Abstract
Background
Cognitive decline and Alzheimer’s disease often affect older adults with Down syndrome (DS) much earlier than those in the general population. There is also growing evidence of the effects of negative life events on the mental health and behavior of individuals with intellectual disability. However, to our knowledge, this is the first study investigating objective cognitive decline following bereavement in aging individuals with DS.
Objective
The objective of this study was to determine whether cognitive decline correlates with bereavement following the recent loss of a caregiver or with behavioral changes in a sample of adult individuals with DS who do not meet the criteria for dementia or depression, using the longitudinal assessment of the Cambridge Cognitive Examination (CAMCOG), together with the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE).
Methods
We evaluated 18 subjects at baseline and over a follow-up period of 14–22 months, attempting to determine whether cognitive decline correlates with bereavement following the recent loss of the main caregiver or with behavioral changes (as assessed with the Neuropsychiatric Inventory).
Results
The mean rate of change in CAMCOG was −1.83 (standard deviation 4.51). Behavioral changes had a significant direct influence on cognitive decline. When bereavement was accompanied by behavioral changes, the probability of cognitive decline was 87% (odds ratio 3.82).
Conclusion
The occurrence of behavioral changes attributed to bereavement following the loss of the primary caregiver significantly increases the probability of cognitive decline in individuals with DS. Longitudinal comparison of the CAMCOG and use of the IQCODE appear to enrich the analysis of cognitive decline in individuals with DS. Further studies involving larger samples are needed in order to corroborate and expand upon our findings, which can have implications for the clinical management of older adults with DS.
Introduction
The genetic relationship between Down syndrome (DS) and the early development of neuropathological characteristics of Alzheimer’s disease (AD), attributed to overexpression of β-amyloid precursor protein on chromosome 21, is well documented.Citation1–Citation4 Although dementia is considered the major problem faced by aging individuals with DS,Citation5 there is a lack of data regarding the distinctions between cognitive decline related to the normal aging process and that characteristic of early indicators of neurodegenerative aspects of the syndrome.Citation6,Citation7 Although it is believed that, after reaching the age of 40 years, all individuals with DS have the neuropathological features of AD,Citation4 it remains unclear why only some of them show early clinical evidence of the disease. In addition, the precursors that hasten cognitive decline and trigger the clinical symptoms of AD in such individuals have yet to be identified.
With the increase in life expectancy, individuals with DS are now more likely to experience the bereavement that follows the loss of a parent or caregiver. However, family members and caregivers still underestimate the extent of the bereavement process in such individuals.Citation8–Citation10 There is considerable scientific evidence regarding the negative effects of stressful events on the physical and mental health of the population in general,Citation11–Citation13 regarding the association between depressive symptoms and a progressive decline in cognitive functionCitation14 and, more specifically, regarding bereavement as a risk factor for health problems in the elderly.Citation15–Citation17 It is also broadly hypothesized that intellectual disability (ID) is a predictor of mental health problems following bereavement.Citation9,Citation18–Citation21 Nevertheless, there have been few prospective, population-based studies of this topic.Citation22–Citation24 In addition, we were unable to identify any studies using objective cognitive measures to determine the effects that bereavement or other negative life events have on cognition in aging individuals with DS or other etiologies of ID. The recently developed concept of complicated grief (also referred to as prolonged grief disorder, traumatic grief, or persistent complex bereavement disorder) indicates the need for further investigations of the pathological grief response, in order to predict long-term functional impairments, especially in vulnerable populations.Citation12,Citation25–Citation27 The DSM-V (Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) includes persistent complex bereavement disorder in Conditions for Further Study,Citation28 encouraging new research that would enable its inclusion with robust criteria in the next edition. The risk of persistent complex bereavement disorder is heightened by increased dependency on the deceased person.Citation28 According to the ICD-10 (Tenth Revision of the International Classification of Diseases),Citation29 symptoms of normal grief occur within 1 month of the death and do not persist for more than 6 months. According to the DSM-V,Citation28 normal grief does not persist for more than 12 months in adults and 6 months in children. The Diagnostic Manual-Intellectual Disability (DM-ID): A Textbook of Diagnosis of Mental Disorders in Persons with Intellectual DisabilityCitation30 offers no adaptation for symptoms considered as part of normal grief in people with ID to those seen in the general population in the text revised edition of the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV).Citation31,Citation32 There is mounting evidence that the onset of grief is delayed and its duration is prolonged in individuals with ID.Citation22,Citation23,Citation33
Many authors had pointed out that individuals with ID are vulnerable to development of pathological responses to the death of a loved one and negative life events, such responses including aberrant behavior and disease.Citation9,Citation18,Citation19,Citation21,Citation24,Citation33 In a study investigating changes in health status, functional ability, and behavior among individuals with ID, parental death was associated with a behavioral change persisting for more than 2 years.Citation23 There is also evidence that changes in the personality and behavior of adult individuals with DS often indicate prodromal (early stages of) AD,Citation34–Citation39 underscoring the importance of investigating the relationships between bereavement, behavioral changes, and cognitive decline.
