Abstract
Background and purpose
The cAMP response element binding protein (CREB) plays an important role in the mechanism of cognitive impairment and is also pivotal in the switch from short-term to long-term memory. Brain-derived neurotrophic factor (BDNF) seems a promising avenue in the treatment of cerebral ischemia injury since this neurotrophin could stimulate structural plasticity and repair cognitive impairment. Several findings have displayed that the dysregulation of the CREB–BDNF cascade has been involved in cognitive impairment. The aim of this study was to investigate the effect of cerebral ischemia on learning and memory as well as on the levels of CREB, phosphorylated CREB (pCREB), and BDNF, and to determine the effect of minocycline on CREB, pCREB, BDNF, and behavioral functional recovery after cerebral ischemia.
Methods
The animal model was established by permanent bilateral occlusion of both common carotid arteries. Behavior was evaluated 5 days before decapitation with Morris water maze and open-field task. Four days after permanent bilateral occlusion of both common carotid arteries, minocycline was administered by douche via the stomach for 4 weeks. CREB and pCREB were examined by Western blotting, reverse transcription polymerase chain reaction, and immunohistochemistry. BDNF was measured by immunohistochemistry and Western blotting.
Results
The model rats after minocycline treatment swam shorter distances than control rats before finding the platform (P=0.0007). The number of times the platform position was crossed for sham-operation rats was more than that of the model groups in the corresponding platform location (P=0.0021). The number of times the platform position was crossed for minocycline treatment animals was significantly increased compared to the model groups in the corresponding platform position (P=0.0016). CREB, pCREB, and BDNF were downregulated after permanent bilateral occlusion of both common carotid arteries in the model group. Minocycline increased the expression of CREB, pCREB, and BDNF, and improved cognitive suffered from impairment of permanent bilateral occlusion of both common carotid arteries.
Conclusion
Minocycline improved cognitive impairment from cerebral ischemia via enhancing CREB, pCREB, and BDNF activity in the hippocampus.
Introduction
Increasing findings have evidenced that cerebral ischemia plays a critical role in the pathogenesis of vascular cognitive impairment, and the reduction of cerebral blood flow correlates with the severity of cognitive impairment.Citation1,Citation2 Various mechanisms of neuronal injury suffered from cerebral ischemia have been proposed, including formation of free radicals, oxidative stress,Citation3,Citation4 mitochondrial dysfunction,Citation5,Citation6 inflammatory processes,Citation7 genetic factors, environmental impact factors,Citation8,Citation9 apoptosis,Citation10 and so on. These factors may interact with and amplify each other in a vicious cycle of toxicity, leading to neuronal dysfunction and cognitive impairment. The transcription factor cyclic AMP response element binding protein (CREB) and neurotrophin brain-derived neurotrophic factor (BDNF) have emerged as molecules that may play an important role in modulating mood, behavior, and memory.Citation11–Citation13 CREB and BDNF are known to be dysregulated in animal models and in patients suffering from cerebral ischemia, and are deemed to be therapeutic targets of cerebral ischemia.Citation14,Citation15
Minocycline, a tetracycline derivative, protects against cerebral ischemia via inhibiting inflammation, oxidative stress, and apoptosis.Citation16,Citation17 Previously, we have found that minocycline retarded astrocytic reactivation, and restrained oxidative stress and neuroinflammation in the hippocampus of cerebral ischemia rats.Citation18,Citation19 In the present study, we observed the expression of CREB, phosphorylated CREB (pCREB), and BDNF in the hippocampus of cerebral ischemia rats with cognitive impairment by permanent bilateral occlusion of both common carotid arteries, and explored the neuroprotective mechanism of minocycline for the treatment of cerebral ischemia injury. We found that CREB, pCREB, and BDNF were downregulated after permanent bilateral occlusion of both common carotid arteries in a model group, and minocycline attenuated cognitive impairment and upregulated CREB, pCREB, and BDNF in the hippocampus of rats with permanent bilateral occlusion of both common carotid arteries. Therefore, a hypothesis was made that minocycline upregulated CREB, pCREB, and BDNF and improved cognitive impairment from cerebral vascular factors.
