Abstract
Though new antiepileptic drugs are emerging, approximately a third of epileptic patients still suffer from recurrent convulsions and cognitive dysfunction. Therefore, we tested whether berberine (Ber), a vegetable drug, has an anticonvulsant property and attenuates memory impairment in a pilocarpine (Pilo)-induced epilepsy model in rats. The rats were injected with 400 mg/kg Pilo to induce convulsions, and Ber 25, 50, and 100 mg/kg were administrated by the intragastric route once daily 7 days before Pilo injection until the experiment was over. Convulsions were observed after Pilo injection. For the rats that developed status epilepticus (SE), malondialdehyde, glutathione levels, superoxide dismutase, and catalase activity in the hippocampus were measured 24 hours after SE. The rats received the Morris water-maze test 2 weeks after SE, and then were killed for fluoro-jade B staining to detect the degenerating neurons. We found Ber delayed latency to the first seizure and the time to develop SE in a dose-dependent manner. Malondialdehyde levels were decreased, while glutathione and catalase activity were strengthened in Ber-injected SE rats. In the Morris water-maze test, Ber decreased escape latency compared to saline-treated SE rats. Additionally, Ber reduced the number of fluoro-jade B-positive cells in the hippocampal CA1 region. Our data suggest that Ber exerts anticonvulsant and neuroprotective effects on Pilo-induced epilepsy in rats. Simultaneously, Ber attenuates memory impairment. The beneficial effect may be partly due to mitigation of the oxidative stress burden.
Introduction
Epilepsy, a neurological disorder characterized by recurrent episodes of seizures due to an imbalance between cerebral excitability and inhibition, affects approximately 50 million people worldwide.Citation1 Seizures in 20%–30% epileptic patients cannot be controlled effectively, though new antiepilepsy drugs (AEDs) are growing in number. A large number of patients suffer from cognitive dysfunction.Citation2–Citation4 The present AEDs can only ameliorate the occurrence of seizures, while they are not helpful for cognitive dysfunction. Moreover, cognitive impairment is one of the side effects of AEDs.Citation5 Therefore, the need for newer and more efficacious AEDs is urgent.
The epilepsy model induced by pilocarpine (Pilo), a potent muscarinic cholinergic agonist, is a useful animal model to reproduce behavioral and electroencephalographic alterations similar to those in human epilepsy.Citation6 The brain processes large amounts of O2 in a relatively small mass, and has a high content of substrates available for oxidation associated with low antioxidant activities, making it extremely susceptible to oxidative damage.Citation7 Under normal conditions, there is a steady-state balance between the production of reactive oxygen species and their destruction by antioxidant systems. However, in the Pilo epilepsy model, this balance has been broken by increased reactive oxygen species production or/and by a decrease in cellular antioxidant systems, such as catalase (CAT) and superoxide dismutase (SOD). This imbalance contributes to irreversible neuronal damage of cell-membrane phospholipid, and it has been suggested as a possible mechanism of epileptic activity.Citation8 Previous studies have suggested that antioxidative therapy may have beneficial effects on epilepsy.Citation9,Citation10
Berberine (Ber) is the major active constituent of Rhizoma coptidis and other plants, and has multiple pharmacological effects, including anticholinergic, antihypotensive, antiar-rhythmic, antiosteoporotic, cardioprotective, antitumor and antimalarial effects.Citation11 In central nervous system diseases, such as cerebral ischemia, Ber has neuroprotective function through its anti-inflammatory, antioxidative and antineuronal apoptosis pharmacological properties.Citation12–Citation14 Recent research has indicated that Ber has an anticonvulsant effect on a pentylenetetrazol–maximal electroshock–kainic acid-induced epileptic model in mice.Citation15 However, the underlying mechanism remains unknown.
The present study was therefore designed to evaluate anticonvulsant activity of Ber in Pilo-induced status epilepticus (SE) rats. We also evaluated the effects of Ber on oxidative stress, memory impairment, and neuronal degeneration in this process.
