Hager K, Baseman AS, Nye JS, Brashear HR, Han J, Sano M, Davis B, Richards HM. Neuropsychiatr Dis Trea. 2014;10:391–401.
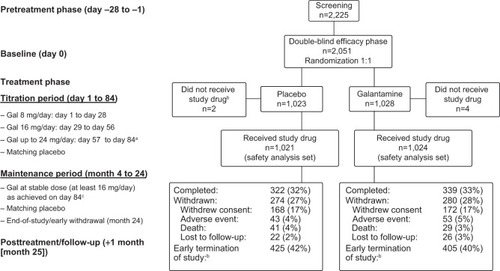
On page 393, Figure 1, “Maintenance period (month 6 to 24)” should be “Maintenance period (month 4 to 24)”; “– Gal at stable dose (at least 18 mg/day) as achieved on day 84c” should be “– Gal at stable dose (at least 16 mg/day) as achieved on day 84c”. The correct figure is shown below.
Abbreviation: Gal, galantamine.