Materials and methods
Animal and drug
Wistar rats (10 weeks old, female, quality 200–250 g, from the Field Zoology Research Institute of the Third Military Medical University of the People’s Republic of China) were randomly divided into sham-operated group (S) (with a mean survival time of 16 weeks), ischemia model group (M) (with permanent bilateral occlusion of both common carotid arteries), and minocycline treatment group (MT) (beginning treatment after 4 days from permanent bilateral occlusion of both common carotid arteries, minocycline was administered by douche via the stomach for 4 weeks). M and MT groups were separately subdivided into 4-, 8-, and 16-week groups. Each group had six animals. The animal model of cerebral ischemia was established with permanent bilateral occlusion of both common carotid arteries for chronic bilateral common carotid artery occlusion (bCCAo).Citation20,Citation21
Rats were anesthetized with 10% chloral hydrate (350 mg/kg, intraperitoneally) and breathed normally throughout the surgical procedure. Both common carotid arteries were exposed via a midline cervical incision and doubly-ligated with silk suture. Sham-operated animals were treated in the same manner, except that the common arteries were not ligated. The investigation was performed according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.Citation22 The animal experiments were performed according to internationally followed ethical standards and approved by the research ethics committee of Chongqing Medical University, Chongqing, People’s Republic of China.
Minocycline (100 mg/capsule; Huishi Pharmaceutical Limited Company, Shanghai, the People’s Republic of China) was diluted to 0.5 mg/mL density by normal saline. S and M groups were given the same volume of normal saline through douche via the stomach. MT group was given 50 mg/kg/d minocycline through douche via the stomach. The minocycline dosage used for animals was as described elsewhere.Citation16,Citation17
Morris water maze task
The Morris water maze task (Chinese Academy of Medical Sciences, People’s Republic of China) includes a place navigation test and spatial probe test, and is widely used in behavioral neuroscience to study spatial learning and memory.Citation23 The rats were placed in a large circular pool with an invisible platform that allows them to escape the water. The time it took a rat to find the platform and escape was measured for up to four trials a day for 5 days. The time it took to find the platform is referred to as escape latency (the earliest learning measure). After training was complete, the spatial probe trial was conducted in which the escape platform was removed from the pool and the animal allowed to swim for 120 seconds. The spatial probe test was for the measurement of preservation-of-experience (memory) capacity, ie, looking for the platform position. The time it took to find the unmoved platform (learning latency) and the times a rat crossed the corresponding position of the removed platform in 120 seconds (memory latency) were recorded.
Immunohistochemical assay
Tissue samples were collected after surgery and immediately frozen with liquid nitrogen. Prior to immunohistochemistry assay, frozen sections were prepared with a cryostat (FACS caliber; Becton Dickinson, Franklin Lakes, NJ, USA) at −20°C, dried at room temperature, and fixed with acetone. The peripheral blood mononuclear cells were routinely isolated and the slides were prepared with a cytospin. The avidin-biotin-peroxidase complex immunohistochemical assay was carried out according to the protocols we described before anti-CREB (Sigma-Aldrich Co., St Louis, MO, USA), anti-pCREB (Sigma-Aldrich Co.), and anti-BDNF (Santa Cruz, LA, CA, USA) were prepared. The second antibody, a goat anti-mouse IgG labeled with biotin, was purchased from Vector Co. (Burlingame, CA, USA). Two hundred cells were counted and the intensity of staining for each of those cells was adjusted. Five grades were employed to express the degrees of staining, which represent five reaction coefficients, respectively. The five products of every coefficient and the corresponding cell number were added up, which resulted in the value of a positive score.Citation16,Citation18 All slides were measured in duplicate. Those samples with a positive score over 10 or a frequency over 5% were considered as positive.
Western blotting
Rat tissues were dissected and homogenized in Tissue Protein Extraction Reagent (T-PER) buffer in the presence of protease inhibitors. After homogenization, the lysates were centrifuged at 100,000 × g, and the supernatants were saved for Western blotting. Equal amounts of lysates were subject to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting analysis using antibodies specific for the following: CREB (1:1,000; BioSource International, Inc., USA), pCREB (1:1,000; BioSource International, Inc.), BDNF (1:500; Sigma-Aldrich Co., St Louis, MO, USA), and β-tublin (1:200; BioSource International Inc., Camarillo, CA, USA). The optical densities of the specific bands were scanned and measured by image analysis software (Tongji Qianping Company, Wuhan, Hubei Province, People’s Republic of China).
