Mental Disorders, fourth edition criteriaCitation26 (the acute period of psychosis), and they were treated with a stable dose of second-generation antipsychotic drugs (risperidone 4–6 mg/day, quetiapine 600–750 mg/day, and olanzapine 15–20 mg/day; duration of treatment 2.0±1.5 months). They did not use antidepressants or mood stabilizers. The mean duration of schizophrenia in the subjects was 4 years.
The control group consisted of 30 healthy subjects – workers and students of the Medical University of Lodz – who were age- and sex-matched. Both groups belonged to the same community, and had similar nutritional habits. Both groups were on a balanced diet without antioxidant supplementation. Patients and control subjects with somatic illnesses were excluded. None of the control subjects or patients received aspirin or any other antiplatelet drugs, and none were alcohol/drug abusers or showed clinical manifestation of allergy or infection. Heavy smokers were excluded from the group of patients. The majority of patients were not smokers (83%), 17% of patients did not smoke in the week before blood drawing. There were no smokers in the control group. The body mass index of all subjects ranged around normal values. For each subject, blood-platelet counts were recorded, as well as medical history and medications used.
PAF, thrombin, and cytochrome C were purchased from Sigma-Aldrich (St Louis, MO, USA). All other reagents were of analytical grade, and were provided by commercial suppliers.
Isolation of blood platelets
Blood samples (2×9 mL) from fasting schizophrenic patients and healthy volunteers were drawn between 8 and 8.30 am from a forearm vein into tubes containing acid citrate dextrose solution (5:1, v/v). Platelets were isolated by differential centrifugation of fresh blood (20 minutes, 200 g, 20°C).Citation27 Platelet-rich plasma was separated and centrifuged for 20 minutes at 1,000 g, 20°C to sediment platelets. The resulting platelet pellet was gently resuspended in modified Tyrode’s buffer (140 mM NaCl, 10 mM glucose, 15 mM Tris/HCl, pH 7.4) in the presence of apyrase (1 mg/mL), and the platelets were subsequently washed and then suspended in the same buffer. The entire washing procedure was performed in plastic tubes and carried out at room temperature. Blood platelets were suspended in modified Tyrode’s buffer at a final concentration of 5×108 platelets/mL. Platelet suspensions were incubated with PAF (1×10−8 M) or with thrombin (0.4 U/mL) for 2 minutes at 37°C, and then the generation of superoxide radicals was determined.Citation28 The platelets in the suspension were counted by the photometric method, as described by Walkowiak et al.Citation29
The protocol was passed by the Committee for Research on Human Subjects of the Medical University of Lodz, number RNN/899/2000. All patients and volunteers included in the study were informed about the aims of the study and the methods implemented, and gave their written informed consent for participation in this study.
Generation of superoxide anion radicals
The generation of superoxide anions in control platelets (from healthy subjects) and in platelets from patients with schizophrenia (resting and PAF- or thrombin-stimulated platelets) was measured by means of superoxide dismutase (1 μg/μL)-inhibitable reduction of cytochrome C, as described by Jahn and Hänsch.Citation28 Briefly, an equal volume of Ca2+/Mg2+ free Tyrode buffer (pH 7.4), containing cytochrome C (160 μmol/L), was added to a 1 mL suspension of platelets. After 2 minutes of incubation, the platelets were sedimented by centrifugation (2000 g for 5 minutes), and the supernatants were transferred to cuvettes. The reduction of cytochrome C was measured spectrophotometrically at 550 nm. The concentration of superoxide anions was evaluated on a basis of the molar extinction coefficient of reduced cytochrome C (ε=18,700 M−1 · cm−1). All samples were in triplicate.
Determination of glutathione peroxidase activity
GPx activity was measured according to the spectrophotometric method of Paglia and Valentine.Citation30 Briefly, the assay mixture consisted of 50 mM Tris HCl, 0.1 mM ethylenediaminetetraacetic acid, pH 7.6 containing 2 mM GSH, 0.14 mM NADPH and 0.7 U/mL GSH reductase. Platelet suspensions were preincubated for 2 minutes at 37°C in the assay mixture, and the reaction was initiated by the addition of 0.2 mM tert-butyl-hydroperoxide. Absorbance at 340 nm was recorded on a spectrophotometer. Results were expressed as nanomoles of NADPH oxidized per minute per milligram of platelet protein, using a molar extinction coefficient of 6.22/cm2/μmol for NADPH.