The instruments used for detection of cognitive decline and dementia in the general population are not well adapted for use in individuals with ID.Citation30,Citation40,Citation41 AD remains one of the most commonly diagnosed and misdiagnosed mental disorders in adults with DS.Citation33 Screening instruments, such as the Mini-Mental State Examination (MMSE)Citation42 and neuropsychological assessment tests, identify signs of dementia by evaluating various cognitive functions but do not take into account pre-existing intellectual deficits, which can negatively affect the performance of individuals on such tests. A recent systematic review showed that, although numerous instruments are used in order to screen for dementia in individuals with ID, the majority of them are not well adapted for use in this population.Citation43 Some adapted instruments have recently been shown to produce consistent results in this population.Citation35,Citation40,Citation41,Citation44,Citation45 However, in many countries, such instruments have yet to be validated, which makes it difficult to assess cognitive decline and to diagnose dementia. This also impedes the referral of patients for pharmacological and psychosocial treatment to mitigate or delay the consequences of progression of the disease. Given the difficulties in assessing dementia and cognitive decline in such individuals, longitudinal comparison of individual performance has proven useful in their clinical evaluation.Citation39,Citation46–Citation49 In heterogeneous populations, such as the population with ID, the use of a cognitive assessment instrument and a scale applied to the informant has proven to be more effective in detecting early and moderate cases of dementia, as well as cognitive decline, than is the use of a single instrument.Citation50 Examples of such instruments include the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) and the Cambridge Cognitive Examination (CAMCOG).Citation51,Citation52
Our hypothesis was that a behavioral change associated with bereavement would increase the probability of cognitive decline in individuals with DS. Therefore, the objective of the present study was to investigate the correlations between cognitive decline, bereavement, and behavioral changes in adult individuals with DS not meeting the criteria for dementia or depression. To that end, we applied the IQCODE and (for longitudinal comparison) the CAMCOG.
Subjects and methods
Subjects
All the subjects were enrolled in the Aging Support Service of the Association of Parents and Friends of Individuals with Intellectual Disability of São Paulo. The service has an interdisciplinary team approach and assists people with ID over the age of 35 years focusing on health and quality of life. Physical, corporal, and artistic activities are offered to enhance cognitive and functional abilities. In 2011, from among 49 individuals with DS over 35 years of age, all enrolled in that service and none meeting the criteria for dementia, we randomly selected 21 subjects. After baseline evaluation, the selected subjects were followed for 14–22 months (mean 18.33, standard deviation [SD] 3.06). The sample size of 21 and the differences in the length of follow-up were determined by limitations related to time constraints and lack of funding, the assessments being performed by one neuropsychologist from the institution who had other responsibilities within the facility, as well as to absences on the part of the subjects (for vacation and convalescence) or loss to follow-up. The diagnosis of DS was confirmed by cytogenetic analysis and by identification of phenotypic characteristics of DS. The level of ID was not analyzed, because none of the instruments designed to assess the degree of moderate and severe disabilities have yet been validated for use in Brazil. Therefore, in Brazil, the level of ID is determined by clinical evaluation, the results of which are often inconclusive or debatable. In the baseline evaluation, a diagnosis of dementia, according to the ICD-10 and DSM-IV criteria, was one of the exclusion criteria. Of the 21 selected subjects, two were suspected of having AD in the baseline evaluation, and that suspicion was confirmed during the follow-up period, and another subject was taking antidepressant medication during follow-up. Those three subjects were therefore excluded from the analyses, and the final sample was composed of 18 individuals. By follow-up, the sample was divided in two groups: six patients with DS who experienced bereavement, and 12 patients with DS who did not. For those who experienced bereavement, the mean length of follow-up was 18.5 (SD 3.09) months compared with 18.16 (SD 3.03) months for the 12 remaining subjects.
The baseline assessments were performed at the facilities of the Aging Support Service of the Association of Parents and Friends of Individuals with Intellectual Disability of São Paulo. During the follow-up period, the majority of subjects and informants were evaluated at the same institution, except for three who were no longer enrolled in the program and were assessed in their homes.
The study was approved by the research ethics committee of the Federal University of São Paulo. All subjects and their legal guardians gave their written informed consent.
Demographic data
Of the 18 subjects, 12 were male and six were female. All were living in private residences with family members, except for one who was living alone with family supervision. The age at baseline ranged from 35 to 55 years (mean 42.44, SD 6.11). Fourteen of the subjects were illiterate. One subject had never attended school. The remaining subjects had studied in schools specialized in educating individuals with ID, although one subject had attended regular school up to the fourth grade.