Statistical analysis
Quantitative data were expressed as mean ± standard deviation. All statistical analyses used the SPSS software for Windows 13.0 (SPSS, Inc., Chicago, IL, USA) and Student’s t-test for intergroup analysis. Student–Newman–Keuls test was performed when variance was equal, and Games–Howell test was performed when variance was not equal. Pearson’s correlation analysis was also performed on some indices. P<0.05 was considered as statistically significant.
Results
Minocycline improved behavioral deficits
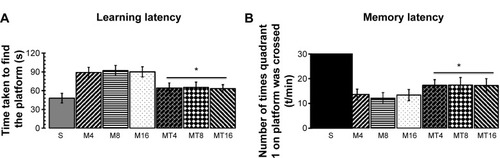
Cerebral ischemia was induced in 10-week-old Wistar rats by bCCAo as described previously.Citation18,Citation19 After the performance of bCCAo, rats were subjected to the Morris water maze. Escape latency decreased after 1 day of training. On days 3, 4, and 5, S animals immediately swam toward the platforms in the water maze, whereas M rats swam longer distances before finding the platform (). In general, escape latency decreased with bCCAo duration (P=0.0004), the M rats, after minocycline treatment, swam shorter distances than control rats before finding the platform (P=0.0007) (). In the probe trials, the number of times the platform position was crossed for the S group was more than for the bCCAo rats groups in the corresponding platform location (P=0.0000) (). The number of times the platform position was crossed for MT animals was significantly increased compared to bCCAo rat groups in the corresponding platform position (P=0.0016) ().
Figure 1 Morris water maze performance.
Abbreviations: bCCAo, bilateral common carotid artery occlusion; M, ischemia model; M4, bCCAo 4 weeks; M8, bCCAo 8 weeks; M16, bCCAo 16 weeks; MT, minocycline treatment; MT4, MT 4 weeks; MT8, MT 8 weeks; MT16, MT 16 weeks; S, sham-operated.
Minocycline upregulated CREB and pCREB
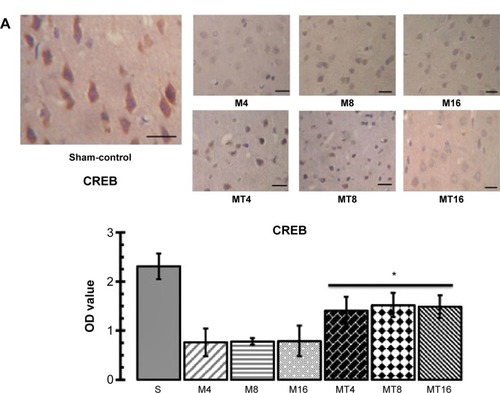
The results of immunohistochemistry and Western blotting showed that expression of CREB and pCREB in the MT group was significantly higher than that of the M group at the corresponding time. Expression of CREB and pCREB by immunohistochemistry in the M groups was more decreased than in the control group (P=0.0009; P=0.0023), whereas expression of CREB and pCREB in the MT groups was more increased than in the control groups (P=0.0001; P=0.0005) (). Expression of CREB and pCREB by Western blotting in the M groups was lowered more than in the control group (P=0.0010; P=0.0031), whereas expression of CREB and pCREB in the MT groups was more enhanced than in the control groups (P=0.0004; P=0.0003) ().
Figure 2 The expression of CREB and pCREB in the hippocampus by immunohistochemistry and Western blotting.
Abbreviations: bCCAo, bilateral common carotid artery occlusion; CREB, cAMP response element binding protein; M4, bCCAo 4 weeks; M8, bCCAo 8 weeks; M16, bCCAo 16 weeks; MT4, minocycline treated 4 weeks; MT8, minocycline treated 8 weeks; MT16, minocycline treated 16 weeks; OD, optical density; pCREB, phosphorylated CREB; S, sham-operation group.