Protein assay
Protein concentration was determined according to Bradfort, using bovine serum albumin as a standard.Citation31
Statistical analysis
Statistical analysis was done by several tests. The Mann–Whitney U test was used for comparisons between the groups of healthy subjects and schizophrenic patients. For comparison of the generation of superoxide anion radicals after stimulation (thrombin or PAF) with resting platelets derived from schizophrenic patients or healthy subjects, the Wilcoxon signed-rank test was used. All values were expressed as means ± standard error of the mean. P-values <0.05 were considered statistically significant.
Results
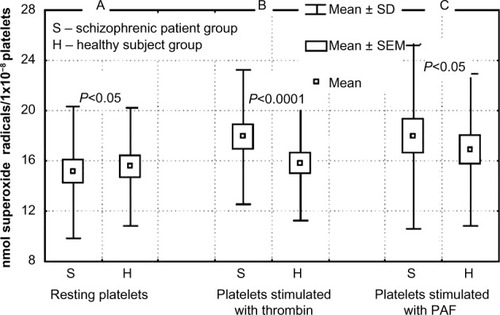
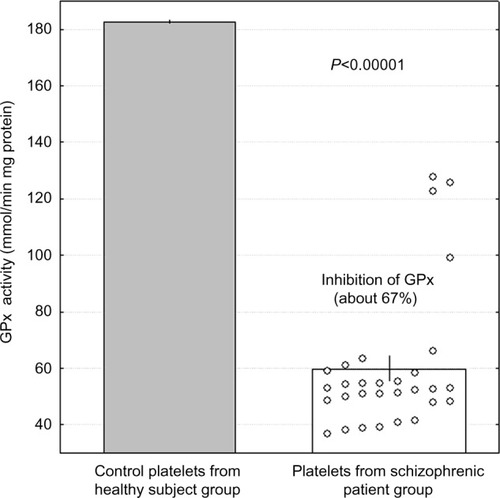
The results showed that in unstimulated resting platelets obtained from patients with schizophrenia, the level of generated superoxide anions was lower than in platelets derived from healthy subjects (, P<0.05). After thrombin stimulation, platelets from patients with schizophrenia generated higher amounts of superoxide anions than those from healthy subjects (, P<0.0001). Platelet-activating factor also caused increased production of superoxide anions in platelets from both schizophrenic patients and healthy volunteers (, P<0.05). In platelets from patients with schizophrenia, the generation of superoxide radicals after thrombin or PAF stimulation was significantly higher than in resting platelets (P<0.00001, P<0.001, respectively, Wilcoxon signed-rank test; ). Differences in the generation of superoxide anions after thrombin or PAF stimulation of platelets in healthy subjects in comparison to resting platelets were not statistically significant. This indicates that platelets from patients with schizophrenia are more sensitive to agonists and produce more superoxide anions than those from healthy subjects. The activity of GPx in platelets from schizophrenic patients was significantly lower (P<0.00001) than in healthy subjects.
Figure 1 (A–C) The effects of thrombin (0.4 U/mL) and platelet-activating factor (PAF; 1×10−8 M) on the level of superoxide radicals in blood platelets isolated from healthy subjects and from schizophrenic schizophrenic patients (n=30). (A) Levels of superoxide radicals in resting platelets. (B) Levels of superoxide radicals in platelets after stimulation by thrombin (0.4 U/mL). (C) Levels of superoxide radicals in platelets after stimulation by PAF. Differences in anion radical generation in resting platelets and after agonist stimulation (thrombin or PAF) between groups calculated by Mann–Whitney U test.
Discussion
Oxidative stress in schizophreniaCitation7 may be responsible for altered platelet function, aggregation, and reactivity.Citation32 It has been established that platelets of patients with schizophrenia have the increased ability to generate superoxide radicals in response to such agonists as thrombin or PAF, and they are particularly sensitive to thrombin stimulation (). The technique used enabled the measurement of superoxide radical production in platelets from schizophrenic patients.