Health status
The diagnosis of dementia or depression was made by a geriatrician through clinical analysis based on the criteria established in the ICD-10 and the DSM-IV.Citation29,Citation32 The attending physician was blinded to the results of the neuropsychological assessments and the cognition scores. None of the subjects met the criteria for a diagnosis of depression. Eight of the subjects had comorbidities: hypothyroidism, in eight; heart disease, in two; pulmonary hypertension, in one; digestive disorder, in one; coagulation disorder, in one; gout, in one; and high cholesterol, in one. Four subjects had only one comorbidity, whereas two had two, one had three, and one had four comorbidities. All cases with hypothyroidism were effectively treated at baseline and follow-up.
Of the 18 subjects, eight were using some type of medication. Of those eight, one was taking a neuroleptic, a medication that affects the central nervous system. The latter subject had not been recently bereaved.
Longitudinal neuropsychological evaluation
CAMCOG
The CAMCOG is a brief neuropsychological battery that is part of the Cambridge Examination for Mental Disorders of the Elderly interview.Citation52 The CAMCOG offers a wide possibility of scores, covering several cognitive functions, as well as incorporating the MMSE. The test has subscales for orientation, language, memory, attention, praxis, calculation, abstract thinking, and perception.
The CAMCOG has been applied in the general population, and it has been shown that there are significant differences between the population without dementia and that of those with early-stage or intermediate-stage dementia, in terms of the total CAMCOG score and each of the subscale scores.Citation53 The CAMCOG has been validated for use in Brazil.Citation54,Citation55 The CAMCOG longitudinal comparison has been investigated by various researchers, who achieved good results for evaluation of normal and pathological declines in the general population.Citation56–Citation58 Researchers in England adapted the CAMCOG for use in individuals with ID,Citation35 and a recent study involving the application of the CAMCOG for DS validated it for use in the ID population of Spain.Citation44
In the present study, longitudinal comparison of neuropsychological performance involved application of the CAMCOG at baseline and after 14–22 months of follow-up. The CAMCOG was administered by a professional properly trained in the use of the instrument and blinded to any previous results. As comparison data for measuring cognitive decline, we employed the results of CAMCOG longitudinal comparisons obtained in studies of populations without DS, including those conducted by Cullum et alCitation57 who reported an annual decline of 1.6 points per year; Brayne et alCitation56 who observed a normal decline of 4.7 points in 5 years; and Jonker et alCitation58 who reported an annual decline of 1.4 points for carriers of the apoE4 allele and 0.4 points for noncarriers. Although we recognize the fact that there are major differences among the studies, in terms of subject ages and sample sizes, we considered it important to cite these studies, because we were unable to identify any reference values for normal cognitive decline in aging individuals with DS. The cognitive decline observed in our study sample was quantified and correlated with the other variables evaluated.
IQCODE
The IQCODE is a widely used instrument that is easily applied to heterogeneous, uneducated populations,Citation51,Citation58,Citation60 as well as being an instrument that is applied to the informant. The IQCODE is a rating scale of cognitive and functional decline that compares the current performance of the subject with that of 10 years ago. By making this comparison over time, the instrument does not take into account a standard of behavior, but rather the standard of functionality achieved by each individual during adulthood. Therefore, the IQCODE is easily adapted for use in individuals with different levels of education or disability. The IQCODE consists of 26 items that are simple and easy to understand. It has been validated for use in Brazil, where it has been shown to be sensitive and specific in screening for dementia in the elderly.Citation60,Citation61
We found no studies using the IQCODE specifically for individuals with DS, although the 16-item short form IQCODECitation59 was used in one study evaluating individuals with ID.Citation62 The authors concluded that the short form IQCODE probably needs to be modified for use in this population, although they acknowledged that some study limitations might have resulted in suboptimal estimates and made their findings questionable. As the authors pointed out, there are many important methodological limitations, such as limited contact between the informant and subject and variations in the time frame of caregiver–subject contact (2–10 years), that is not in accordance with the procedures outlined for the IQCODE, which state that the informant (the primary caregiver) must have had daily contact with the subject for at least 10 years. In our study, we aimed to avoid the same missteps. Certain social and cultural aspects of our sample allow us to hypothesize that the IQCODE could be a suitable tool for use in our population, because individuals with DS in Brazil often live with their families, rather than being institutionalized.
In the present study, the IQCODE was administered by a trained professional. All informants who completed the questionnaire had daily contact with the subject, relationships established at least 10 years prior, and were the primary caregivers at the time of evaluation. To categorize the degree of decline, as investigated by the IQCODE, we used a total IQCODE score >3 (any cognitive decline) and the cut-off scores for dementia of ≥3.16 reported for the Brazilian population by Perroco et al.Citation60
Medication use and comorbidities
The study protocol involved collecting data related to the use of medications at baseline and during follow-up and identifying comorbidities, especially hypothyroidism.