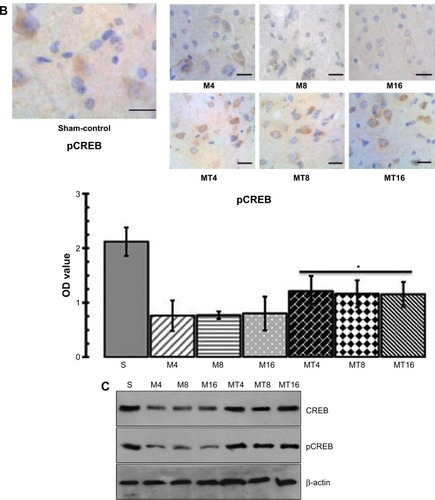
Minocycline enhanced BDNF activity
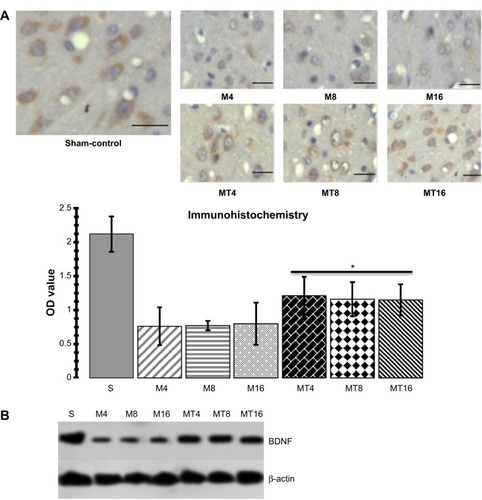
The results of immunohistochemistry showed that expression of BDNF in the MT animals was higher than that of the M ones (P=0.0005), whereas expression of BDNF in the M groups was decreased compared to the control group (P=0.0001). Western blotting analysis found that BDNF in the MT groups was higher than that of the M group (P=0.0006), while expression of BDNF in the M groups was more reduced than in the control group (P=0.0000) ().
Figure 3 Assessment of BDNF.
Abbreviations: bCCAo, bilateral common carotid artery occlusion; BDNF, brain-derived neurotrophic factor; M, ischemia model; M4, bCCAo 4 weeks; M8, bCCAo 8 weeks; M16, bCCAo 16 weeks; MT, minocycline treatment; MT4, MT 4 weeks; MT8, MT 8 weeks; MT16, MT 16 weeks; OD, optical density; S, sham-operation.
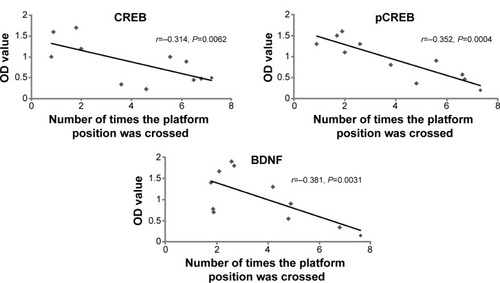
Correlation between cognition and CREB, pCREB, and BDNF
The number of times the platform position was crossed during the probe trial for the S group was higher than for M rats (P=0.0021). Therefore, we determined correlation analysis between the number of times the platform position was crossed and CREB, pCREB, and BDNF expression. Linear correlation analysis shows that the optical density (OD) values of immunoblotting protein for CREB, pCREB, and BDNF were negatively correlated with the number of platform position crossings (r=−0.314, P=0.0062; r=−0.352, P=0.0004; r=−0.381, P=0.0031) respectively (). We inferred that downregulation of CREB, pCREB, and BDNF contributed to cognitive impairment from chronic cerebral ischemia.
Figure 4 Correlation between cognition and CREB, pCREB, and BDNF.
Abbreviations: BDNF, brain-derived neurotrophic factor; CREB, cAMP response element binding protein; OD, optical density; pCREB, phosphorylated CREB.
Discussion
Minocycline, a semisynthetic tetracycline antibiotic that effectively crosses the blood–brain barrier, has been reported to have significant neuroprotective effects in cognitive impairment,Citation24,Citation25 schizophrenia,Citation26 cerebral ischemia,Citation27 amyotrophic lateral sclerosis,Citation28 Alzheimer’s disease,Citation25,Citation29 Huntington’s disease,Citation30,Citation31 and Parkinson’s diseases.Citation32 Minocycline can inhibit ischemic-induced inflammation,Citation33,Citation34 astrocyte reactivation,Citation35 microglia activation,Citation32 oxidative stress,Citation36,Citation37 apoptosis,Citation37,Citation38 and so on. One common manifestation after brain ischemic damage is cognitive impairment. In this present study, we established the cerebral ischemia model by a permanent bilateral occlusion of both common carotid arteries. The results from Morris water maze test showed that cognitive impairment occurred with the ischemic brain damage model, and cognitive impairment of control animals had been attenuated after minocycline administration. Furthermore, minocycline increased the levels of CREB, pCREB, and BDNF in the hippocampus of rats by a permanent bilateral occlusion of both common carotid arteries.