Materials and methods
Animals and experimental procedures
Male Sprague Dawley rats, weighing between 220 and 250 g were used in this study. The rats were housed in a controlled environment (21°C±1°C and 60% humidity, under a 12-hour light/dark cycle, lights on at 8 am). Food and water were available ad libitum. All animal procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
The rats were randomly divided into six groups in this experiment: 1) control group (n=12), administered saline alone; 2) Ber 100 group (n=12), administered Ber (100 mg/kg) alone; 3) Pilo group (n=20), administered Pilo; 4) Pilo + Ber 25 group (n=20), administered both Ber (25 mg/kg) and Pilo; 5) Pilo + Ber 50 group (n=20), administered both Ber (50 mg/kg) and Pilo; 6) Pilo + Ber 100 group (n=20), administered with both Ber (100 mg/kg) and Pilo.
Ber was dissolved in 0.9% saline and administered by intragastric route once daily for 7 days before Pilo injection until the experiment was over. In the control group, equivalent saline was administered instead of Ber. All rats received methylscopolamine (1 mg/kg, intraperitoneally) 45 minutes before Pilo injection to minimize the peripheral effects of Pilo. Rats were continuously monitored to detect any behaviors indicative of seizure activity for 120 minutes following Pilo administration. Epileptic activity was graded according to the Racine scale.Citation16 An animal was considered to be in SE when continuous generalized seizure activity was observed without normal behavior in seizure episodes.Citation17 When rats had experienced SE for 1 hour, seizures were terminated by intraperitoneal injection of 10 mg/kg diazepam. All SE animals were given water-soaked food until they were able to eat normal dry-food pellets.
At 24 hours after SE, rats were killed for the valuation of lipid peroxidation, GSH, SOD, and CAT activity. The surviving SE rats received the Morris water maze (MWM) test 14 days after SE, and were killed for fluoro-jade B (FJB) stains after the MWM test.
Drugs and chemicals
All drugs were purchased from Sigma (St Louis, MO, USA). MDA, GSH, SOD, and CAT activity assay kits were purchased from Jiancheng Bioengineering Institute (Nanjing, People’s Republic of China).
Tissue preparation
The animals were deeply anesthetized (10% chloral hydrate, 350 mg/kg, intraperitoneally) and killed by decapitation. Their brains were rapidly dissected on ice to remove the hippocampus for determination of MDA, GSH, SOD, and CAT activities. The brain samples were weighed and kept at −70°C until analyzed. Each brain sample was then homogenized in 5% w/v 20 mM phosphate buffer, pH 7.4.
For the FJB stain, rats were deeply anesthetized and perfused transcardially with 0.9% saline (100 mL), followed by 4% paraformaldehyde (200 mL). Brains were removed and postfixed overnight in 4% paraformaldehyde, and then in a 30% sucrose solution at 4°C. When brains sank to the bottom of the solution, serial coronal sections (30 μm thickness) were made through the entire hippocampus using a sliding microtome.
Lipid peroxidation level
According to a previous experiment,Citation18 lipid peroxidation level was determined by measuring the level of thiobarbituric reactive species. This is expressed as nanomoles of MDA/g wet tissue.
Catalase activity
This was assessed according to the methods of Aebi,Citation19 in which the CAT reaction is stopped after 1 minute with a CAT inhibitor. This is expressed as mmol/min/mg protein.
Superoxide dismutase activity
As described by Misra and Fridovich,Citation20 SOD activity is expressed as U/mg protein.
Reduced glutathione level
GSH level was assayed spectrophotometrically as described by Beutler et alCitation21 and is expressed as nmol/mg protein.
FJB staining
FJB staining of brain slices was carried out to identify neurons undergoing degeneration.Citation22 Floating sections were mounted on slides and dried at room temperature. Sections were then rehydrated by sequential soaking in 100% ethanol (5 minutes), 70% ethanol (2 minutes), and distilled water (2 minutes). After 15 minutes, sections were incubated in 0.06% potassium permanganate and rinsed for 1 minute in distilled water, then immersed in a solution containing 0.0004% FJB and 0.1% acetic acid for 30 minutes. After washing, the sections were coverslipped. The number of FJB positive neuronal cells was calculated in per millimeter length of the hippocampal CA1 pyramidal cell layer.