Behavioral changes
During the follow-up period, reports of behavioral changes in the last 2 years, as measured by the Neuropsychiatric Inventory,Citation63,Citation64 were collected from the staff of the institution and from family members. In our analysis, behavioral changes were defined as any type of behavioral change identified by the Neuropsychiatric Inventory (delusions, hallucinations, dysphoria, anxiety, agitation/aggression, euphoria, disinhibition, irritability/lability, apathy, or aberrant motor activity).
Bereavement
We collected information regarding the deaths of primary caregivers during the 2 years prior to follow-up. All of our assessments were made at least 6 months after those deaths. The length of time between bereavement and the follow-up assessment tests for each patient was 24, 18, 12, 6, 12, and 24 months (n=6, mean 16, SD 6.63). The information was obtained from the individual who was the primary caregiver at the time of evaluation.
Statistical analysis
Assumptions of homogeneity between groups of demographic features were verified with the Chi-square test. We observed a heterogeneous sex distribution only for the variables ΔCAMCOG score and IQCODE cut-off score of 3.16. Therefore, for those variables, we used backward stepwise logistic regression adjusted for sex to determine the relevance of the other variables, calculating odds ratios and estimated probabilities.
To identify differences between the baseline and final evaluations, in terms of the CAMCOG subscale scores, we used the nonparametric Wilcoxon test.
We constructed receiver operating characteristic curves (ROC curves) in order to determine the relevance of the IQCODE score in relation to the degree of cognitive decline determined with the CAMCOG. For the equivalence of mean difference in CAMCOG with other studies, we used one-sample t-tests and confidence intervals.
All selected data were analyzed using Statistical Package for the Social Sciences version 14.0 software for Windows (SPSS Inc., Chicago, IL, USA). The level of statistical significance was set at 5%.
Results
Measures of cognitive decline
CAMCOG scores
Of the 18 subjects included in the study, eleven showed some degree of cognitive decline during the 14–22 months of follow-up, as evidenced by the difference between the baseline and final evaluations, in terms of the ΔCAMCOG score (mean −4.63, SD 3.17). Of the seven subjects who showed no cognitive decline, six actually showed some improvement in cognitive function and one remained the same (mean ΔCAMCOG score for the subgroup 2.57, SD 1.98). The mean ΔCAMCOG score for the sample as a whole was −1.83 points (SD 4.51, 95% confidence interval [CI] −4.07 to 0.41). This value is comparable with the −1.88 (95% CI −2.36 to −1.36, P=0.966), −2.7 (95% CI −3.58 to 1.82, P=0.427), and −3.2 (95% CI −4.0 to −2.2, P=0.216) reported for the general elderly population in a linear estimation of 2 years by Brayne et al,Citation56 Jonker et al,Citation58 and Cullum et al,Citation57 respectively.
The areas of greatest cognitive decline on the CAMCOG scale, as measured by the Wilcoxon test, were the domains of language (P=0.025), abstract thinking (P=0.046), and perception (P=0.034). Of the 18 subjects, eleven (61%) exhibited some decline in the language domain, mainly in comprehension (P=0.020).
IQCODE scores
Eleven of the 18 informants had observed a functional decline in some area, as measured by the IQCODE, within the last 10 years. In our box-plot analysis of the mean IQCODE scores, we identified three outliers indicating a functional gain (IQCODE score <2). When those three outliers were excluded, the mean IQCODE score was 3.12 (n=16, 95% CI 3.02–3.22).
CAMCOG versus IQCODE
The receiver operating characteristic curve in which the dependent variable was CAMCOG-determined cognitive decline showed that the IQCODE discriminates well between groups (ROC curve area 0.734, 95% CI 0.498–0.970).
Responses indicative of worsening on the IQCODE items related to memory (items 1–10, 15, 16, and 21) and were found to correlate significantly with a decline in the CAMCOG memory subscale score (P=0.013).
The IQCODE items that had the highest rates of “not applicable” responses were items 24 (mean 0.83, 95% CI 0.64–1.02), 23 (mean 0.72, 95% CI 0.49–0.95), 20 (mean 0.61, 95% CI 0.36–0.86), and 21 (mean 0.56, 95% CI 0.30–0.81). shows which IQCODE items had the greatest influence on the decline in the total IQCODE score, the distribution of responses by item, and the relative risk of positive response in each item, stratified by the IQCODE score (≥3.16).
Table 1 Informant Questionnaire on Cognitive Decline in the Elderly items and its relationship to cognitive decline
Behavioral changes, bereavement, and cognitive change
None of our 18 subjects exhibited delusions, hallucinations, or aberrant motor activity. A total of eleven subjects exhibited at least one behavioral change, six exhibited only one, four exhibited two, and one exhibited four. Three of the subjects exhibited agitation/aggression, one exhibited euphoria, one exhibited anxiety, two exhibited apathy, two exhibited dysphoria, four exhibited disinhibition, and five exhibited irritability/lability. Six subjects lost their primary caregiver during the study period, and all of them exhibited behavioral changes after the loss.