The results from the Morris water maze test showed that cognitive impairment occurred with chronic cerebral ischemia injury, and minocycline reduced cognitive impairment caused by permanent bilateral occlusion of both common carotid arteries. To further examine the mechanism by which cognitive impairment occurred with chronic cerebral ischemia and by which minocycline improved behavioral deficits, the expression of CREB, a bio-marker of memory,Citation39 was examined in the hippocampus tissue of rats. CREB, belonging to the family of leucine zipper transcription factors, is critical to induce its effects at phosphorylation of a serine residue (S133) in its kinase-inducible domain. Phosphorylation of CREB can be accomplished by a number of upstream signaling cascades.Citation40,Citation41 Studies indicate that these pathways are perturbed in patients suffering from cognitive impairment and they are also known to be influenced by anti-cognitive impairment treatment.Citation42,Citation43 CREB has a role to play in the pathogenesis of cognitive impairment and in anti-cognitive impairment action.Citation39,Citation44,Citation45 In the present study, both CREB and pCREB were downregulated after cerebral ischemia injury, and downregulation of CREB and pCREB contributed to cognitive impairment from cerebral ischemia injury by correlation analysis. Both CREB and pCREB were upregulated in the hippocampus tissue after minocycline administration. In addition, the cAMP–CREB signaling cascade is critical to the generation of new neurons in the rodent hippocampus, and also facilitates their subsequent morphological maturation. Thus, CREB’s neuroprotective and survival-enhancing properties can act in a manner analogous to that of anti-cognitive impairment. Therefore, minocycline can mediate overexpression of CREB in the hippocampus and has an anti-cognitive impairment-like effect in the process of cerebral ischemia injury.
To further clarify the mechanism by which minocycline improved behavioral deficits, the expression of BDNF (a neurotrophin that play a critical role in the development of the brain and continues to have a seminal role in shaping plasticity in the mature nervous system) was investigated. BDNF is the most widely expressed member of the nerve growth factor family of growth regulators, collectively termed the neurotrophins.Citation46–Citation48 The neurotrophins play a critical role in the development of the brain and continue to have a seminal role in shaping plasticity in the mature nervous system.Citation49 BDNF has also been shown to elicit rapid action potentials, thus influencing neuronal excitability, and it has a demonstrable role in activity-dependent synaptic plasticity events like long-term potentiation, learning tasks, and memory.Citation50,Citation51 BDNF is involved in structural remodeling, neuronal plasticity, and synaptic restructuring,Citation52,Citation53 and is promising as a candidate molecule underlying the structural changes associated with cerebral ischemia damage, and as a potential target for cerebral ischemia damage.Citation54 In the present study, BDNF was downregulated in the hippocampus tissue after chronic cerebral ischemia injury, whereas BDNF was upregulated after minocycline administration. Thus, it is speculated that minocycline has an anti-cognitive impairment-like effect in behavioral models of vascular cognitive impairment through enhancing the expression of BDNF in the hippocampus.
The hippocampus is a key limbic region whose structure and function is compromised in cognition disorders. In the hippocampus, increased activity of the CREB–BDNF cascade results in anti-cognition responses.Citation13,Citation55,Citation56 Hippocampal overexpression of BDNF and CREB is capable of mimicking both the structural consequences of sustained anti-cognition treatment as well as exerting anti-cognition-like behavioral effects.Citation56 Activation of the cAMP–CREB cascade results in increased neurogenesis of dentate granule cell progenitors, and increased dendritic length and branching. It is possible that CREB, a transcriptional activator of BDNF, recruits this neurotrophin to mediate its effects on structural plasticity.Citation57,Citation58 BDNF, in addition to being a target of CREB, can itself recruit this particular transcription factor by activating the MAP kinase cascade, thus setting up a potential positive feedback loop. Taken together, elevated CREB–BDNF, through its protective influences on vulnerable hippocampal neurons and ability to directly promote structural reorganization, could result in repair of the region known to be damaged in cognitive impairment. Moreover, the well-established role of BDNF and CREB in hippocampal-dependent learning and memory may play a critical role in ameliorating the cognitive symptoms.Citation13,Citation59 In the present study, minocycline efficiently improved behavioral deficits and increased CREB, pCREB, and BDNF that had been down-regulated by cerebral ischemia. It is possible that minocycline recruits CREB–BDNF cascade to mediate its effects on structural plasticity and set up a potential positive CREB–BDNF feedback loop. Taken together, elevated CREB–BDNF activity by minocycline, through its protective influences on vulnerable hippocampal neurons and ability to directly promote structural reorganization, could result in repair of the region known to be damaged in cognitive impairment.