Morris water-maze test
The MWM test is widely used for assessment of spatial memory. A test pool (diameter 140 cm, 60 cm deep) was filled with water (water temperature 22°C±1°C) made opaque by the addition of a white nontoxic paint. A platform (10×10 cm) was placed 1.5 cm below the surface of the water. The rats were placed into the pool at four different starting points. Then, rats were tested for their ability to locate the hidden platform. Rats were given two trials per day, and a trial ended when the rat escaped onto the platform. The escape latency for each trial was recorded. Each trial was started and ended manually by the experimenter. The movement of the rats was monitored by a digital camera (HVR-S270C; Sony, Tokyo, Japan).
Statistical analysis
All measurement data are expressed as means ± standard error of mean. One-way analysis of variance (ANOVA) followed by the least significant difference post hoc test was used for the analysis of FJB stain and oxidative stress study data. Two-way repeated-measure ANOVA was used to analyze data in the MWM test. For the analysis of categorical data, Fisher’s exact test was used. P<0.05 was considered statistically significant.
Results
Berberine relieved pilocarpine-induced convulsions in rats
After Pilo injection, all rats inhibited convulsive behaviors, such as head bobbing, scratching, and chewing. In the Pilo group, the first episode of seizures occurred at 16.7±2.37 minutes. However, 25 mg/kg Ber treatment significantly delayed latency to first seizure (P<0.05). In the 50 mg/kg and 100 mg/kg Ber groups, latency to first seizure was 33.8±2.75 min and 45.2±2.70 min, respectively. Compared to the Pilo group rats, latency was significantly longer (P<0.01). Time to SE in the Pilo + Ber 25 group rats was delayed significantly compared with that in the Pilo group rats (P<0.05). After Ber 50 mg/kg and Ber 100 mg/kg injection, time to SE increased to 40.8±3.13 and 50.5±3.34 minutes, respectively. They both increased significantly compared with that in the Pilo group. Rats in the control and Ber 100 groups showed no seizures in the 2 hours after saline or Ber injection. Nineteen of 20 rats developed SE and nine rats died in the Pilo group, while 14 of 20 rats developed SE and three rats died in the Pilo + Ber 50 group. The percentage SE between the two groups was significantly different (P<0.05). The incidence of SE also significantly decreased in the Pilo + Ber 100 group compared to that in the Pilo group (P<0.01). Percentage survival in Ber 100 mg/kg-treated SE rats was 90%. The experimental data are shown in .
Table 1 Effects of berberine on pilocarpine-induced convulsions in rats
Berberine decreased the degree of oxidative stress in the hippocampus 24 hours after pilocarpine-induced SE in rats
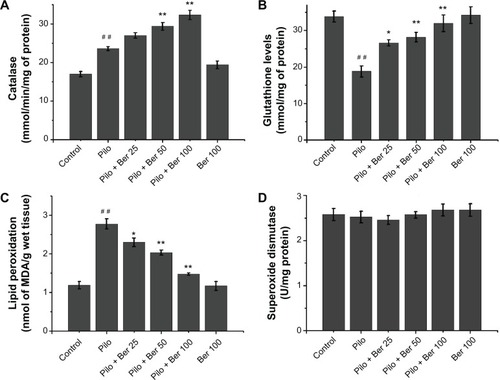
At 24 hours after SE, the CAT level of hippocampus significantly increased in Pilo-group rats compared to saline-treated control rats (P<0.01, four to six rats per group, ). In the Ber 50 and Ber 100 groups, hippocampal CAT levels were significantly higher than in the saline-treated SE rats (P<0.01, four to six rats per group, ). There was a significant fall in GSH levels in hippocampal tissue of SE rats compared to saline-treated control rats (P<0.01, four to six rats per group, ). In the Ber 25 group, GSH levels in the hippocampus significantly increased compared with saline-treated SE rats (P<0.05, four to six rats per group, ). With increased Ber dose, there were significantly growing GSH levels of hippocampal tissue in the 50 and 100 mg/kg Ber treatment groups (both P<0.01, four to six rats per group, ). The effects of administration of Ber on lipid peroxidation are depicted in . Compared with saline-treated control rats, there was a significant rise in MDA levels in the hippocampus in SE rats. Ber (25, 50 and 100 mg/kg) significantly reduced MDA levels in the hippocampus compared to SE rats in the Pilo group (P<0.05, P<0.01, and P<0.01, respectively, four to six rats per group, ). As in our previous study,Citation23 the SOD level in hippocampus showed no significant change between SE rats and saline-treated control rats. Three doses of Ber injection had no effect on SOD level (all P>0.05, four to six rats per group, ). Ber 100 mg/kg per se did not influence MDA, CAT, SOD, or GSH levels.