We found that behavioral changes had a direct influence on cognitive decline (P=0.049). For the eleven subjects who exhibited behavioral changes, the mean ΔCAMCOG score was −3.81 (SD 3.78), compared with 1.28 (SD 3.90) for the seven remaining subjects (P=0.035), indicating that the subjects exhibiting no behavioral changes also showed no cognitive decline.
Six subjects had recently gone through a period of bereavement after the death of their primary caregiver, and all of those subjects exhibited subsequent behavioral changes, compared with only half of the subjects who had not recently been bereaved. Five of those six subjects (83%) also showed a decline in their CAMCOG score. For those who experienced bereavement, the mean ΔCAMCOG was −4.16 (SD 4.41, 95% CI −8.43, −0.10), compared with −0.66 (SD 4.07, 95% CI −3.47, 2.14) for the 12 remaining subjects (comparison not considered statistically significant). shows the differences in the subscale scores after follow-up between the two groups, and describes the differences including demographic characteristics, length of follow-up time, CAMCOG total score, and subscale scores, as well as behavioral changes.
Figure 1 Differences in subscale scores and total score on the CAMCOG, as well as the MMSE score, between bereaved and not bereaved groups after 2 years of follow-up in 18 individuals with Down syndrome.
Abbreviations: CAMCOG, Cambridge Cognitive Examination; MMSE, Mini-Mental State Examination; n, number of subjects.
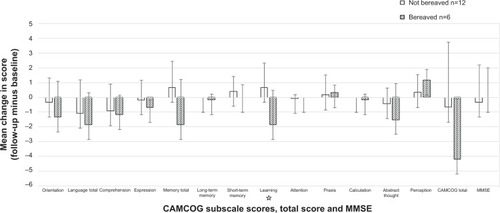
Table 2 Differences between groups: bereaved and not bereaved
Using the Kolmogorov–Smirnov test, we found that the IQCODE data did not follow a normal distribution. Therefore, to assess the relevance of the variables to the IQCODE score, we stratified the results into two categories: worse performance (IQCODE score >3) and the same or better performance (IQCODE score ≤3). Using logistic regression in which the dependent variable was an IQCODE score >3, the independent variables being behavioral change and bereavement, we found that behavioral changes worsened IQCODE performance. When there was a behavioral change, the probability of a cognitive decline, as measured by the IQCODE, was 48% in the absence of bereavement (odds ratio 16.00, 95% CI 1.13–12.93, P=0.030) compared with 87% in the presence of bereavement (odds ratio 3.82, 95% CI 1.31–194.62, P=0.031).
Additional variables
Age and the MMSE score showed no statistically significant correlation with the CAMCOG scores or with a worsening of the IQCODE score. Cognitive change, whether determined by IQCODE, CAMCOG, or MMSE, was not found to correlate significantly with the length of the follow-up period, literacy, sex, medication use, or comorbid hypothyroidism.
Discussion
The major finding of this study is that behavioral changes associated with death of the primary caregiver significantly increased the probability of cognitive decline in adult individuals with DS. Our results suggest that clinicians should take behavior and bereavement into consideration when dealing with aging individuals with DS. Because of the small size of our study sample, this finding should be interpreted with caution. Further studies are needed in order to corroborate our results, warranting replication and representing encouragement on future investigation of the intersection of DS with aging and thanatology, a small but growing field in terms of the interest shown by researchers.Citation65 In addition, the investigation of bereavement in individuals with DS or other forms of ID could also aggregate knowledge to further the understanding of persistent complex bereavement disorder, as proposed in the DSM-V.Citation28
Another important finding of the present study is that behavioral change was identified as a major variable in relation to occurrence of cognitive decline as measured by CAMCOG and IQCODE. On the basis of this finding and the observation that a lack of behavioral change appeared to correspond with a lack of cognitive decline, we believe that, in individuals with DS, behavioral changes precede or occur concomitantly with cognitive decline, in agreement with the results of other cross-sectional and longitudinal studies involving larger samples.Citation13,Citation35–Citation39 Nevertheless, DM-IDCitation30 calls attention to the fact that there is need for more research to better define which behaviors are related to cognitive decline and dementia. Cognitive change measured by IQCODE was significantly different between the bereaved and not bereaved groups, indicating a greater decline for those who were bereaved. Although the difference in CAMCOG was not significantly different between the groups, it is possible to observe that the range interval for those who were bereaved is always negative, while for the remaining subjects the interval is centered at zero, suggesting that results with larger samples could reach significance.