The increased ROS production in stimulated platelets of patients with schizophrenia suggests the involvement of free radicals in the etiopathogenesis of this disease. After binding of the agonist to specific receptors on the platelet surface, several biochemical reactions occur together with ROS production. These include receptor-mediated activation of phospholipase A2 and C and formation of diacylglycerol, activation of protein kinase C, formation of inositol-1,4,5-trisphosphate, and increased calcium-ion levels in cytosol. The platelet stimulation is accompanied by changes in platelet shape, aggregation, and secretion.Citation33 In platelets from patients with schizophrenia, increased activity of phospholipase A2 changed phosphoinositide metabolism, and peroxidation of lipids has also been shown.Citation2,Citation3,Citation34
Platelet lipid peroxidation is a process that leads to membrane damage and changes in platelet function, with altered platelet reactivity to agonists. The response of platelets from patients with schizophrenia to the action of a strong platelet agonist found in this study could be also associated with disturbances of the biochemical pathways in platelets caused by oxidative stress. Platelet response to the action of thrombin or PAF in schizophrenic patients may depend upon the modulated access of specific platelet surface receptors, which can result from changes in platelet membrane. Walsh et alCitation35 demonstrated that the number of platelet integrin receptors (αIIb/β3) involved in platelet aggregation in schizophrenic patients is significantly higher in comparison with the platelets of healthy subjects.Citation35 The increased expression of platelet integrin receptors for fibrinogen in schizophrenic patients may be responsible for increased platelet reactivity, which probably contributes to the increased risk of cardiovascular diseases in schizophrenia.Citation36–Citation38 The peroxidation of PUFAs in platelet membranes can cause a decrease in eicosanoid concentration. Eicosanoids and their precursors, PUFAs, may be involved not only in the pathophysiology but also in the treatment of schizophrenia. Antipsychotics such as clozapine influence thromboxane and chlorpromazine production, and may therefore influence platelet function and exert therapeutic effects in schizophrenic patients. Changes in the concentration of several PUFAs (mainly arachidonic acid) and phospholipase A2 activity were found in erythrocyte and platelet membranes in patients with schizophrenia.Citation2,Citation34,Citation39 There is a growing body of evidence that the generation of free radicals and the changed antioxidant defense may play important roles in schizophrenia.Citation2,Citation3,Citation7,Citation34,Citation40–Citation42 Defense mechanisms against oxidative stress include such antioxidant enzymes as superoxide dismutase (SOD), catalase, or GPx.Citation43 SOD activity is decreased in the platelets of patients with schizophrenia.Citation2 The results of the present study showed that in platelets from schizophrenic patients, the activity of GPx was significantly lower than in platelets from healthy subjects (controls). This is in agreement with other studies.Citation11,Citation44
These data reveal that antioxidative defense mechanisms might be impaired in schizophrenic patients. GPx provides an effective mechanism against cytosolic oxidative injury, because it eliminates hydroperoxide and lipid peroxides by reduction utilizing GSH. The low activity of GPx, which is a selenoenzyme, might be partly dependent on a defect in a selenium-transport protein described in schizophrenia,Citation45 on the oxidative/nitrosative alteration of the enzyme,Citation46 or suppressed GSH level in platelets when oxidative stress occurs.Citation44,Citation46 It should be underlined that in platelets there are two main members of the seleno-GPx family: the cytosolic enzyme cGPx (GPx-1), and phospholipid hydroperoxide GPx (GPx-4), which reduces lipid hydroperoxides in membranes.
Low SOD activity was established in our earlier study,Citation45 but this study presents low GPx activity in platelets (). In several studies, it has been demonstrated that total antioxidant status of plasma in schizophrenic patients was reduced with impaired antioxidant-enzyme activities in schizophrenia.Citation1,Citation4–Citation6,Citation8,Citation47–Citation50 In schizophrenia, a defect in the antioxidant defense system under conditions of oxidative stress can lead to oxidative damage to different cells, especially membranes, including blood platelets.Citation19,Citation20 In a previous study, it was shown that in patients with schizophrenia, oxidative stress, expressed as the increase of the isoprostane concentration, was elevated.Citation51 In platelets of patients with schizophrenia lipid peroxidation, expressed as the concentration of thiobarbituric acid reactive substances, is enhanced, with SOD activity being simultaneously reduced.Citation2 The decreased activity of platelet SOD in subjects with psychosis with prominent positive symptoms correlated with the increase of oxidative stress, expressed as the increased level of lipid peroxidation in platelets and production of other ROS.Citation2 It appears that the lack of significant increase of superoxide anions generated in resting platelets from patients with schizophrenia presented in this study may be associated with the mechanism that removes the superoxide radicals in the reaction with nitric oxide.Citation19 The superoxide anion is a highly reactive radical, and may be decomposed by SOD into H2O2, or it may produce peroxynitrite as the result of its reaction with nitric oxide (NO).Citation52 Peroxynitrite (ONOO−) is a highly reactive, short-lived oxidant, which can mediate the oxidation process (nitration and oxidation biomolecules), leading to changes of signal transduction and functioning of platelets.Citation19,Citation20,Citation44,Citation52 The role of nitric oxide in schizophrenia has been studied; however, few studies indicate that the generation of nitric oxide in patients with such a diagnosis is increased compared to healthy subjects.Citation8 After the reaction of superoxide anion radicals with nitric oxide, the level of superoxide anions is reduced, but lipid peroxidation in platelets can be increased as a result of the reaction initiated also by peroxynitrite.Citation19,Citation44,Citation52 The data obtained in this study demonstrate that in platelets from patients with schizophrenia, the generation of superoxide anions after thrombin or PAF stimulation is significantly augmented, suggesting that platelets from schizophrenic patients are more sensitive to platelet agonists. It indicates that activation of platelets in schizophrenia associated with O2•−2 production with a diminished antioxidant defense system may lead to oxidative stress. It seems that the changes in the activities of such peripheral antioxidant enzymes as SOD or GPx in platelets could be considered a biological indicator of severity of the schizophrenia symptoms.Citation6,Citation42,Citation47,Citation48
Figure 2 The activity of glutathione peroxidase (GPx) in platelets from patients with schizophrenia (n=30) and healthy subjects (control, n=30). Control group mean 182.5, standard error of mean 0.4 (minimum 178.9, maximum 186.8).