Conclusion
In conclusion, this study is the first to evaluate the influence of minocycline on the transcription factors (CREB, pCREB, and BDNF) as potential key players in the treatment of vascular cognitive impairment in the process of cerebral ischemia injury. From a clinical point of view, the ability of minocycline to modulate cognitive impairment may be of great importance in the selection of neuroprotective agents, especially in chronic cerebral ischemia procedures.
Acknowledgments
This work was supported by the Youth Provincial Nature Science Foundation of Heilongjiang grant (QC2011C053) to Dr Yu Zhao, the Provincial Nature Science Foundation of Anhui grant (1308085MH158) to Dr Zhiyou Cai, and funding for basic research from the Ministry of Civil Affairs (Number: 2007-18-3-05) to Dr Zhiyou Cai.
Disclosure
The authors report no conflicts of interest in this work.
References
- BrionesTLWoodsJWadowskaMChronic neuroinflammation and cognitive impairment following transient global cerebral ischemia: role of fractalkine/CX3CR1 signalingJ Neuroinflammation2014111324447880
- LeiYGuoQLiYJiangHNiWGuYCharacteristics of cognitive impairment in adults with cerebral ischemiaZhonghua Yi Xue Za Zhi20149413984989 Chinese24851684
- GePZhaoJLiSDingYYangFLuoYInhalation of hydrogen gas attenuates cognitive impairment in transient cerebral ischemia via inhibition of oxidative stressNeurol Res201234218719422333294
- RazLZhangQGZhouCFRole of Rac1 GTPase in NADPH oxidase activation and cognitive impairment following cerebral ischemia in the ratPLoS One201059e1260620830300
- LiJMaXYuWReperfusion promotes mitochondrial dysfunction following focal cerebral ischemia in ratsPLoS One201279e4649823029539
- LiJYuWLiXTQiSHLiBThe effects of propofol on mitochondrial dysfunction following focal cerebral ischemia-reperfusion in ratsNeuropharmacology20147735836824035920
- FengXYangSLiuJElectroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured ratsMol Med Rep2013751516152223525450
- WinocurGThompsonCHakimAGreenwoodCThe effects of high- and low-risk environments on cognitive function in rats following 2-vessel occlusion of the carotid arteries: a behavioral studyBehav Brain Res201325214415623742800
- GobboOLO’MaraSMImpact of enriched-environment housing on brain-derived neurotrophic factor and on cognitive performance after a transient global ischemiaBehav Brain Res2004152223124115196790
- ChengZLiLMoXNon-invasive remote limb ischemic postconditioning protects rats against focal cerebral ischemia by upregulating STAT3 and reducing apoptosisInt J Mol Med201434495796625092271
- YuSChengQLiLLiuMYangYDingF2-(4-Methoxyphenyl) ethyl-2-acetamido-2-deoxy-β-d-pyranoside confers neuroprotection in cell and animal models of ischemic stroke through calpain1/PKA/CREB-mediated induction of neuronal glucose transporter 3Toxicol Appl Pharmacol2014277325926924726522
- ChungJYKimMWBangMSKimMIncreased expression of neurotrophin 4 following focal cerebral ischemia in adult rat brain with treadmill exercisePLoS One201383e5246123526925
- GumusluEMutluOSunnetciDThe Antidepressant Agomelatine Improves Memory Deterioration and Upregulates CREB and BDNF Gene Expression Levels in Unpredictable Chronic Mild Stress (UCMS)-Exposed MiceDrug Target Insights20148112124634580
- YaoCZhangJLiuGChenFLinYNeuroprotection by (−)-epigallocatechin-3-gallate in a rat model of stroke is mediated through inhibition of endoplasmic reticulum stressMol Med Rep201491697624193141
- QinLJingDParaudaSAn adaptive role for BDNF Val66Met polymorphism in motor recovery in chronic strokeJ Neurosci20143472493250224523540
- CaiZYanYWangYMinocycline alleviates beta-amyloid protein and tau pathology via restraining neuroinflammation induced by diabetic metabolic disorderClin Interv Aging201381089109523983461