Due to their ID, our subjects performed poorly on some of the CAMCOG subscales in the initial and final evaluations, which confirms the appropriateness of adaptation of the instrument to the specifics of the population of individuals with ID.Citation35 Similarly, we observed that there was a high rate of “does not apply” responses to the IQCODE items related to handling money and dealing with finances (items 23 and 24), which had no impact on the total IQCODE score, but suggests that the instrument should be modified for use in this population. Although longitudinal analyses using tools adapted to this population might be more sensitive to detailed analysis of cognition of adults with DS, we can conclude that the use of the CAMCOG and the IQCODE would be useful to complement assessment of cognitive decline and assist in the diagnosis of dementia.
The fact that none of our subjects were diagnosed with depression (as defined in the ICD-10 and DSM-IV), together with the evidence that cognitive decline is associated with bereavement and behavioral changes, raises the question of whether the diagnostic criteria for depression are appropriate and sufficient for use in this population, since assessment of mood is considered a challenge when dealing with patients with ID.Citation30,Citation33
The main limitations of our study were its small sample size and failure to assess the degree of ID prior to inclusion of the subjects. In addition, the lack of instruments adapted for use in the target population can be considered a limitation, as can the lack of validated quantitative instruments to examine in detail the experience of bereavement in the subjects themselves, rather than solely on the basis of information obtained from the primary caregiver. It is also important to consider that although we were careful about length of time between bereavement and assessment according to ICD-10 and DSM-IV criteria, there is no consensus about the length of time needed to capture changes after bereavement in people with ID. Some authors point to the possibility that the normal bereavement period could be extendedCitation22 and its consequences can often appear after years of loss through other triggers.Citation33 Because of this, future research with constant follow-up could allow a better understanding of the duration of grief and its expression in cognition.
Considering the complexity of the theme investigated, we can state that our study raised many questions that merit further investigation. Should individuals with ID be considered at risk for developing persistent complex bereavement disorder? Can the cognitive decline associated with bereavement be considered a reversible feature caused by reactive depression in individuals with DS? Does the use of the current diagnostic criteria result in depression being underdiagnosed in individuals with DS? Is it possible to distinguish the effects of grief from those of dealing with structural changes following the death of a primary caregiver? Is bereavement truly a risk factor for dementia in aging individuals with DS?
Future longitudinal cognitive studies in larger samples could be useful in estimating the average expected cognitive decline in individuals with DS at various ages, in comparison with the general population, establishing criteria for differentiating between normal and pathological processes, and broadening our understanding of the mild cognitive impairment concept for this population.
Acknowledgments
The authors thank the Association of Parents and Friends of Individuals with Intellectual Disability of São Paulo for their partnership and contribution to the study, as well as all subjects and their families. This study was supported by Fundacao de Amparo a Pesquisa do Estado de São Paulo (grant 2013/11571-9) and Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (grant 2010/305512).
Disclosure
The authors report no conflicts of interest in this work.
References
- KimuraRKaminoKYamamotoMThe DYRK1A gene, encoded in chromosome 21 Down syndrome critical region, bridges between beta-amyloid production and tau phosphorylation in Alzheimer diseaseHum Mol Genet200716152317135279
- StantonLRCoetzeeRHDown syndrome and dementiaAdv Psychiatr Treat2004105057
- ZigmanWBAtypical aging in Down syndromeDev Disabil Res Rev201318516723949829
- WisniewskiKEWisniewskiHMWenGYOccurrence of neuropathological changes and dementia of Alzheimer disease in Down’s syndromeAnn Neurol1985172782823158266
- CoppusAEvenhuisHVerberneGJDementia and mortality in persons with Down syndromeJ Intellect Disabil Res20065076877716961706
- Krinsky-McHaleSJSilvermanWDementia and mild cognitive impairment in adults with intellectual disability: issues of diagnosisDev Disabil Res Rev201318314223949827
- SilvermanWPZigmanWBKrinsky-McHaleSJRyanRSchupfNIntellectual disability, mild cognitive impairment, and risk for dementiaJ Policy Pract Intellect Disabil201310245251
- CluteMAKobayashiRLooking within and reaching out: bereavement counselor perceptions of grieving adults with IDAm J Hosp Palliat Care20122958359022514033