Figure 1 Effects of berberine (Ber) on catalase (A), glutathione level (B), lipid peroxidation level (C), and superoxide dismutase (D) activities in hippocampus 24 hours after Pilo-induced status epilepticus in rats (n=4–6 in each group). Results are expressed as means ± standard error of mean.
Abbreviations: MDA, malondialdehyde; Pilo, pilocarpine; Ber 25, berberine 25 mg/kg; Ber 50, berberine 50 mg/kg; Ber 100, berberine 100 mg/kg.
Berberine prevented memory impairment 2 weeks after pilocarpine-induced SE in rats
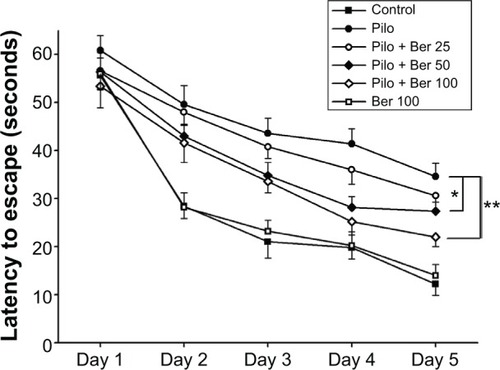
The mean escape latency for the trained rats decreased over the course of the learning trials in all the groups. Two-way repeat-measure ANOVA revealed that Ber (50 and 100 mg/kg) significantly decreased escape latency in SE rats compared to saline-treated SE rats (P<0.05 and P<0.01, respectively, five rats per group). However, Ber 25 mg/kg had no effect on escape latency. There was no significant difference in escape latency between saline-treated control rats and Ber 100 mg/kg alone-treated rats (P>0.05, five rats per group, ).
Figure 2 Effects of berberine (Ber) on latency to escape in Morris water-maze test 14 days after pilocarpine (Pilo)-induced status epilepticus (SE) in rats. Two-way repeat-measure analysis of variance revealed that Ber (50 and 100 mg/kg) significantly decreased escape latency in SE rats compared to saline-treated SE rats (P<0.05 and P<0.01, respectively; n=5 in each group). Results are expressed as means ± standard error of mean.
Abbreviations: Ber 25, berberine 25 mg/kg; Ber 50, berberine 50 mg/kg; Ber 100, berberine 100 mg/kg; SE, status epilepticus.
Berberine alleviated the number of FJB-positive cells in hippocampal CA1 20 days after pilocarpine-induced SE in rats
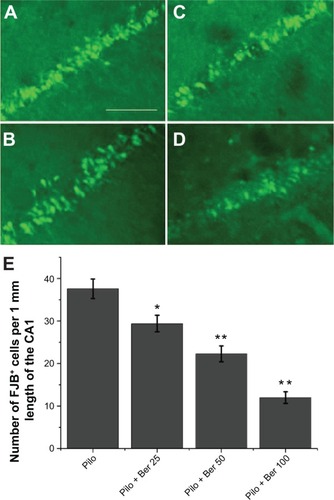
At 20 days after SE, no FJB-positive cells (degenerating neurons) were detected in hippocampus CA1 in control rats or Ber 100 mg/kg alone-treated rats. Abundant FJB-positive cells were observed in CA1 of saline-treated SE rats (). Compared with the saline-treated SE rats, the Ber 100 mg/kg-treated SE rats had significantly lower amounts of FJB-positive cells in CA1 (P<0.01, five rats per group, ). The other two Ber dosages also decreased the FJB-positive cells in CA1 compared to the saline-treated SE rats (Ber 50 versus Pilo group, P<0.01; Ber 25 versus Pilo group, P<0.05; respectively; five rats per group, ).