- CaiZZhaoYYaoSBin ZhaoBIncreases in β-amyloid protein in the hippocampus caused by diabetic metabolic disorder are blocked by minocycline through inhibition of NF-κB pathway activationPharmacol Rep201163238139121602593
- CaiZYYanYSunSQMinocycline attenuates cognitive impairment and restrains oxidative stress in the hippocampus of rats with chronic cerebral hypoperfusionNeurosci Bull200824530531318839024
- CaiZYYanYChenRMinocycline reduces astrocytic reactivation and neuroinflammation in the hippocampus of a vascular cognitive impairment rat modelNeurosci Bull2010261283620101270
- ZhengPZhangJLiuHXuXZhangXAngelica injection reduces cognitive impairment during chronic cerebral hypoperfusion through brain-derived neurotrophic factor and nerve growth factorCurr Neurovasc Res200851132018289017
- KantorOSchmitzCFeiserJModerate loss of cerebellar Purkinje cells after chronic bilateral common carotid artery occlusion in ratsActa Neuropathol2007113554955817308915
- NIH committee recommends against revising the GuideThe Physiologist20075012317366943
- WenkGLAssessment of spatial memory using the radial arm maze and Morris water mazeCurr Protoc Neurosci2004 Chapter 8:Unit 8.5A
- KohmanRABhattacharyaTKKilbyCBuckoPRhodesJSEffects of minocycline on spatial learning, hippocampal neurogenesis and microglia in aged and adult miceBehav Brain Res2013242172423274840
- ChoiYKimHSShinKYMinocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s disease modelsNeuropsychopharmacology200732112393240417406652
- MatteiDDjodari-IraniAHadarRMinocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophreniaBrain Behav Immun20143817518424509090
- XuLFaganSCWallerJLLow dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion-reperfusion in ratsBMC Neurol20044715109399
- YáñezMMatías-GuiuJArranz-TagarroJAThe neuroprotection exerted by memantine, minocycline and lithium, against neurotoxicity of CSF from patients with amyotrophic lateral sclerosis, is antagonized by riluzoleNeurodegener Dis2014132–317117924356417
- FerrettiMTAllardSPartridgeVDucatenzeilerACuelloACMinocycline corrects early, pre-plaque neuroinflammation and inhibits BACE-1 in a transgenic model of Alzheimer’s disease-like amyloid pathologyJ Neuroinflammation201296222472085
- KumarAChaudharyTMishraJMinocycline modulates neuroprotective effect of hesperidin against quinolinic acid induced Huntington’s disease like symptoms in rats: behavioral, biochemical, cellular and histological evidencesEur J Pharmacol20137201–3162824211676
- KaloniaHMishraJKumarATargeting neuro-inflammatory cytokines and oxidative stress by minocycline attenuates quinolinic-acid-induced Huntington’s disease-like symptoms in ratsNeurotox Res201222431032022392362
- ZhuFZhengYDingYQMinocycline and risperidone prevent microglia activation and rescue behavioral deficits induced by neonatal intrahippocampal injection of lipopolysaccharide in ratsPLoS One201494e9396624705495
- AbrahamJFoxPDCondelloCBartoliniAKohSMinocycline attenuates microglia activation and blocks the long-term epileptogenic effects of early-life seizuresNeurobiol Dis201246242543022366182
- SolimanGMChoiAOMaysingerDWinnikFMMinocycline block copolymer micelles and their anti-inflammatory effects on microgliaMacromol Biosci201010327828819937662
- KellerAFGravelMKrizJTreatment with minocycline after disease onset alters astrocyte reactivity and increases microgliosis in SOD1 mutant miceExp Neurol20112281697921168408
- HinwoodMTynanRJCharnleyJLBeynonSBDayTAWalkerFRChronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocyclineCereb Cortex20132381784179722710611
- ChenSDYinJHHwangCSTangCMYangDIAnti-apoptotic and anti-oxidative mechanisms of minocycline against sphingomyelinase/ceramide neurotoxicity: implication in Alzheimer’s disease and cerebral ischemiaFree Radic Res201246894095022583533