- DowlingSHubertJWhiteSHollinsSBereaved adults with intellectual disabilities: a combined randomized controlled trial and quantitative study of two community-based interventionsJ Intellect Disabil Res20065027728716507032
- McRitchieRMcKenzieKQuayleEHarlinMNeumannKHow adults with an intellectual disability experience bereavement and grief: a qualitative explorationDeath Stud20143817918524524546
- ChaoLLYaffeKSamuelsonKNeylanTCHippocampal volume is inversely related to PTSD durationPsychiatry Res201422211912324742925
- ShearMKGetting straight about griefDepress Anxiety20122946146422730310
- HollandJMNeimeyerRABoelenPAPrigersonHGThe underlying structure of grief: a taxometric investigation of prolonged and normal reactions to lossJ Psychopathol Behav Assess200931190201
- MagariFSicoloMSpinelliLAggressive behavior, cognitive impairment, and depressive symptoms in elderly subjectsNeuropsychiatr Dis Treat2012834735322888255
- CareyIMShahSMDewildeSHarrisTVictorCRCookDGIncreased risk of acute cardiovascular events after partner bereavement: a matched cohort studyJAMA Intern Med201417459860524566983
- GrimbyABereavement among elderly individuals: grief reactions, post-bereavement hallucinations and quality of lifeActa Psychiatr Scand19938772808424323
- XavierFMFerrazMPTrentiniCMFreitasNKMoriguchiEHBereavement-related cognitive impairment in an oldest-old community-dwelling Brazilian sampleJ Clin Exp Neuropsychol20022429430111992212
- BrickellCMunirKGrief and its complications in individuals with intellectual disabilityHarv Rev Psychiatry20081611218306095
- DoddPDowlingSHollinsSA review of the emotional, psychiatric and behavioural responses to bereavement in individuals with intellectual disabilitiesJ Intellect Disabil Res20054953754315966961
- DoddPCGuerinSGrief and bereavement in individuals with intellectual disabilitiesCurr Opin Psychiatry20092244244619535983
- Hulbert-WilliamsLHastingsRPLife events as a risk factor for psychological problems in individuals with intellectual disabilities: a critical reviewJ Intellect Disabil Res2008588389518671807
- Bonell-PascualEHuline-DickensSHollinsSBereavement and grief in adults with learning disabilities. A follow up studyBr J Psychiatry199917534835010789302
- EsbensenAJSeltzerMMKraussMWStability and change in health, functional abilities, and behavior problems among adults with and without Down syndromeAm J Ment Retard200811326327718564887
- Hulbert-WilliamsLHastingsROwenDMExposure to life events as a risk factor for psychological problems in adults with intellectual disabilities: a longitudinal designJ Intellect Disabil Res201458486023627774
- MaerckerABrewinCRBryantRADiagnosis and classification of disorders specifically associated with stress: proposals for ICD-11World Psychiatry20131219820624096776
- PrigersonHGHorowitzMJJacobsSCProlonged grief disorder: psychometric validation of criteria proposed for DSM-V and ICD-11PLoS Med200968
- SimonNMTreating complicating griefJAMA201331041642323917292
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders5th edWashington, DC, USAAmerican Psychiatric Association2013
- World Health OrganizationICD-10 International Statistical Classification of Diseases and Related Health ProblemsGeneva, SwitzerlandWorld Health Organization1992
- FletcherRLoschenEStavrakakiCFirstMDiagnostic Manual-Intellectual Disability: A Text Book of Diagnosis of Mental Disorders in Persons with Intellectual DisabilityNew York, NY, USANAAD Press/National Association for the Dually Diagnosed NADD2007
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental DisordersFourth Edition Text RevisionWashington, DC, USAAmerican Psychiatric Association2000
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders4th edWashington, DC, USAAmerican Psychiatric Association1994
- McGuireDChicoineBMental Wellness in Adults with Down Syndrome: A Guide to Emotional and Behavioral Strengths and ChallengesBethesda, MD, USAWoodbine House2006
- AkahoshiKMatsudaHFunahashiMHanaokaTSuzukiYAcute neuropsychiatric disorders in adolescents and young adults with Down syndrome: Japanese case reportsNeuropsychiatr Dis Treat2012833934522888254
- BallSLHollandAJHuppertFATreppnerPWatsonPHonJThe modified CAMDEX informant interview is a valid and reliable tool for use in the diagnosis of dementia in adults with Down’s syndromeJ Intellect Disabil Res20044861162015312062
- BallSLHollandAJHonJHuppertFATreppnerPWatsonPCPersonality and behaviour changes mark the early stages of Alzheimer’s disease in adults with Down’s syndrome: findings from a prospective population-based studyInt J Geriatr Psychiatry20062166167316802281
- BallSLHollandAJWatsonPCHuppertFATheoretical exploration of the neural bases of behavioural disinhibition, apathy and executive dysfunction in preclinical Alzheimer’s disease in individuals with Down’s syndrome: potential involvement of multiple frontal-subcortical neuronal circuitsJ Intellect Disabil Res20105432033620202073