Figure 3 Effects of berberine (Ber) on number of fluoro-jade B (FJB)-positive cells in hippocampal CA1 region 20 days after pilocarpine (Pilo)-induced status epilepticus (SE) in rats (n=5 in each group). The non-SE control rats and Ber 100 mg/kg alone-treated rats had no FJB-positive cells in hippocampal CA1 (data not shown). FJB-positive cells were abundant in the CA1 of saline-treated SE rats (A). There were significantly fewer FJB-positive cells in the CA1 in the Pilo + Ber 50 group (B), Pilo + Ber 25 group (C), and Pilo + Ber 100 group (D) compared to saline-treated SE rats. Quantitative analysis of FJB-positive cells demonstrated that Ber reduced FJB-positive cells in CA1 (E).
Abbreviations: Ber 25, berberine 25 mg/kg; Ber 50, berberine 50 mg/kg; Ber 100, berberine 100 mg/kg.
Discussion
In this study, our data suggested that Ber exerts dose-dependent anticonvulsive activity in a Pilo-induced epilepsy model in rats. Simultaneously, Ber reduced the degree of oxidative stress in the hippocampus, and alleviated neuronal degeneration in hippocampal CA1 region in SE rats. Memory impairment in SE rats was also reduced by the administration of Ber.
Ber 25, 50, and 100 mg/kg all performed an anticonvulsant function. They delayed the onset of first seizure, and reduced time to SE. Ber 100 mg/kg decreased the numbers of rats developing SE and the mortality of SE rats. This result was similar to a previous study.Citation15 The dosage of Ber used in the previous study was much lower than that in our experiment. In our preliminary dosage studies, low dosage, such as 10 mg/kg, had no effects on the seizure activity (data not shown). This may be attributed to differences in experimental animals, epileptic model, and pathway of administration.
Oxidative stress, an imbalance between prooxidants and antioxidants, has been indicated to play an important role in the pathogenesis of seizures.Citation8 Abnormal values of oxidants and antioxidant enzymes are detected in the blood of children with refractory epilepsy.Citation24 Reactive oxygen species have been implicated in the development of seizures and SE induced by Pilo.Citation25,Citation26 In our study, there was an increase in lipid peroxidation in the hippocampus of rats 24 hours after SE, which was in line with previous studies.Citation23,Citation25 The increased lipid peroxidation may do harm to the protein, lipids, and deoxyribonucleic acid in the brain.Citation27 It may account for the underlying mechanisms for the deficits in cognitive function.Citation28 Our data indicated that Ber can markedly prevent the rise in brain-lipid peroxidation demonstrated by decreased MDA level. It suggested that Ber had antioxidative potential in a Pilo-induced SE model in rats, and this pharmacological property may be dose-dependent.
CAT and SOD are important antioxidative enzymes in the brain. They can protect neuronal cells through the removal of free radicals. At 24 hours after SE, the level of CAT significantly increased in the hippocampus, in contrast to the control rats. This result is consistent with previous studies.Citation23,Citation25 The increase in CAT activity may be a compensatory mechanism accompanied by an immediate increase in free radicals.Citation29 However, the activity of SOD remained unaltered at 24 hours after SE. Ber treatment had no effect on level of SOD, which suggested Ber cannot strengthen SOD activity in this condition.
GSH is another important antioxidant agent in the brain. In the present study, Pilo-induced SE decreased the GSH level in the rat hippocampus, which damaged the antioxidant defense system and may partially have been responsible for seizures and neuronal degeneration. Freitas et al also observed that hippocampal GSH contents were decreased 24 hours after SE.Citation25 Ber significantly increased GSH levels in a dose-dependent manner compared with Pilo-induced SE rats. This demonstrated that Ber may strengthen hippocampal antioxidative ability through increasing the level of CAT and GSH, but not SOD, at this time point in Pilo-induced SE rats.