Further research is necessary in order to elucidate the effects of different antipsychotics on antioxidant-enzyme activities and oxidative stress in peripheral cells, such as platelets from schizophrenic patients.
Acknowledgments
The authors are grateful to Dr Bogdan Kontek from the Department of General Biochemistry, University of Lodz for technical assistance. This work was supported by a grant from the Medical University of Lodz (502/03-1-155-02/502-14-109).
Disclosure
The authors report no conflicts of interest in this work.
References
- CiobicaAPadurariuMDobrinIStefanescuCDobrinROxidative stress in schizophrenia – focusing on the main markersPsychiatr Danub20112323724521963690
- Dietrich-MuszalskaAOlasBRabe-Jabłon´skaJOxidative stress in blood platelets from schizophrenic patientsPlatelets20051638639116236599
- FendriCMechriAKhiariGOthmanAKerkeniAGahaLOxidative stress involvement in schizophrenia pathophysiology: a reviewEncephale200632244252 French16910626
- LiHCChenQZMaYZhouJFImbalanced free radicals and antioxidant defense systems in schizophrenia: a comparative studyJ Zhejiang Univ Sci B2006798198617111467
- MicóJARojas-CorralesMOGibert-RaholaJReduced antioxidant defense in early onset first-episode psychosis: a case-control studyBMC Psychiatry2011112621320302
- RaffaMAtigFMhallaAKerkeniAMechriADecreased glutathione levels and impaired antioxidant enzyme activities in drug-naïve first-episode schizophrenic patientsBMC Psychiatry20111112421810251
- ReddyRYaoJKFree radical pathology in schizophrenia: a reviewProstaglandins Leukot Essent Fatty Acids19965533438888121
- YaoJKReddyROxidative stress in schizophrenia: pathogenetic and therapeutic implicationsAntioxid Redox Signal2011151999200221194354
- YaoJKReddyRMcElhinnyLGvan KammenDPReduced status of plasma total antioxidant capacity in schizophreniaSchizoph Res19983218
- YaoJKReddyRvan KammenDPOxidative damage and schizophreniaCNS Drugs20011528721011463134
- BerkMPleinHBelshamBThe specificity of platelet glutamate receptor supersensitivity in psychotic disordersLife Sci2000662427243210894085
- CamachoADimsdaleJEPlatelets and psychiatry: lessons learned from old and new studiesPsychosom Med20006232633610845346
- DreuxCLaunayJMBlood platelets: neuronal model in psychiatric disordersEncephale1985115764 French2862006
- PleinHBerkMThe platelet as a peripheral marker in psychiatric illnessHum Psychopharmacol20011622923612404575
- CarotenutoMEspositoMNutraceuticals safety and efficacy in migraine without aura in a population of children affected by neurofibromatosis type INeurol Sci2013341905190923532548
- EspositoMRubertoMPascottoACarotenutoMNutraceutical preparations in childhood migraine prophylaxis: effects on headache outcomes including disability and behaviourNeurol Sci2012331365136822437495
- IulianoLColavitaARLeoRPraticòDVioliFOxygen free radicals and platelet activationFree Radic Biol Med19972299910069034239
- WachowiczBOlasBZbikowskaHMBuczyn′skiAGeneration of reactive oxygen species in blood plateletsPlatelets20021317518212180500
- OlasBWachowiczBRole of reactive nitrogen species in blood platelets functionsPlatelets20071855556517852770
- OlasBNowakPKolodziejczykJWachowiczBThe effects of antioxidants on peroxynitrite-induced changes in platelet proteinsThromb Res200411339940615226095
- DoKQTrabesingerAHKirsten-KrügerMSchizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivoEur J Neurosci2000123721372811029642
- DoKQLauerCJSchreiberWγ-Glutamylglutamine and taurine concentrations are decreased in the cerebrospinal fluid of drug-naive patients with schizophrenic disordersJ Neurochem199565265226627595563
- BerkMNgFDeanODoddSBushAIGlutathione: a novel treatment target in psychiatryTrends Pharmacol Sci20082934635118538422
- DeanOGiorlandoFBerkMN-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of actionPsychiatry Neurosci2011367886
- SheehanDVLecrubierYSheehanKHThe Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10J Clin Psychiatry19985922339881538