- DebSHareMPriorLSymptoms of dementia among adults with Down’s syndrome: a qualitative studyJ Intellect Disabil Res20075172673917845241
- HollandAJHonJHuppertFAStevensFIncidence and course of dementia in individuals with Down’s syndrome: findings from population-based studyJ Intellect Disabil Res20004413814610898377
- DebSHareMPriorLBhaumikSDementia screening questionnaire for individuals with intellectual disabilitiesBr J Psychiatry200719044044417470960
- PrasherVFarooqAHolderRThe Adaptive Behaviour Dementia Questionnaire (ABDQ): screening questionnaire for dementia in Alzheimer’s disease in adults with Down syndromeRes Dev Disabil20042538539715193672
- FolsteinMFFolsteinSEMcHughPRMini-mental state. A practical method for grading the cognitive state of patients for the clinicianJ Psychiatr Res1975121891981202204
- ZeilingerELStiehlKAWeberGA systematic review on assessment instruments for dementia in person with intellectual disabilitiesRes Dev Disabil2013343962397724025441
- Esteba-CastilloSDalmau-BuenoARibas-VidalNVila-AlsinaMNovell-AlsinaRGarcia-AlbaJAdaptation and validation of CAMDEX-DS (Cambridge Examination for Mental Disorders of Older Individuals with Down’s Syndrome and Others with Intellectual Disabilities) in Spanish population with intellectual disabilitiesRev Neurol201357337346 Spanish24081888
- EvenhuisHMEvaluation of a screening instrument for dementia in ageing mentally retarded personsJ Intellect Disabil Res1992363373471525439
- DasJPMishraRKAssessment of cognitive decline associated with aging: a comparison of individuals with Down syndrome and other etiologiesRes Dev Disabil19951611257701089
- Margallo-LanaMLMoorePBKayDWFifteen-year follow up of 92 hospitalized adults with Down’s syndrome: incidence of cognitive decline, its relationship to age and neuropathologyJ Intellect Disabil Res20075146347717493029
- McKensieKMurayGMcKensieSMunirJA prospective, longitudinal study of functional decline in individuals with Down’s syndromeJ Intellect Disabil1998298104
- McCarronMMcCallionPReillyEMulryanNA prospective 14-year longitudinal follow up of dementia in persons with Down syndromeJ Intellect Disabil Res201458617023902161
- BottinoCMZevallos-BustamanteSELopesMACombined instruments for the screening of dementia in older individuals with low educationArq Neuropsiquiatr20096718519019547806
- JormAFJacombPAThe Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some normsPsychol Med198919101510222594878
- RothMTymEMountjoyCOHuppertHVermaSGoddardRCAMDEX: a standardized instrument for the diagnosis of mental disorders in the elderly with special reference to the early detection of dementiaBr J Psychiatry19861496987093790869
- HuppertFAJormAFBrayneCPsychometric properties of the CAMCOG and its efficacy in the diagnosis of dementiaNeuropsychol Dev Cogn B Aging Neuropsychol Cogn19963201214
- BottinoCMAlmeidaOPTamaiSForlenzaOVScalcoMZCarvalhoIACAMDEX: The Cambridge Examination for Mental Disorders of the ElderlySão Paulo, BrazilInstituto de Psiquiatria1999 Portuguese
- BottinoCMStoppeAJrScalcoAZFerreiraRCHototianSRScalcoMZValidade e confiabilidade da versão brasileira do CAMDEX [Validity and Reliability of the Brazilian version of CAMDEX]Arq Neuropsiquiatr20015920 Portuguese
- BrayneCBestNMuirMRichardsSJGillCFive-year incidence and prediction of dementia and cognitive decline in a population sample of women aged 70–79 at baselineInt J Geriatr Psychiatry199712110711189427095
- CullumSHuppertFMcGeeMDecline across different domains of cognitive function in normal ageing: results of a longitudinal population-based study using CAMCOGInt J Geriatr Psychiatry20001585386210984733
- JonkerCSchmandBLindeboomJHaveskesLMLaunerLJAssociation between apolipoprotein e4 and the rate of cognitive decline in community-dwelling elderly individuals with and without dementiaArch Neurol199855106510699708956
- JormAFA short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic and cross-validationPsychol Med1994241451538208879
- PerrocoTRBustamanteSEMorenoMPPerformance of Brazilian long and short IQCODE on the screening of dementia in elderly people with low educationInt Psychogeriatr20092153153819323868
- SanchezMALourençoRAInformant Questionnaire on Cognitive Decline in the Elderly (IQCODE): adaptação transcultural para uso no Brasil [Transcultural Adaptation for the Brazilian Population]Cad Saude Publica200925714551465 Portuguese19578566
- ShultsJMAmanMGRojahnJPsychometric evaluation of a measure of cognitive decline in elderly individuals with mental retardationRes Dev Disabil19981963719472135
- CummingsJLMegaMGrayKRosenberg-ThompsonSCarusiBSGornbeinJThe Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementiaNeurology199444230823147991117
- CamozzatoALKochhannRSimeoniCKonrathCACarvalhoAChavesMLReliability of the Brazilian Portuguese version of the Neuropsychiatric Inventory (NPI) for patients with Alzheimer’s disease and their caregiversInt Psychogeriatr20082038339318257965
- ToddSBernakJForrester-JonesRDeath, dying and intellectual disability researchJ Appl Res Intellect Disabil20132618318523580204