- LiuXSuHChuTHGuoAWuWMinocycline inhibited the pro-apoptotic effect of microglia on neural progenitor cells and protected their neuronal differentiation in vitroNeurosci Lett2013542303623518153
- KimJKwonJTKimHSJosselynSAHanJHMemory recall and modifications by activating neurons with elevated CREBNat Neurosci2014171657224212670
- Scott BitnerRCyclic AMP response element-binding protein (CREB) phosphorylation: a mechanistic marker in the development of memory enhancing Alzheimer’s disease therapeuticsBiochem Pharmacol201283670571422119240
- SuzukiAFukushimaHMukawaTUpregulation of CREB-mediated transcription enhances both short- and long-term memoryJ Neurosci201131248786880221677163
- WangBZhaoJYuMDisturbance of Intracellular calcium homeostasis and CaMKII/CREB signaling is associated with learning and memory impairments induced by chronic aluminum exposureNeurotox Res2014261526324366850
- NamSMChoiJHYooDYEffects of curcumin (Curcuma longa) on learning and spatial memory as well as cell proliferation and neuroblast differentiation in adult and aged mice by upregulating brain-derived neurotrophic factor and CREB signalingJ Med Food201417664164924712702
- KidaSSeritaTFunctional roles of CREB as a positive regulator in the formation and enhancement of memoryBrain Res Bull2014105172424811207
- KimJKwonJTKimHSHanJHCREB and neuronal selection for memory traceFront Neural Circuits201374423519079
- AgermanKHjerling-LefflerJBlanchardMPBDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system developmentDevelopment200313081479149112620975
- YuHZhangZJShiYMCognitive function, serum BDNF levels and BDNF gene Val66Met polymorphism in amnestic mild cognitive impairmentZhong Nan Da Xue Xue Bao Yi Xue Ban2008334321325 Chinese18460776
- KiprianovaISandkühlerJSchwabSHoyerSSprangerMBrain-derived neurotrophic factor improves long-term potentiation and cognitive functions after transient forebrain ischemia in the ratExp Neurol1999159251151910506522
- HellwegRJockers-ScherüblMNeurotrophic factors in memory disordersLife Sci19945525–26216521697997075
- NacmiasBPicciniCBagnoliSBrain-derived neurotrophic factor, apolipoprotein E genetic variants and cognitive performance in Alzheimer’s diseaseNeurosci Lett2004367337938315337270
- FossatiPRadtchenkoABoyerPNeuroplasticity: from MRI to depressive symptomsEur Neuropsychopharmacol200414Suppl 5S503S51015550349
- DumanRSPathophysiology of depression: the concept of synaptic plasticityEur Psychiatry200217Suppl 330631015177086
- AlonsoMMedinaJHPozzo-MillerLERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neuronsLearn Mem200411217217815054132
- PizarroJMLumleyLAMedinaWAcute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in miceBrain Res200410251–2102015464739
- BerryAGrecoAGiorgioMDeletion of the lifespan determinant p66(Shc) improves performance in a spatial memory task, decreases levels of oxidative stress markers in the hippocampus and increases levels of the neurotrophin BDNF in adult miceExp Gerontol200843320020818065182
- JainVBaitharuIPrasadDIlavazhaganGEnriched environment prevents hypobaric hypoxia induced memory impairment and neurodegeneration: role of BDNF/PI3K/GSK3β pathway coupled with CREB activationPLoS One201385e6223523704876
- HattiangadyBRaoMSShettyGAShettyAKBrain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampusExp Neurol2005195235337116002067
- FaverjonSSilveiraDCFuDDBeneficial effects of enriched environment following status epilepticus in immature ratsNeurology20025991356136412427884
- ChenDYBambah-MukkuDPolloniniGAlberiniCMGlucocorticoid receptors recruit the CaMKIIα-BDNF-CREB pathways to mediate memory consolidationNat Neurosci201215121707171423160045