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders4th edWashingtonAmerican Psychiatric Press1994
- WachowiczBKustronJEffect of cisplatin on lipid peroxidation in pig blood plateletsCytobios19927041471511628
- JahnBHänschGMOxygen radical generation in human platelets: dependence on 12-lipoxygenase activity and on the glutathione cycleInt Arch Allergy Appl Immunol19909373791964932
- WalkowiakBMichalakEKoziolkiewiczWCierniewskiCSRapid photometric method for estimation of platelet count in blood plasma or platelet suspensionThromb Res1989567637762633404
- PagliaDEValentineWNStudies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidaseJ Lab Clin Med1967701581696066618
- BradfortMMA rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye bindingAnal Biochem197672248254942051
- Dietrich-MuszalskaAOlasBThe changes of aggregability of blood platelets in schizophreniaWorld J Biol Psychiatry20091017117619514099
- LapetinaEGThe signal transduction induced by thrombin in human plateletsFEBS Lett19902684004042166695
- RípováDStruneckáAPlatilováVHöschlCPhosphoinositide signalling system in platelets of schizophrenic patients and the effect of neuroleptic therapyProstaglandins Leukot Essent Fatty Acids19996112512910509868
- WalshMTRyanMHillmannAElevated expression of integrin alpha(IIb), beta(IIIa) in drug-naïve, first-episode schizophrenic patientsBiol Psychiatry20025287487912399140
- AllebeckPSchizophrenia: a life-shortening illnessSchizophr Bull19891581892717890
- AllebeckPWistedtBMortality in schizophrenia. A ten-year follow-up based on the Stockholm Country inpatient registerArch Gen Psychiatry1986436506533718167
- RuschenaDMullenPEBurgessPSudden death in psychiatric patientsBr J Psychiatry19981723313369715336
- RossBMPhospholipid and eicosanoid signaling disturbances in schizophreniaProstaglandins Leukot Essent Fatty Acids200369640741214623494
- KulogluMUstundagBAtmacaMCanatanHTezcanAECinkilincNLipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorderCell Biochem Funct20022017111711979513
- MahadikSPMukherjeeSFree radical pathology and antioxidant defence in schizophrenia: a reviewSchizoph Res199619117
- RaffaMMechriAOthmanLBFendriCGahaLKerkeniADecreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patientsProg Neuropsychopharmacol Biol Psychiatry2009331178118319576938
- BuckmanTDKlingASutphinMSSteinbergAEidusonSPlatelet glutathione peroxidase and monoamine oxidase activity in schizophrenics with CT scan abnormalities: relation to psychosocial variablesPsychiatry Res1990311141969170
- NowakPOlasBBaldEGłowackiRWachowiczBPeroxynitrite-induced changes of thiol groups in human blood plateletsPlatelets20031437537914602551
- BerryTA selenium transport protein model of a sub-type of schizophreniaMed Hypotheses1994434094147739414
- Dietrich-MuszalskaAOlasBModification of blood platelet proteins of patients with schizophreniaPlatelets200920909619235050
- MiljevicCNikolicMNikolic-KokicALipid status, anti-oxidant enzyme defence and haemoglobin content in the blood of long-term clozapine-treated schizophrenic patientsProg Neuropsychopharmacol Biol Psychiatry20103430330719962416
- PadurariuMCiobicaADobrinIStefanescuCEvaluation of antioxidant enzymes activities and lipid peroxidation in schizophrenic patients treated with typical and atypical antipsychoticsNeurosci Lett201047931732020561936
- RaffaMBarhoumiSAtigFFendriCKerkeniAMechriAReduced antioxidant defense systems in schizophrenia and bipolar I disorderProg Neuropsychopharmacol Biol Psychiatry20123937137522841966
- ZhangMZhaoZHeLWanCA meta-analysis of oxidative stress markers in schizophreniaSci China Life Sci20105311212420596963
- Dietrich-MuszalskaAOlasBIsoprostanes as indicators of oxidative stress in schizophreniaWorld J Biol Psychiatry2007817
- BartoszGPeroxynitrite: mediator of the toxic action of nitric oxideActa Biochim Pol1996436456599104501