Cognitive impairment is frequently observed in epileptic patients. Though the underlying mechanisms are not well known, growing evidence indicates that oxidative stress is one of the important causes leading to memory impairment after SE.Citation30 Oxidative damage to the rat synapse in the hippocampus has been previously reported to contribute to the deficit of cognitive functions.Citation31–Citation33 In our study, Ber alleviated memory impairment in rats with epilepsy induced by Pilo. Ber had no effect on the memory function in non-SE control rats. This suggests that this dosage of Ber can suppress seizure activity while having no side effect of memory impairment. On the other hand, neuron loss is one of the mechanisms underlying cognitive impairment. Ber alleviated hippocampal neuronal degeneration, demonstrated by decreasing CA1 FJB-positive cells, in our study. It also performed a neuroprotective function in other central nervous system diseases. Besides, antioxidation, anti-inflammation, and antiapoptosis may be involved in this process.Citation12,Citation13,Citation34 Additionally, Ber blocked the transient outward potassium current and delayed the rectifier potassium current in a concentration-dependent manner in acutely isolated CA1 pyramidal neurons.Citation35 It is possible that a block of potassium currents is one of the mechanisms underlying its neuroprotective action.
In conclusion, the results of this study revealed that Ber exerts an anticonvulsant effect against Pilo-induced seizures in rats. Simultaneously, Ber attenuates memory impairment and neuronal degeneration in the hippocampus. At least in part, its beneficial effect is due to its potential to mitigate the oxidative stress burden. Therefore, this study further confirms the anticonvulsant activity of Ber, although more studies are necessary to elucidate the complete mechanism involved in these effects.
Acknowledgments
This work was supported by grants from the Xi’an Science and Technology Bureau (No.YF07168), the Shaanxi Health and Family Planning Commission (No.2010H27), the National Natural Science Foundation of China (No. 81301937) and the International Cooperation Foundation of Shaanxi Province of China (No. 2013KW-27-03).
Disclosure
The authors report no conflicts of interest in this work.
References
- DuaTde BoerHMPrilipkoLLSaxenaSEpilepsy care in the world: results of an ILAE/IBE/WHO global campaign against epilepsy surveyEpilepsia20064771225123116886987
- KapurNTransient epileptic amnesia – a clinical update and a reformulationJ Neurol Neurosurg Psychiatry19935611118411908229029
- ThompsonPJTrimbleMRAnticonvulsant serum levels: relationship to impairments of cognitive functioningJ Neurol Neurosurg Psychiatry19834632272336842230
- JambaquéIDellatolasGDulacOPonsotGSignoretJLVerbal and visual memory impairment in children with epilepsyNeuropsychologia19933112132113378127430
- OrtinskiPMeadorKJCognitive side effects of antiepileptic drugsEpilepsy Behav20045Suppl 1S60S6514725848
- CuriaGLongoDBiaginiGJonesRSAvoliMThe pilocarpine model of temporal lobe epilepsyJ Neurosci Methods2008172214315718550176
- de FreitasRLSantosIMde SouzaGFTome AdaRSaldanhaGBde FreitasRMOxidative stress in rat hippocampus caused by pilocarpine-induced seizures is reversed by buspironeBrain Res Bull2010814–550550919800952
- SudhaKRaoAVRaoAOxidative stress and antioxidants in epilepsyClin Chim Acta20013031–2192411163018
- KamidaTFujikiMOobaHAnanMAbeTKobayashiHNeuroprotective effects of edaravone, a free radical scavenger, on the rat hippocampus after pilocarpine-induced status epilepticusSeizure2009181717518672383
- ShinEJJeongJHChungYHRole of oxidative stress in epileptic seizuresNeurochem Int201159212213721672578
- ImanshahidiMHosseinzadehHPharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberinePhytother Res2008228999101218618524
- HongJSChuYKLeeHEffects of berberine on hippocampal neuronal damage and matrix metalloproteinase-9 activity following transient global cerebral ischemiaJ Neurosci Res201290248949722052603
- ZhangXWangCLiYNeuroprotection of early and short-time applying berberine in the acute phase of cerebral ischemia: Up-regulated pAkt, pGSK and pCREB, down-regulated NF-κB expression, ameliorated BBB permeabilityBrain Res20121459617022560097
- GuLLiNYuWBerberine reduces rat intestinal tight junction injury induced by ischemia-reperfusion associated with the suppression of inducible nitric oxide synthesisAm J Chin Med20134161297131224228602
- BhutadaPMundhadaYBansodKDixitPUmatheSMundhadaDAnticonvulsant activity of berberine, an isoquinoline alkaloid in miceEpilepsy Behav201018320721020638957
- RacineRJModification of seizure activity by electrical stimulation. II. Motor seizureElectroencephalogr Clin Neurophysiol19723232812944110397
- ChuKJungKHLeeSTErythropoietin reduces epileptogenic processes following status epilepticusEpilepsia200849101723173218479396
- Ruiz-LarreaMBLealAMLizaMLacortMde GrootHAntioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomesSteroids19945963833887940617
- AebiHCatalase in vitroMethods Enzymol19841051211266727660
- MisraHPFridovichIThe role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutaseJ Biol Chem197224710317031754623845
- BeutlerEDuronOKellyBMImproved method for the determination of blood glutathioneJ Lab Clin Med19636188288813967893
- SchmuedLCHopkinsKJFluoro-jade: novel fluorochromes for detecting toxicant-induced neuronal degenerationToxicol Pathol2000281919910668994
- LiuYFGaoFLiXWThe anticonvulsant and neuroprotective effects of baicalin on pilocarpine-induced epileptic model in ratsNeurochem Res20123781670168022528832
- SaadKHammadEHassanAFBadryRTrace element, oxidant, and antioxidant enzyme values in blood of children with refractory epilepsyInt J Neurosci2014124318118623919524
- FreitasRMVasconcelosSMSouzaFCVianaGSFontelesMMOxidative stress in the hippocampus after pilocarpine-induced status epilepticus in Wistar ratsFEBS J200527261307131215752349
- FreitasRMSousaFCVasconcelosSMVianaGSFontelesMMPilocarpine-induced status epilepticus in rats: lipid peroxidation level, nitrite formation, GABAergic and glutamatergic receptor alterations in the hippocampus, striatum and frontal cortexPharmacol Biochem Behav200478232733215219774
- LiangLPPatelMSeizure-induced changes in mitochondrial redox statusFree Radic Biol Med200640231632216413413
- KellerJNSchmittFAScheffSWEvidence of increased oxidative damage in subjects with mild cognitive impairmentNeurology20056471152115615824339
- FrantsevaMVVelazquezJLHwangPACarlenPLFree radical production correlates with cell death in an in vitro model of epilepsyEur J Neurosci20001241431143910762371
- AncelinMLChristenYRitchieKIs antioxidant therapy a viable alternative for mild cognitive impairment? Examination of the evidenceDement Geriatr Cogn Disord200724111917495472
- BiesselsGJKamalARamakersGMPlace learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic ratsDiabetes1996459125912668772732
- FukuiKOnoderaKShinkaiTSuzukiSUranoSImpairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidative defense systemsAnn N Y Acad Sci200192816817511795507
- GispenWHBiesselsGJCognition and synaptic plasticity in diabetes mellitusTrends Neurosci2000231154254911074263
- HuJChaiYWangYPI3K p55γ promoter activity enhancement is involved in the anti-apoptotic effect of berberine against cerebral ischemia-reperfusionEur J Pharmacol20126742–313214222119079
- WangFZhaoGChengLZhouHYFuLYYaoWXEffects of berberine on potassium currents in acutely isolated CA1 pyramidal neurons of rat hippocampusBrain Res20049991919714746925
